Construction of a miRNA Panel for Differentiating Lung Adenocarcinoma Brain Metastases and Glioblastoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Tissue Sample Disruption and RNA Extraction
2.3. Library Preparation and Next-Generation Sequencing (NGS)
2.4. Identification of Differentially Expressed miRNAs
2.5. Functional Annotation and Enrichment Analysis
2.6. Verification of miRNA Expression Levels Through Quantitative Real-Time PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
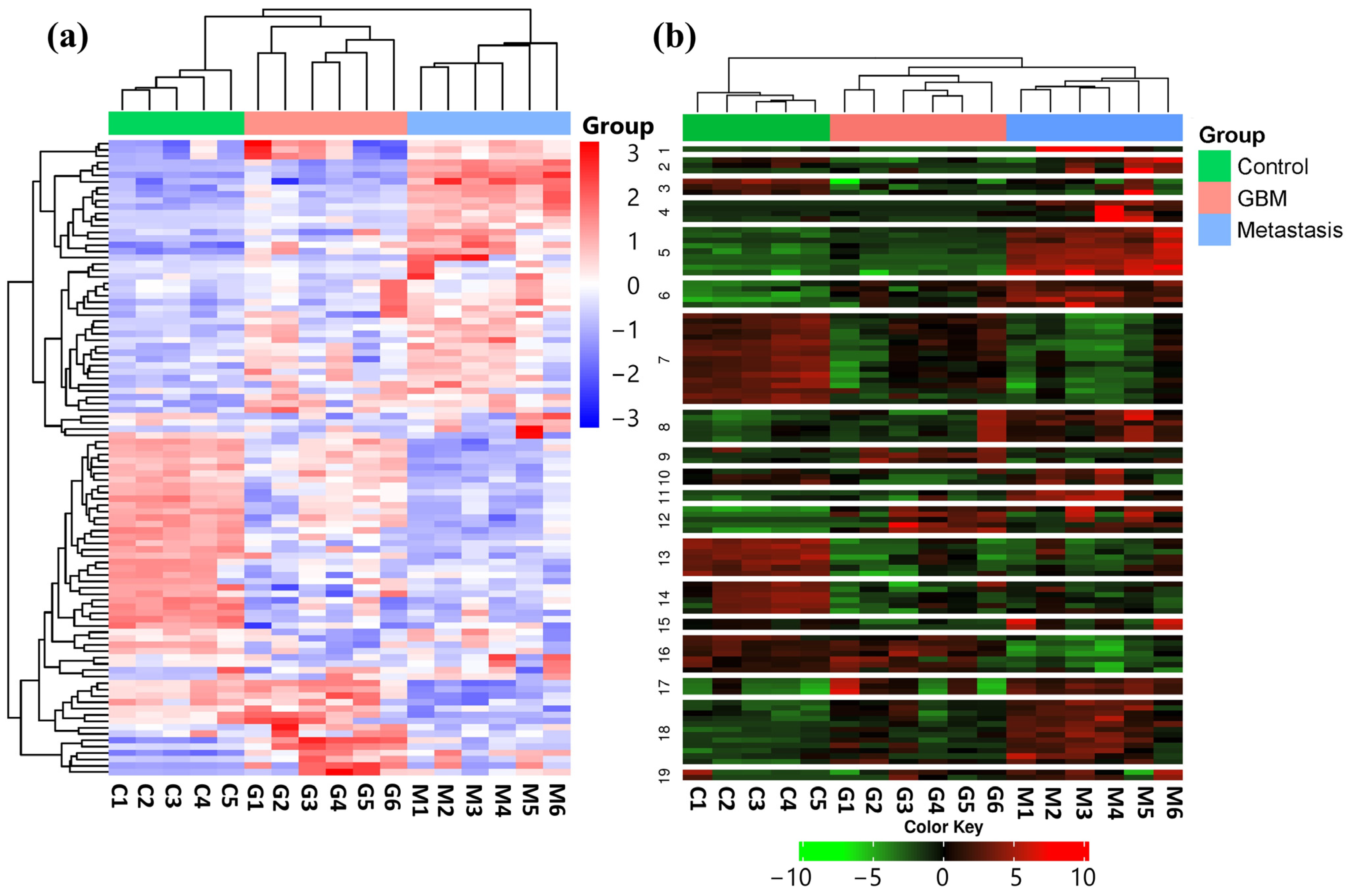
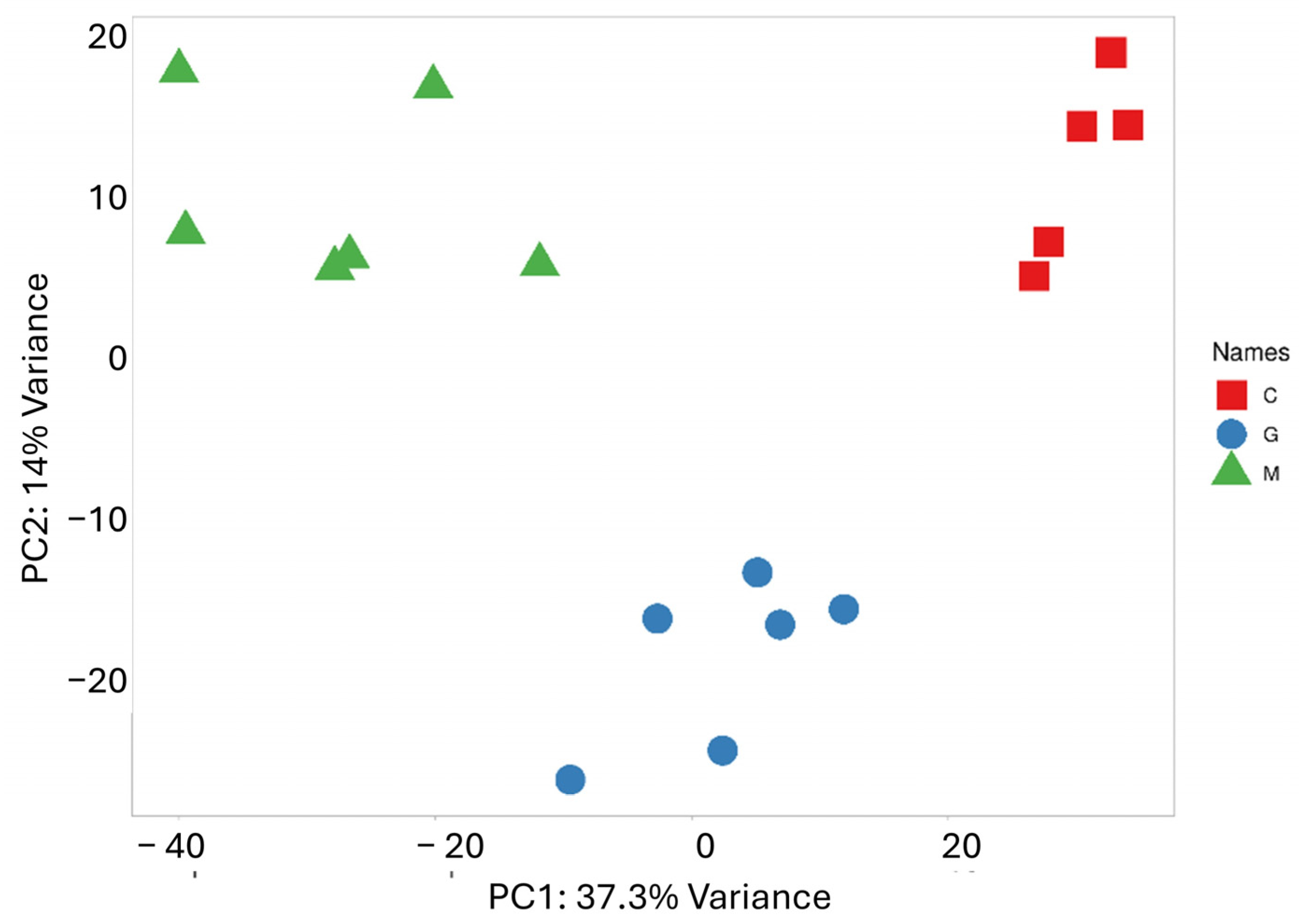
3.1. Hierarchical Clustering, K-Means Clustering, and PCA of Normalized Mirna-Seq Data
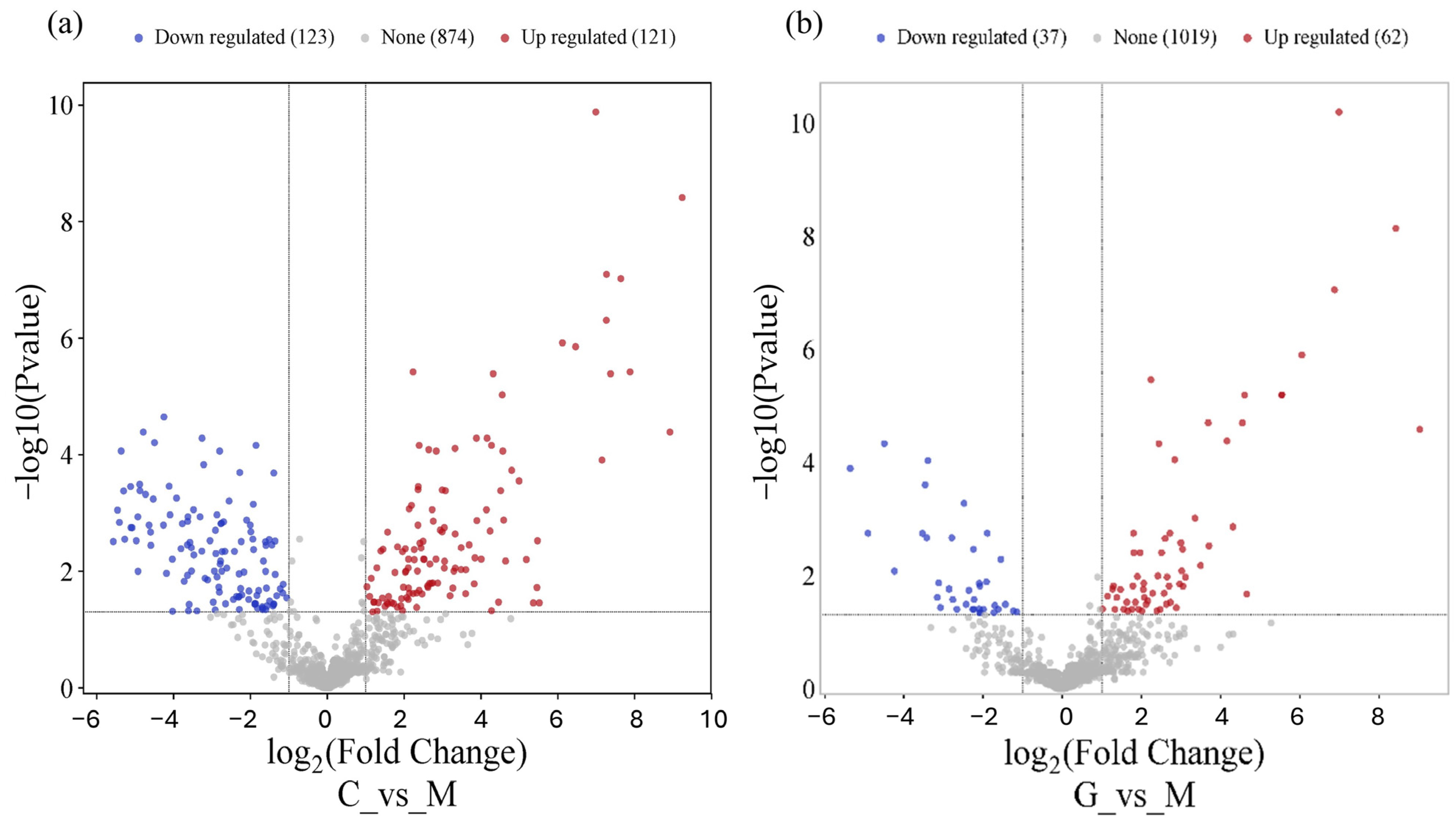
3.2. miRNA Differential Expression Analysis
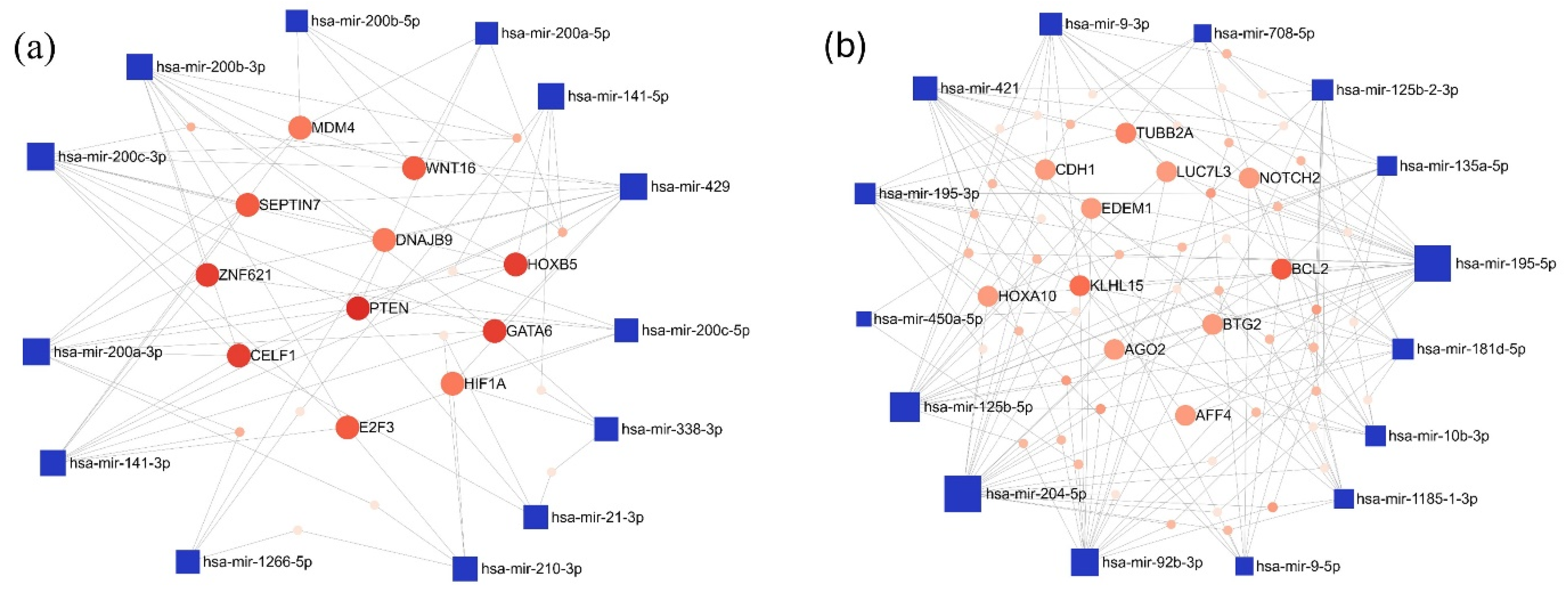
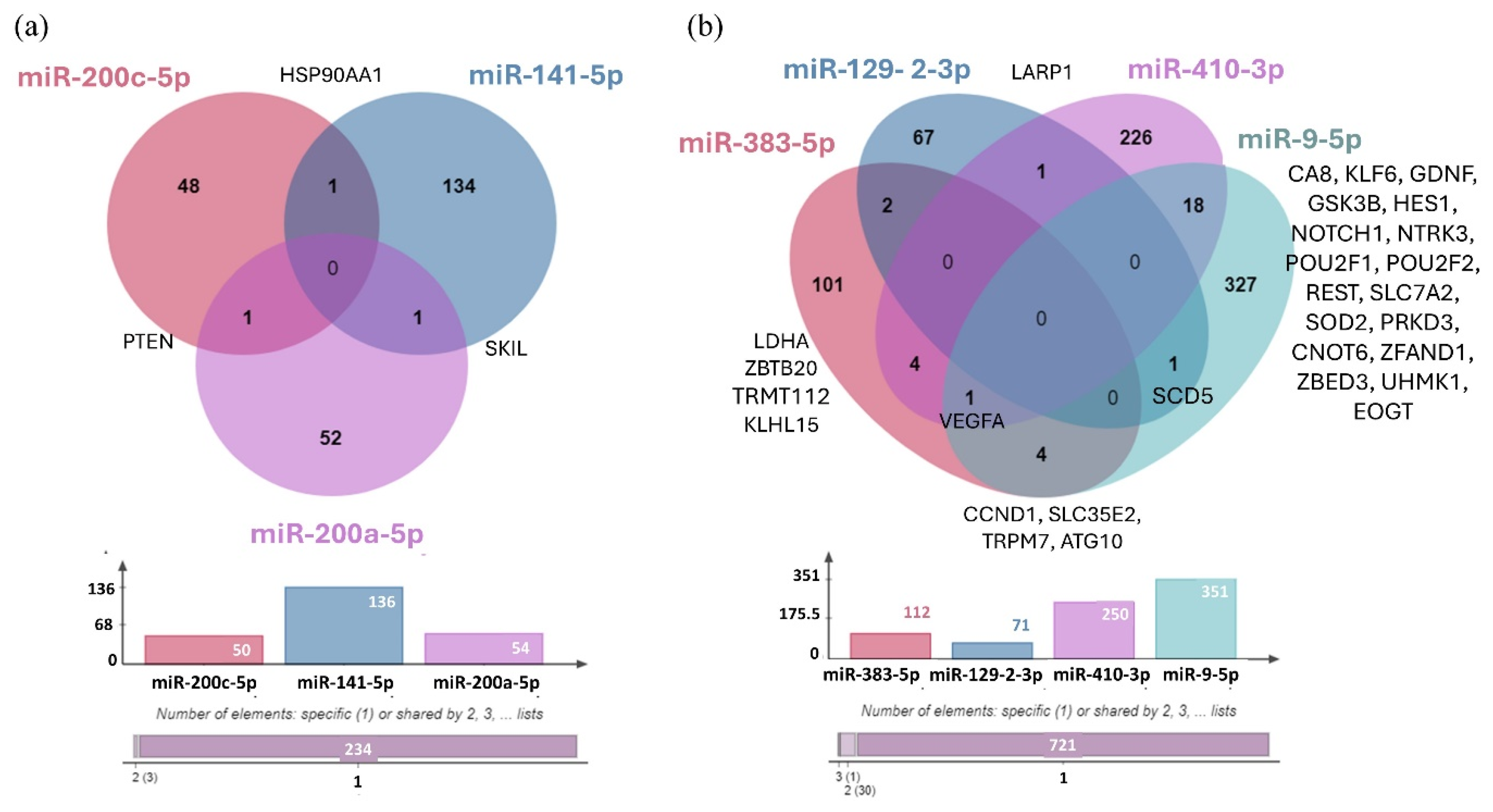
3.3. Network-Based Analysis of Differentially Expressed miRNAs
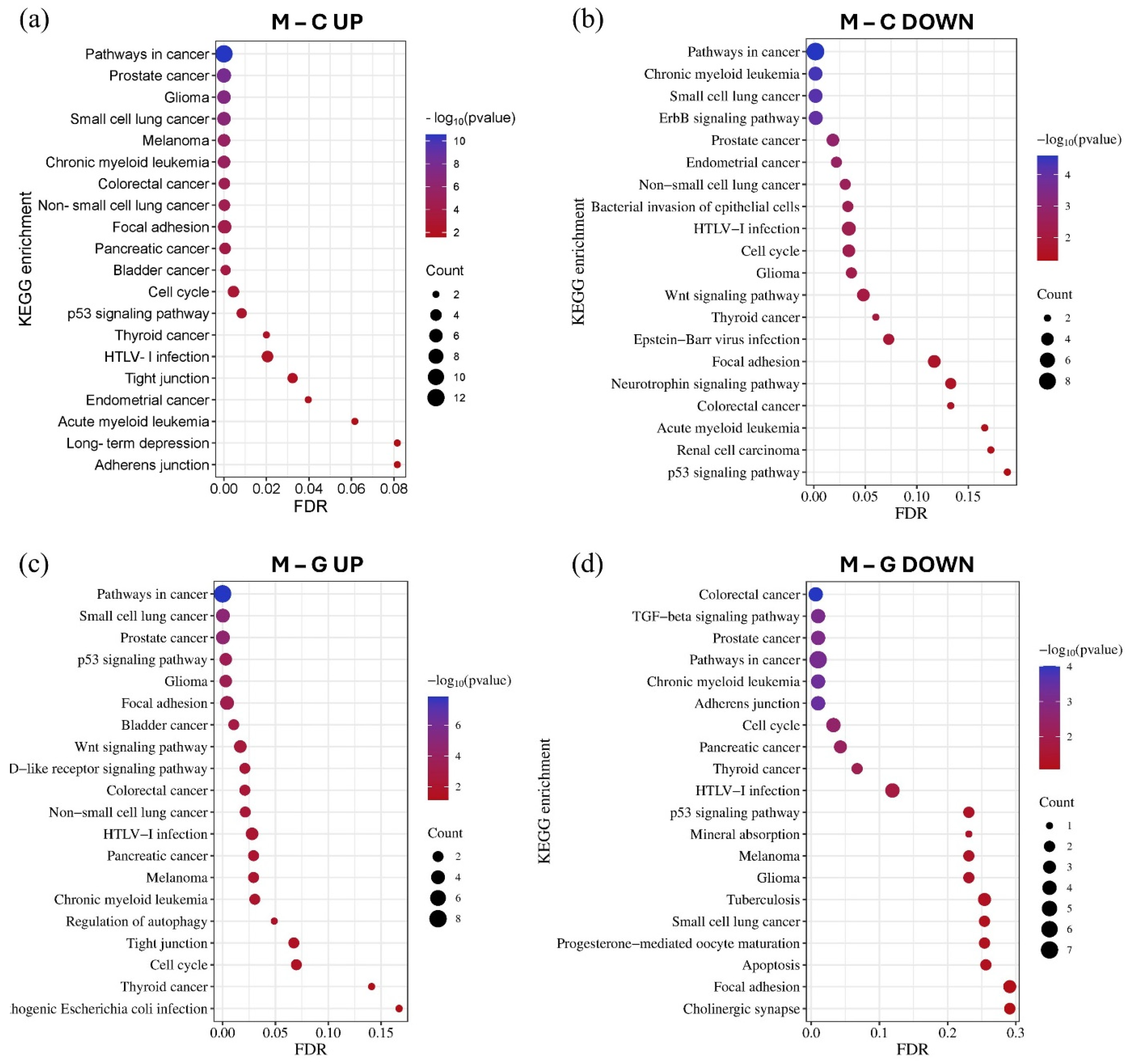
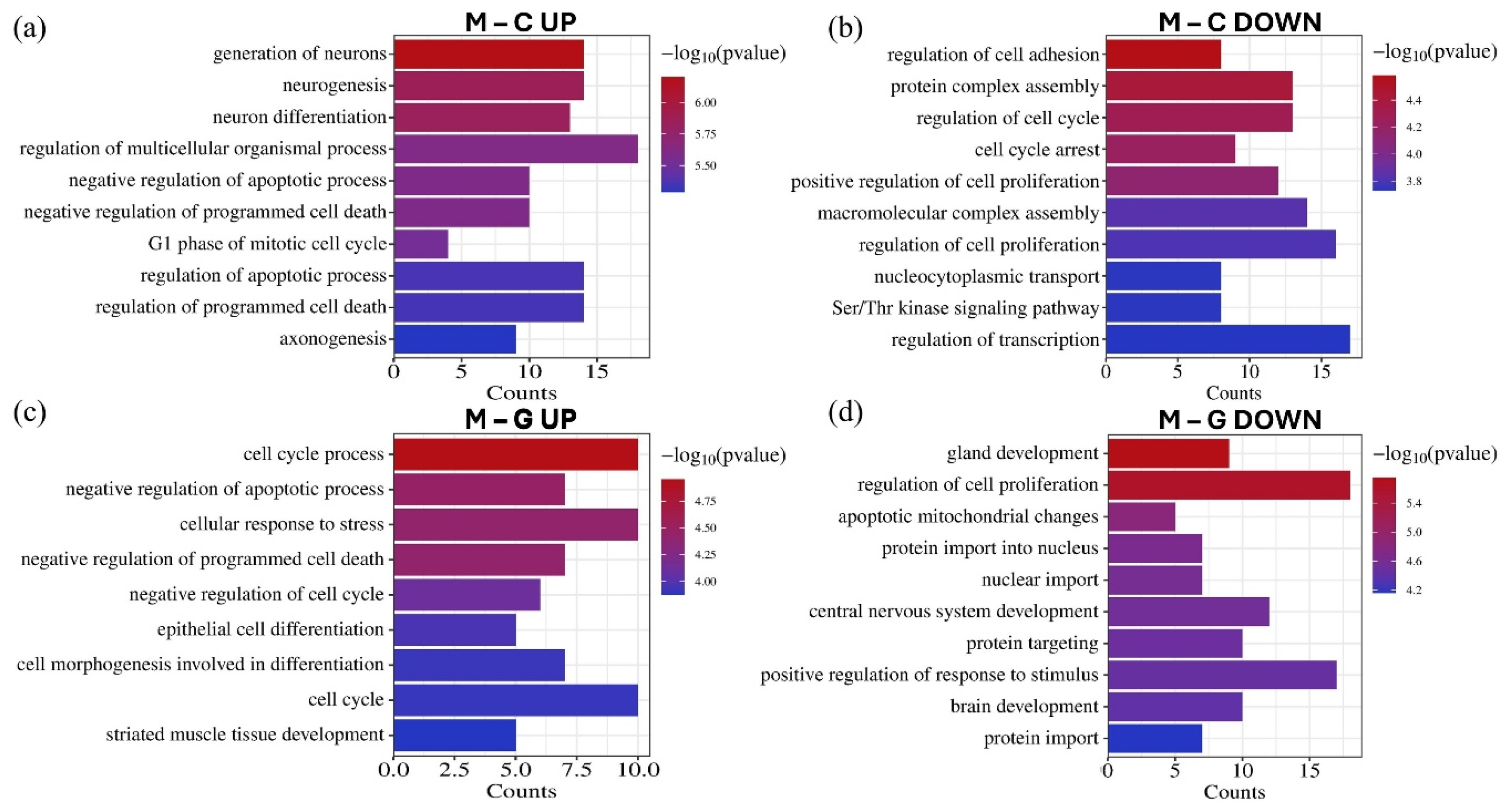
3.4. Gene Ontology (GO) and Pathway Enrichment Analysis of MiRNA
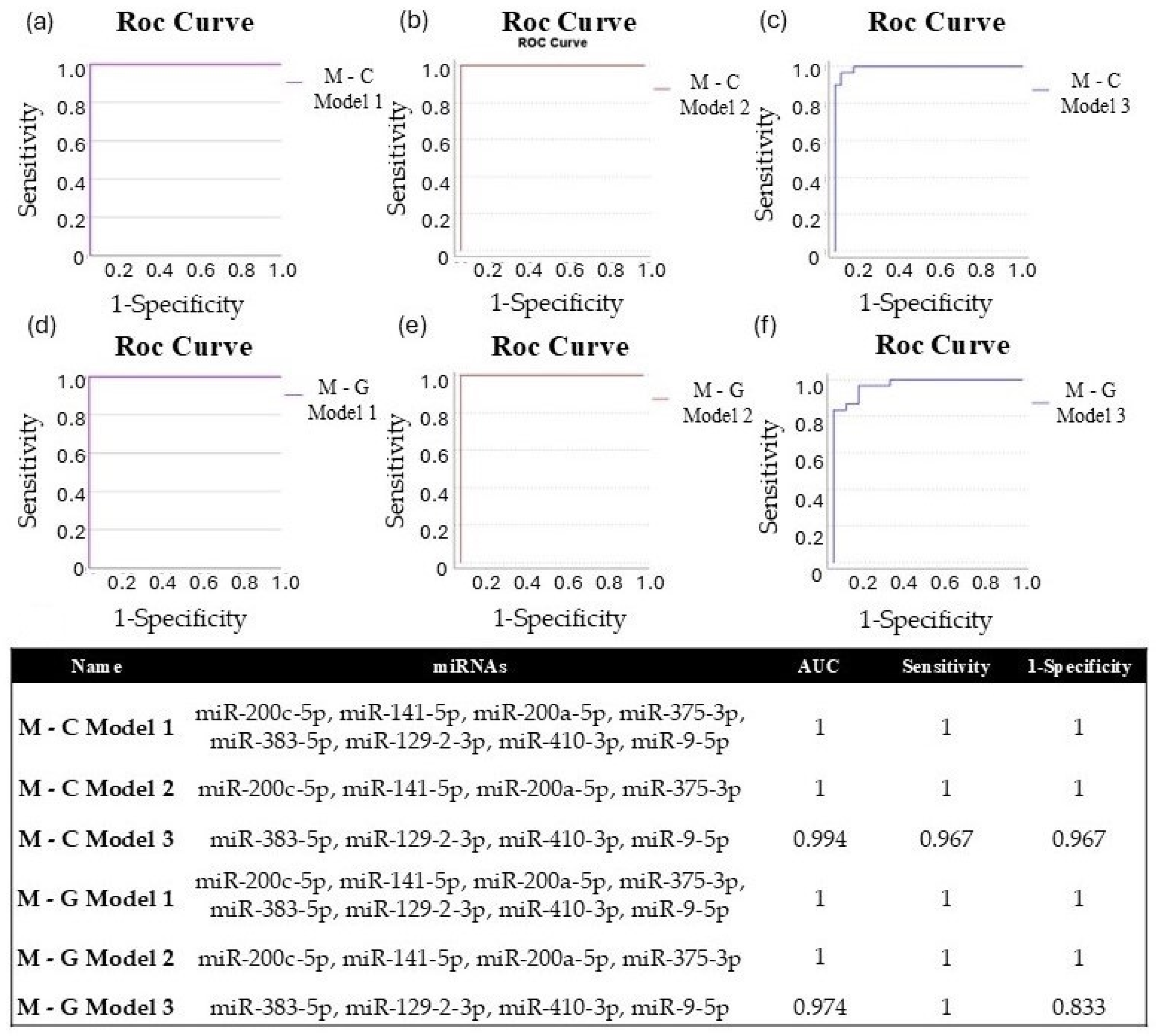
3.5. Validation of Differentially Expressed miRNAs by RT-qPCR in Tissue Samples
4. Discussion
4.1. Identification of Differentially Expressed miRNAs and Pathway Enrichment Analysis
4.2. Validation of Differentially Expressed miRNAs by RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Antonio, C.; Passaro, A.; Gori, B.; Del Signore, E.; Migliorino, M.R.; Ricciardi, S.; Fulvi, A.; de Marinis, F. Bone and brain metastasis in lung cancer: Recent advances in therapeutic strategies. Ther. Adv. Med. Oncol. 2014, 6, 101–114. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-Oncology 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Duan, W.; Xu, M.; Li, M.; Tang, M.; Wei, S.; Lin, M.; Li, E.; Liu, W.; Wang, Q. Mesothelin promotes brain metastasis of non-small cell lung cancer by activating MET. J. Exp. Clin. Cancer Res. 2024, 43, 103. [Google Scholar] [CrossRef]
- Fox, B.D.; Cheung, V.J.; Patel, A.J.; Suki, D.; Rao, G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 2011, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Bexelius, C.; Munk, V.; Leighl, N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat. Rev. 2016, 45, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.J.; Nayyar, N.; Bihun, I.; Dagogo-Jack, I.; Gill, C.M.; Aquilanti, E.; Bertalan, M.; Kaplan, A.; D’Andrea, M.R.; Chukwueke, U. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat. Genet. 2020, 52, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, M.M.; Hoz, S.; Lee, A.; Taha, M. Management of patients with multiple brain metastases. Egypt. J. Neurosurg. 2024, 39, 64. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Hviid, C.V.B.; Meldgaard, P.; Sorensen, B.S.; Sandfeld-Paulsen, B. Neurofilament Light Chain as A Biomarker for Brain Metastases. Cancers 2020, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma; Exon Publications: Brisbane, Australia, 2017; pp. 143–153. [Google Scholar]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of glioblastoma multiforme–literature review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.H.; Synowitz, M.; Badakshi, H.; Lohkamp, L.N.; Wüstefeld, J.; Schäfer, M.L.; Wiener, E. Glioblastoma multiforme versus solitary supratentorial brain metastasis: Differentiation based on morphology and magnetic resonance signal characteristics. Rofo 2013, 185, 235–240. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, X.; Zhang, S.; Ruan, X.; Gao, C.; Liu, Z.; Wei, X. Discrimination Between Solitary Brain Metastasis and Glioblastoma Multiforme by Using ADC-Based Texture Analysis: A Comparison of Two Different ROI Placements. Acad. Radiol. 2019, 26, 1466–1472. [Google Scholar] [CrossRef]
- Lee, E.J.; Ahn, K.J.; Lee, E.K.; Lee, Y.S.; Kim, D.B. Potential role of advanced MRI techniques for the peritumoural region in differentiating glioblastoma multiforme and solitary metastatic lesions. Clin. Radiol. 2013, 68, e689–e697. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Meng, L.; Yang, J.; Liu, Y.; Zhang, Y.; Peng, H.; Li, J.; Xiao, G.; Zhang, Z.; et al. Handcrafted and Deep Learning-Based Radiomic Models Can Distinguish GBM from Brain Metastasis. J. Oncol. 2021, 2021, 5518717. [Google Scholar] [CrossRef] [PubMed]
- Fordham, A.-J.; Hacherl, C.-C.; Patel, N.; Jones, K.; Myers, B.; Abraham, M.; Gendreau, J. Differentiating Glioblastomas from Solitary Brain Metastases: An Update on the Current Literature of Advanced Imaging Modalities. Cancers 2021, 13, 2960. [Google Scholar] [CrossRef]
- Kamimura, K.; Kamimura, Y.; Nakano, T.; Hasegawa, T.; Nakajo, M.; Yamada, C.; Akune, K.; Ejima, F.; Ayukawa, T.; Ito, S.; et al. Differentiating brain metastasis from glioblastoma by time-dependent diffusion MRI. Cancer Imaging 2023, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Hopkins, K.; Tonn, J.C.; Stupp, R.; Falini, A.; Cohen-Jonathan-Moyal, E.; Frappaz, D.; Henriksson, R.; Balana, C.; et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014, 15, e395–e403. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Shimato, S.; Mitsudomi, T.; Kosaka, T.; Yatabe, Y.; Wakabayashi, T.; Mizuno, M.; Nakahara, N.; Hatano, H.; Natsume, A.; Ishii, D.; et al. EGFR mutations in patients with brain metastases from lung cancer: Association with the efficacy of gefitinib. Neuro-Oncology 2006, 8, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Komaki, R.; Amini, A.; Munsell, M.F.; Unger, W.; Allen, P.K.; Chang, J.Y.; Wefel, J.S.; McGovern, S.L.; Garland, L.L.; et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 895–902. [Google Scholar] [CrossRef]
- Mathe, A.; Scott, R.J.; Avery-Kiejda, K.A. MiRNAs and Other Epigenetic Changes as Biomarkers in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2015, 16, 28347–28376. [Google Scholar] [CrossRef]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef]
- Pawelka, D.; Laczmanska, I.; Karpinski, P.; Supplitt, S.; Witkiewicz, W.; Knychalski, B.; Pelak, J.; Zebrowska, P.; Laczmanski, L. Machine-learning-based Analysis Identifies miRNA Expression Profile for Diagnosis and Prediction of Colorectal Cancer: A Preliminary Study. Cancer Genom. Proteom. 2022, 19, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Veryaskina, Y.A.; Titov, S.E.; Zhimulev, I.F. Reference Genes for qPCR-Based miRNA Expression Profiling in 14 Human Tissues. Med. Princ. Pract. 2022, 31, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lee, H.; Um, S.W.; Kim, K.; Zo, J.I.; Shim, Y.M.; Jung Kwon, O.; Lee, K.S.; Ahn, M.J.; Kim, H. Incidence of brain metastasis in lung adenocarcinoma at initial diagnosis on the basis of stage and genetic alterations. Lung Cancer 2019, 129, 28–34. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Arbiser, J.; Zelnak, A.; Shu, H.K.; Shim, H.; Robin, A.M.; Kalkanis, S.N.; Whitsett, T.G.; Salhia, B.; Tran, N.L.; et al. Current approaches to the treatment of metastatic brain tumours. Nat. Rev. Clin. Oncol. 2014, 11, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Park, M.N.; Rahman, M.H.; Rashid, M.M.; Islam, R.; Uddin, M.J.; Hannan, M.A.; Kim, B. p53 Modulation of Autophagy Signaling in Cancer Therapies: Perspectives Mechanism and Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 761080. [Google Scholar] [CrossRef]
- Thapa, N.; Wen, T.; Cryns, V.L.; Anderson, R.A. Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. Biomolecules 2023, 13, 1430. [Google Scholar] [CrossRef]
- Soini, Y. Tight junctions in lung cancer and lung metastasis: A review. Int. J. Clin. Exp. Pathol. 2012, 5, 126–136. [Google Scholar] [PubMed]
- Li, H.J.; Ke, F.Y.; Lin, C.C.; Lu, M.Y.; Kuo, Y.H.; Wang, Y.P.; Liang, K.H.; Lin, S.C.; Chang, Y.H.; Chen, H.Y.; et al. ENO1 Promotes Lung Cancer Metastasis via HGFR and WNT Signaling-Driven Epithelial-to-Mesenchymal Transition. Cancer Res. 2021, 81, 4094–4109. [Google Scholar] [CrossRef]
- Fuxe, J.; Vincent, T.; Garcia de Herreros, A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010, 9, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, re8. [Google Scholar] [CrossRef]
- Kyuno, D.; Takasawa, A.; Kikuchi, S.; Takemasa, I.; Osanai, M.; Kojima, T. Role of tight junctions in the epithelial-to-mesenchymal transition of cancer cells. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183503. [Google Scholar] [CrossRef]
- Ma, Y.; Semba, S.; Khan, R.I.; Bochimoto, H.; Watanabe, T.; Fujiya, M.; Kohgo, Y.; Liu, Y.; Taniguchi, T. Focal adhesion kinase regulates intestinal epithelial barrier function via redistribution of tight junction. Biochim. Biophys. Acta 2013, 1832, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. Int. J. Mol. Sci. 2012, 13, 4564–4590. [Google Scholar] [CrossRef]
- Li, Y.; Bai, W.; Zhang, J. MiR-200c-5p suppresses proliferation and metastasis of human hepatocellular carcinoma (HCC) via suppressing MAD2L1. Biomed. Pharmacother. 2017, 92, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, Z.; Jin, X.; Chen, W. Prognostic value and potential regulatory relationship of miR-200c-5p in colorectal cancer. J. Biochem. Mol. Toxicol. 2024, 38, e23770. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Yang, L.; Li, Y.; Wang, Z.; Ye, C. circ-ZEB1 regulates epithelial-mesenchymal transition and chemotherapy resistance of colorectal cancer through acting on miR-200c-5p. Transl. Oncol. 2023, 28, 101604. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Zhang, Y.; Yan, C.; Yang, M.; Wang, Z.; Li, W.; Li, F.; Wang, W.; Yang, Y.; et al. MicroRNA-200c-5p Regulates Migration and Differentiation of Myoblasts via Targeting Adamts5 in Skeletal Muscle Regeneration and Myogenesis. Int. J. Mol. Sci. 2023, 24, 4995. [Google Scholar] [CrossRef]
- Gu, J.; Chen, J.; Feng, J.; Liu, Y.; Xue, Q.; Mao, G.; Gai, L.; Lu, X.; Zhang, R.; Cheng, J.; et al. Overexpression of ADAMTS5 can regulate the migration and invasion of non-small cell lung cancer. Tumour Biol. 2016, 37, 8681–8689. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, B.; He, M.; Kang, Y.; Zhang, G. MicroRNA biomarkers and their use in evaluating the prognosis of lung cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 16753–16761. [Google Scholar] [CrossRef]
- Roskova, I.; Vecera, M.; Radova, L.; Trachtova, K.; Siegl, F.; Hermanova, M.; Hendrych, M.; Kren, L.; Vybihal, V.; Valekova, H.; et al. Small RNA Sequencing Identifies a Six-MicroRNA Signature Enabling Classification of Brain Metastases According to their Origin. Cancer Genom. Proteom. 2023, 20, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Qu, X.; Zhao, C.; Xu, L.; Hou, K.; Liu, Y.; Zhang, N.; Feng, J.; Shi, S.; Zhang, L.; et al. FEN1 mediates miR-200a methylation and promotes breast cancer cell growth via MET and EGFR signaling. FASEB J. 2019, 33, 10717–10730. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Jin, H.; Wang, S.; Zhang, Y.; Tang, X.; Tang, G. Screening miRNAs for early diagnosis of colorectal cancer by small RNA deep sequencing and evaluation in a Chinese patient population. OncoTargets Ther. 2016, 9, 1159–1166. [Google Scholar]
- Xie, K.; Wang, C.; Qin, N.; Yang, J.; Zhu, M.; Dai, J.; Jin, G.; Shen, H.; Ma, H.; Hu, Z. Genetic variants in regulatory regions of microRNAs are associated with lung cancer risk. Oncotarget 2016, 7, 47966–47974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.; Li, X.; Jiang, D.; Yu, H.; Wu, Q.; Gao, C.; Wu, Z. A potential biomarker hsa-miR-200a-5p distinguishing between benign thyroid tumors with papillary hyperplasia and papillary thyroid carcinoma. PLoS ONE 2018, 13, e0200290. [Google Scholar] [CrossRef] [PubMed]
- Bilski, M.; Ciesielka, M.; Orzechowska, M.; Jarosz, B.; Całka, P.; Bilska, S.; Banach, A.; Czaja, G.; Fijuth, J.; Kuncman, Ł. miR-200 family as new potential prognostic factor of overall survival of patients with WHO G2 and WHO G3 brain gliomas. Sci. Rep. 2024, 14, 29345. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Sun, J.; Wang, W.; Yong, H. Long Noncoding RNA MLK7-AS1 Promotes Non-Small-Cell Lung Cancer Migration and Invasion via the miR-375-3p/YWHAZ Axis. Front. Oncol. 2021, 11, 626036. [Google Scholar] [CrossRef]
- Mao, S.; Zheng, S.; Lu, Z.; Wang, X.; Wang, Y.; Zhang, G.; Xu, H.; Huang, J.; Lei, Y.; Liu, C.; et al. Exosomal miR-375-3p breaks vascular barrier and promotes small cell lung cancer metastasis by targeting claudin-1. Transl. Lung Cancer Res. 2021, 10, 3155–3172. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, X.; Zang, S.; Li, Y.; Feng, X.; Yuan, X. MicroRNA-383-5p acts as a prognostic marker and inhibitor of cell proliferation in lung adenocarcinoma by cancerous inhibitor of protein phosphatase 2A. Oncol. Lett. 2017, 14, 3573–3579. [Google Scholar] [CrossRef]
- Mu, X.; Wu, H.; Liu, J.; Hu, X.; Wu, H.; Chen, L.; Liu, W.; Luo, S.; Zhao, Y. Long noncoding RNA TMPO-AS1 promotes lung adenocarcinoma progression and is negatively regulated by miR-383-5p. Biomed. Pharmacother. 2020, 125, 109989. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xia, L. Resveratrol inhibits the proliferation, invasion, and migration, and induces the apoptosis of human gastric cancer cells through the MALAT1/miR-383-5p/DDIT4 signaling pathway. J. Gastrointest. Oncol. 2022, 13, 985–996. [Google Scholar] [CrossRef]
- Li, F.; Li, F.; Chen, W. Propofol Inhibits Cell Proliferation, Migration, and Invasion via mir-410-3p/Transforming Growth Factor-β Receptor Type 2 (TGFBR2) Axis in Glioma. Med. Sci. Monit. 2020, 26, e919523. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; Fang, M.; Zhou, L.; Chen, Y.; Zhou, J.; Song, Y.; Kong, G.; Zhang, B.; Jiang, B.; et al. MicroRNA-129-2-3p directly targets Wip1 to suppress the proliferation and invasion of intrahepatic cholangiocarcinoma. J. Cancer 2020, 11, 3216–3224. [Google Scholar] [CrossRef]
- Tao, L.; Song, P.; Shao, L.; Gao, H.; Ji, K.; Ren, Y.; Wang, F.; Wang, M. miR-129-2-3p inhibits colon cancer cell proliferation by down-regulating the expression of BZW1. Arab. J. Gastroenterol. 2024, 25, 42–50. [Google Scholar] [CrossRef]
- Zhu, K.; Lin, J.; Chen, S.; Xu, Q. miR-9-5p Promotes Lung Adenocarcinoma Cell Proliferation, Migration and Invasion by Targeting ID4. Technol. Cancer Res. Treat. 2021, 20, 15330338211048592. [Google Scholar] [PubMed]
- Zhang, H.; Li, Y.; Tan, Y.; Liu, Q.; Jiang, S.; Liu, D.; Chen, Q.; Zhang, S. MiR-9-5p Inhibits Glioblastoma Cells Proliferation Through Directly Targeting FOXP2 (Forkhead Box P2). Front. Oncol. 2019, 9, 1176. [Google Scholar]
- Rawlings-Goss, R.A.; Campbell, M.C.; Tishkoff, S.A. Global population-specific variation in miRNA associated with cancer risk and clinical biomarkers. BMC Med. Genom. 2014, 7, 53. [Google Scholar]
- Ying, L.; Lu, T.; Tian, Y.; Guo, H.; Wu, C.; Xu, C.; Jin, J.; Zhu, R.; Liu, P.; Yang, Y.; et al. A predictive model for prognostic risk stratification of early-stage NSCLC based on clinicopathological and miRNA panel. Lung Cancer 2024, 195, 107902. [Google Scholar] [PubMed]
- Li, Q.; Yang, J.; Yu, Q.; Wu, H.; Liu, B.; Xiong, H.; Hu, G.; Zhao, J.; Yuan, X.; Liao, Z. Associations between single-nucleotide polymorphisms in the PI3K-PTEN-AKT-mTOR pathway and increased risk of brain metastasis in patients with non-small cell lung cancer. Clin. Cancer Res. 2013, 19, 6252–6260. [Google Scholar] [PubMed]
- Orhan, C.; Bulut, P.; Dalay, N.; Ersen, E.; Buyru, N. Downregulation of TCEAL7 expression induces CCND1 expression in non-small cell lung cancer. Mol. Biol. Rep. 2019, 46, 5251–5256. [Google Scholar]
- Baykara, O.; Dalay, N.; Bakir, B.; Bulut, P.; Kaynak, K.; Buyru, N. The EMSY Gene Collaborates with CCND1 in Non-Small Cell Lung Carcinogenesis. Int. J. Med. Sci. 2017, 14, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Köles, L.; Ribiczey, P.; Szebeni, A.; Kádár, K.; Zelles, T.; Zsembery, Á. The Role of TRPM7 in Oncogenesis. Int. J. Mol. Sci. 2024, 25, 719. [Google Scholar] [CrossRef]
- Meng, S.; Alanazi, R.; Ji, D.; Bandura, J.; Luo, Z.W.; Fleig, A.; Feng, Z.P.; Sun, H.S. Role of TRPM7 kinase in cancer. Cell Calcium 2021, 96, 102400. [Google Scholar] [PubMed]
- Ayanlaja, A.A.; Zhang, B.; Ji, G.; Gao, Y.; Wang, J.; Kanwore, K.; Gao, D. The reversible effects of glial cell line-derived neurotrophic factor (GDNF) in the human brain. Semin. Cancer Biol. 2018, 53, 212–222. [Google Scholar]
- Lam, X.J.; Maniam, S.; Cheah, P.S.; Ling, K.H. REST in the Road Map of Brain Development. Cell Mol. Neurobiol. 2023, 43, 3417–3433. [Google Scholar] [CrossRef]
- Hudson, K.; Mondia, M.W.; Zhang, Y.; Saha, S.; Gibert, M.K., Jr.; Dube, C.; Sun, Y.; Marcinkiewicz, P.; Fadul, C.; Abounader, R. The role of microRNAs in brain metastasis. J. Neurooncol. 2024, 166, 231–241. [Google Scholar] [CrossRef]
- Nassar, F.J.; Talhouk, R.; Zgheib, N.K.; Tfayli, A.; El Sabban, M.; El Saghir, N.S.; Boulos, F.; Jabbour, M.N.; Chalala, C.; Boustany, R.-M.; et al. microRNA Expression in Ethnic Specific Early Stage Breast Cancer: An Integration and Comparative Analysis. Sci. Rep. 2017, 7, 16829. [Google Scholar] [CrossRef]
- Dluzen, D.F.; Noren Hooten, N.; Zhang, Y.; Kim, Y.; Glover, F.E.; Tajuddin, S.M.; Jacob, K.D.; Zonderman, A.B.; Evans, M.K. Racial differences in microRNA and gene expression in hypertensive women. Sci. Rep. 2016, 6, 35815. [Google Scholar] [CrossRef]




| Characreristic | Gender | Age | Immunohistochemical Characteristics |
|---|---|---|---|
| LUAD-BM_1 | M | 67 | CK7 +, TTF-1+, CD×2 − |
| LUAD-BM_2 | M | 71 | CK7 +, TTF-1+, CD×2 − |
| LUAD-BM_3 | M | 73 | CK7 +, TTF-1+, CD×2 − |
| LUAD-BM_4 | F | 66 | CK7 +, TTF-1+, CD×2 − |
| LUAD-BM_5 | F | 71 | CK7 +, TTF-1+, CD×2 − |
| LUAD-BM_6 | F | 59 | CK7 +, TTF-1+, CD×2 − |
| GBM_1 | M | 60 | IDH wild type |
| GBM_2 | M | 56 | IDH wild type |
| GBM_3 | M | 65 | IDH wild type |
| GBM_4 | F | 69 | IDH wild type |
| GBM_5 | F | 57 | IDH wild type |
| GBM_6 | F | 70 | IDH wild type |
| Controll_1 | M | 70 | - |
| Controll_2 | M | 52 | - |
| Controll_3 | M | 52 | - |
| Controll_4 | F | 71 | - |
| Controll_5 | F | 80 | - |
| Controll_6 | F | 61 | - |
| Differentially Expressed miRNAs | UP | DOWN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| miR-200c-5p | miR-141-5p | miR-375-3p | miR-200a-5p | miR-383-5p | miR-129-2-3p | miR-410-3p | miR-9-5p | ||
| Mann-Whitney U test | M ↔ C | <0.00001 | <0.00001 | <0.00001 | <0.00001 | <0.00001 | <0.00001 | <0.00001 | <0.00001 |
| AUC | 1 | 1 | 0.993 | 0.99 | 0.934 | 0.907 | 0.916 | 0.989 | |
| Optimal Cut-Off Point | 13.05 | 17.92 | 4.19 5.31 | 13.18 | 12.52 | 8.5 | 9.87 | −1.37 | |
| Sensitivity | 1 | 1 | 0.967 | 1 | 0.867 | 0.967 | 0.867 | 1 | |
| Specificity | 1 | 1 | 0.967 | 0.967 | 0.867 | 0.833 | 0.867 | 0.933 | |
| Mann-Whitney U test | M ↔ G | <0.00001 | <0.00001 | <0.00001 | <0.00001 | <0.00001 | 0.00026 | <0.00001 | <0.00001 |
| AUC | 1 | 1 | 0.991 | 1 | 0.791 | 0.776 | 0.838 | 0.971 | |
| Optimal Cut-Off Point | 12.46 | 13.29 | 5.88 | 11.12 | 12.72 | 10.99 | 10.43 | −0.75 | |
| Sensitivity | 1 | 1 | 1 | 1 | 0.867 | 0.833 | 0.8 | 1 | |
| Specificity | 1 | 1 | 0.967 | 1 | 0.7 | 0.7 | 0.8 | 0.833 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torner, B.; Géczi, D.; Klekner, Á.; Balogh, I.; Penyige, A.; Birkó, Z. Construction of a miRNA Panel for Differentiating Lung Adenocarcinoma Brain Metastases and Glioblastoma. Cancers 2025, 17, 581. https://doi.org/10.3390/cancers17040581
Torner B, Géczi D, Klekner Á, Balogh I, Penyige A, Birkó Z. Construction of a miRNA Panel for Differentiating Lung Adenocarcinoma Brain Metastases and Glioblastoma. Cancers. 2025; 17(4):581. https://doi.org/10.3390/cancers17040581
Chicago/Turabian StyleTorner, Bernadett, Dóra Géczi, Álmos Klekner, István Balogh, András Penyige, and Zsuzsanna Birkó. 2025. "Construction of a miRNA Panel for Differentiating Lung Adenocarcinoma Brain Metastases and Glioblastoma" Cancers 17, no. 4: 581. https://doi.org/10.3390/cancers17040581
APA StyleTorner, B., Géczi, D., Klekner, Á., Balogh, I., Penyige, A., & Birkó, Z. (2025). Construction of a miRNA Panel for Differentiating Lung Adenocarcinoma Brain Metastases and Glioblastoma. Cancers, 17(4), 581. https://doi.org/10.3390/cancers17040581






