Preoperative Diaphragm Muscle Atrophy Increases the Likelihood of Postoperative Pulmonary Complications After Lung Cancer Resection: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Participants
2.3. Diaphragm Ultrasound Protocol
2.4. Diaphragm Dysfunction Assessment
2.5. Surgical Procedures
2.6. Data Collection
2.6.1. Preoperative and Intra-Operative Data
2.6.2. Post-Operative Data
2.6.3. Postoperative Pulmonary Complications (PPCs)
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.M. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives—State of the Art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Deng, T.; Song, J.; Tuo, J.; Wang, Y.; Li, J.; Suen, L.K.P.; Liang, Y.; Ma, J.; Chen, S. Incidence and Risk Factors of Pulmonary Complications after Lung Cancer Surgery: A Systematic Review and Meta-Analysis. Heliyon 2024, 10, e32821. [Google Scholar] [CrossRef] [PubMed]
- Lugg, S.T.; Agostini, P.J.; Tikka, T.; Adams, K.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B. Long-Term Impact of Developing a Postoperative Pulmonary Complication after Lung Surgery. Thorax 2016, 71, 171–176. [Google Scholar] [CrossRef]
- Bravo Iñiguez, C.E.; Armstrong, K.W.; Cooper, Z.; Weissman, J.S.; Ducko, C.T.; Wee, J.O.; Martinez, M.P.; Bueno, R.; Jaklitsch, M.T.; Wiener, D.C. Thirty-Day Mortality after Lobectomy in Elderly Patients Eligible for Lung Cancer Screening. Ann. Thorac. Surg. 2016, 101, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.H.; Pons, A.; De Sousa, P.; Proli, C.; Jordan, S.; Begum, S.; Buderi, S.; Anikin, V.; Finch, J.; Asadi, N.; et al. Determining the Optimal Time to Report Mortality after Lobectomy for Lung Cancer: An Analysis of the Time-Varying Risk of Death. JTCVS Open 2023, 16, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Baar, W.; Semmelmann, A.; Knoerlein, J.; Weber, F.; Heinrich, S.; Loop, T.; Working Group of the German Thorax Registry. Risk Factors for Postoperative Pulmonary Complications Leading to Increased In-Hospital Mortality in Patients Undergoing Thoracotomy for Primary Lung Cancer Resection: A Multicentre Retrospective Cohort Study of the German Thorax Registry. J. Clin. Med. 2022, 11, 5774. [Google Scholar] [CrossRef]
- Agostini, P.; Cieslik, H.; Rathinam, S.; Bishay, E.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B. Postoperative Pulmonary Complications following Thoracic Surgery: Are There Any Modifiable Risk Factors? Thorax 2010, 65, 815–818. [Google Scholar] [CrossRef]
- Canet, J.; Gallart, L. Predicting Postoperative Pulmonary Complications in the General Population. Curr. Opin. Anaesthesiol. 2013, 26, 107–115. [Google Scholar] [CrossRef]
- Roungeris, L.; Devadze, G.; Talliou, C.; Griva, P. Prediction of Postoperative Complications after Major Lung Resection: A Literature Review. Anesth. Res. 2024, 1, 146–156. [Google Scholar] [CrossRef]
- Gupta, H.; Ramanan, B.; Gupta, P.K.; Fang, X.; Miller, W.J.; Munns, P.M.; Pagni, S.; Sainath, J.; Forse, R.A.; Pryor, J.P. Impact of COPD on Postoperative Outcomes: Results from a National Database. Chest 2013, 143, 1599–1606. [Google Scholar] [CrossRef]
- Rudra, A.; Das, S. Postoperative Pulmonary Complications. Indian J. Anaesth. 2006, 50, 89–98. [Google Scholar]
- Dubé, B.P.; Dres, M. Diaphragm Dysfunction: Diagnostic Approaches and Management Strategies. J. Clin. Med. 2016, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.; Skaarup, S.H.; Bendixen, M.; Tankisi, H.; Mørkved, A.L.; Juhl-Olsen, P. Diaphragmatic Dysfunction is Associated with Postoperative Pulmonary Complications and Phrenic Nerve Paresis in Patients Undergoing Thoracic Surgery. J. Anesth. 2024, 38, 386–397. [Google Scholar] [CrossRef]
- Daniel, M.; Lang, E.; Huynh, T.M.; Martin, J.; Brebion, M.; Guessous, K.; Brazzale, D.; Puyraveau, M.; Tournoy, K.; Janssens, J.-P.; et al. Prevalence and Time-Course of Diaphragmatic Dysfunction following Lung Resection: A Repeated Ultrasonic Assessment. Anaesth. Crit. Care Pain Med. 2022, 41, 101024. [Google Scholar] [CrossRef]
- Vesovic, R.; Milosavljevic, M.; Punt, M.; Aulovska, B.; Dimitrov, G.; Krstevska-Konstantinova, S.; Jovanovic, B.; Zivkovic, M. The Role of the Diaphragm in Prediction of Respiratory Function in the Immediate Postoperative Period in Lung Cancer Patients Using a Machine Learning Model. World J. Surg. Oncol. 2023, 21, 393. [Google Scholar] [CrossRef]
- Laghlam, D.; Naudin, C.; Srour, A.; Monsonego, R.; Malvy, J.; Rahoual, G.; Squara, P.; Nguyen, L.S.; Estagnasié, P. Persistent Diaphragm Dysfunction after Cardiac Surgery is Associated with Adverse Respiratory Outcomes: A Prospective Observational Ultrasound Study. Can. J. Anaesth. 2023, 70, 228–236. [Google Scholar] [CrossRef]
- Laghlam, D.; Lê, M.P.; Srour, A.; Monsonego, R.; Estagnasié, P.; Brusset, A.; Squara, P. Diaphragm Dysfunction after Cardiac Surgery: Reappraisal. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3241–3247. [Google Scholar] [CrossRef]
- Diehl, J.L.; Lofaso, F.; Deleuze, P.; Similowski, T.; Lemaire, F.; Brochard, L. Clinically Relevant Diaphragmatic Dysfunction after Cardiac Operations. J. Thorac. Cardiovasc. Surg. 1994, 107, 487–498. [Google Scholar] [CrossRef]
- Bruni, A.; Garofalo, E.; Pasin, L.; Serraino, G.; Cammarota, G.; Longhini, F.; Gatti, S.; Agnoletti, V.; Zambelli, R.; Spadaro, S.; et al. Diaphragmatic Dysfunction after Elective Cardiac Surgery: A Prospective Observational Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3336–3344. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Orwat, S.; Henke, C.; Kabitz, H.J.; Katsianos, S.; Borrelli, C.; Hecker, M.; Probst, C.; Schulz, R.; Sorichter, S. Inspiratory Muscle Dysfunction and Restrictive Lung Function Impairment in Congenital Heart Disease: Association with Immune Inflammatory Response and Exercise Intolerance. Int. J. Cardiol. 2020, 318, 45–51. [Google Scholar] [CrossRef]
- Kocjan, J.; Rydel, M.; Czyżewski, D.; Adamek, M. Comparison of Early Postoperative Diaphragm Muscle Function after Lobectomy via VATS and Open Thoracotomy: A Sonographic Study. Life 2024, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, T.; Akimoto, Y.; Takashima, T.; Oto, J. Ultrasonographic Assessment of the Diaphragm. Diagnostics 2024, 14, 1481. [Google Scholar] [CrossRef] [PubMed]
- McCool, F.D.; Tzelepis, G.E. Dysfunction of the Diaphragm. N. Engl. J. Med. 2012, 366, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Esper, R.; Pérez-Calatayud, Á.A.; Arch-Tirado, E.; Díaz-Carrillo, M.A.; Garrido-Aguilar, A.J.; Tapia-Venegas, S. Standardization of Sonographic Diaphragm Thickness Evaluations in Healthy Volunteers. Respir. Care 2016, 61, 920–924. [Google Scholar] [CrossRef]
- Sarwal, A.; Walker, F.O.; Cartwright, M.S. Neuromuscular Ultrasound for Evaluation of the Diaphragm. Muscle Nerve 2013, 47, 319–329. [Google Scholar] [CrossRef]
- Boon, A.J.; Harper, C.J.; Ghahfarokhi, L.S.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-Dimensional Ultrasound Imaging of the Diaphragm: Quantitative Values in Normal Subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar] [CrossRef]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Moreno, R.P.; Hoeft, A.; Walder, B.; ESA-ESICM Joint Taskforce on Perioperative Outcome Measures. Standards for Definitions and Use of Outcome Measures for Clinical Effectiveness Research in Perioperative Medicine: European Perioperative Clinical Outcome (EPCO) Definitions. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [CrossRef]
- Liu, F.; Wen, Q.; Yang, Y.; Liu, Y.; Zheng, X.; Lin, H.; Liu, B.; Feng, S.; Luo, J. Diaphragmatic Dysfunction is Associated with Postoperative Pulmonary Complications in the Aged Patients Underwent Radical Resection of Esophageal Cancer: A Prospective Observational Study. J. Thorac. Dis. 2024, 16, 3623–3635. [Google Scholar] [CrossRef]
- Ssouni, O.; Ghannam, A.; El-Ahmadi, B.; Belkhadir, Z.; Abidi, K.; Oualim, Z. The Predictive Value of Diaphragm Thickness Fraction on Postoperative Pulmonary Complications after Digestive Cancer Curative Surgery. Arch. Surg. Clin. Res. 2023, 7, 35–45. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Eljaiek, R.; Rodrigue, É.; Chagnon, J.L.; Lamarche, Y.; Denault, A.Y. Preoperative Diaphragm Function is Associated with Postoperative Pulmonary Complications after Cardiac Surgery. Crit. Care Med. 2019, 47, e966–e974. [Google Scholar] [CrossRef]
- Yu, J.; Lee, Y.; Park, J.Y.; Hwang, J.H.; Kim, Y.K. Diaphragm Thickening Fraction as a Prognostic Imaging Marker for Postoperative Pulmonary Complications in Robot-Assisted Laparoscopic Prostatectomy Requiring the Trendelenburg Position and Pneumoperitoneum. Dis. Markers 2021, 2021, 9931690. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, Z.; Wang, L.; Lv, G.; Cheng, Y.; Wang, B.; Zhang, Z.; Jin, X.; Kang, Y.; Zhou, Y.; et al. Increased Diaphragm Echodensity Correlates with Postoperative Pulmonary Complications in Patients after Major Abdominal Surgery: A Prospective Observational Study. BMC Pulm. Med. 2022, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Grasso, S.; Dres, M.; Volta, C.A.; Aguiar Santos, L.; Rauseo, M.; Karbing, D.S.; Dondossola, D.; Dianti, J.; Vetrugno, L.; et al. Point of Care Ultrasound to Identify Diaphragmatic Dysfunction after Thoracic Surgery. Anesthesiology 2019, 131, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Nucci, R.A.B.; de Souza, R.R.; Suemoto, C.K.; Oliveira, L.V.; Cendoroglo, M.S.; Bortolloti, M.T.; Monteiro, C.R.; Leite, R.V. Cigarette Smoking Impairs the Diaphragm Muscle Structure of Patients without Respiratory Pathologies: An Autopsy Study. Cell Physiol. Biochem. 2019, 53, 648–655. [Google Scholar] [CrossRef]
- Batihan, G.; Ceylan, K.C.; Kaya, S.U.; Usluer, O. Video-Assisted Thoracoscopic Surgery vs. Thoracotomy for Non-Small Cell Lung Cancer Greater Than 5 cm: Is VATS a Feasible Approach for Large Tumors? J. Cardiothorac. Surg. 2020, 15, 261. [Google Scholar] [CrossRef]
- von Meyenfeldt, E.M.; Marres, G.M.H.; van Thiel, E.; Damhuis, R.A.M. Variation in Length of Hospital Stay after Lung Cancer Surgery in the Netherlands. Eur. J. Cardiothorac. Surg. 2018, 54, 560–564. [Google Scholar] [CrossRef]
- Nath, T.S.; Mohamed, N.; Gill, P.K.; Khan, S.; Nath, T.S. A Comparative Analysis of Video-Assisted Thoracoscopic Surgery and Thoracotomy in Non-Small-Cell Lung Cancer in Terms of Their Oncological Efficacy in Resection: A Systematic Review. Cureus 2022, 14, e25443. [Google Scholar] [CrossRef]

| Variables | N (%) or Mean ± SD |
|---|---|
| Surgical approach: | |
| VATS | 19 (42.2%) |
| Conventional thoracotomy | 26 (57.8%) |
| Involved side: | |
| Left | 21 (53.3%) |
| Right | 24 (46.7%) |
| Resected lobe: | |
| Right upper | 11 (24.4%) |
| Right middle | 3 (6.7%) |
| Right lower | 11 (24.4%) |
| Left upper | 8 (17.8%) |
| Left lower | 12 (26.7%) |
| Complete radical lymphadenectomy: | |
| Yes | 45 (100%) |
| No | 0 (0%) |
| TNM staging: | |
| IA | 14 (31.1%) |
| IB | 8 (17.8%) |
| IIA | 4 (8.9%) |
| IIB | 11 (24.4%) |
| IIIA | 8 (17.8%) |
| Drains [days]: | 5.32 ± 4.96 |
| Length of hospital stay [days]: | 9.19 ± 5.80 |
| Postoperative blood parameters: | |
| Erythrocyte [106/uL] | 4.50 ± 0.40 |
| Hemoglobin [g/dL] | 13.91 ± 1.34 |
| Hematocrit [%] | 40.95 ± 3.42 |
| Postoperative blood gas parameters: | |
| pH | 7.4 ± 0.04 |
| PaCo2 [mmHg] | 37.74 ± 6.09 |
| HCO3 [mEq/L] | 22.71 ± 2.41 |
| BE [mmol/L] | −1.63 to 2.0 |
| Variables | Diaphragm Atrophy | Diaphragm Paralysis | Diaphragm Weakness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 19) | No (n = 26) | p | Yes (n = 10) | No (n = 35) | p | Yes (n = 31) | No (n = 14) | p | |
| PPCs | |||||||||
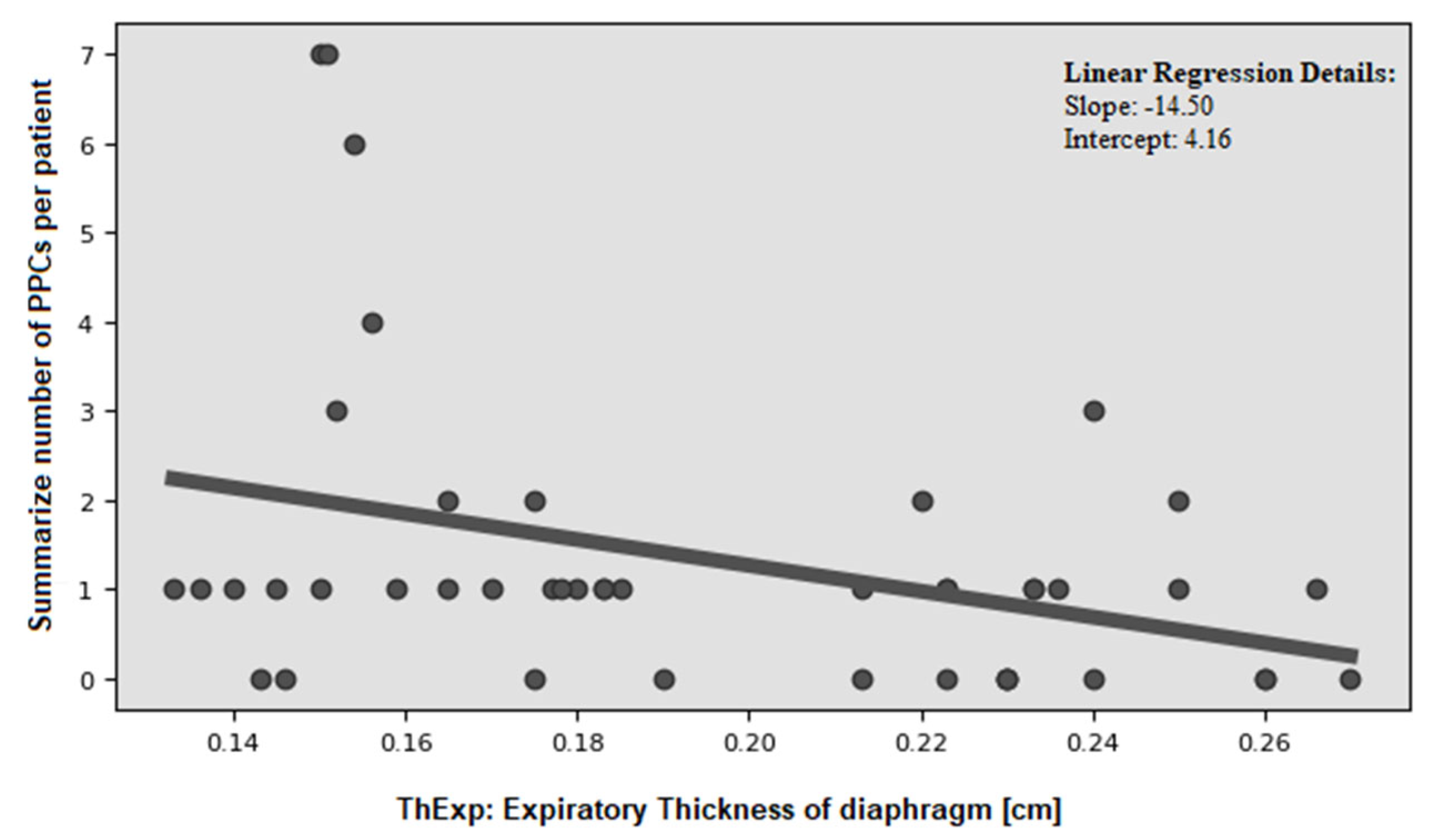
| mean number of PPCs: | 2.1 ± 2.2 | 0.9 ± 1.0 | 0.0163 | 2.5 ± 2.6 | 1.0 ± 1.2 | 0.0117 | 1.0 ± 1.1 | 1.0 ± 1.1 | 0.5766 |
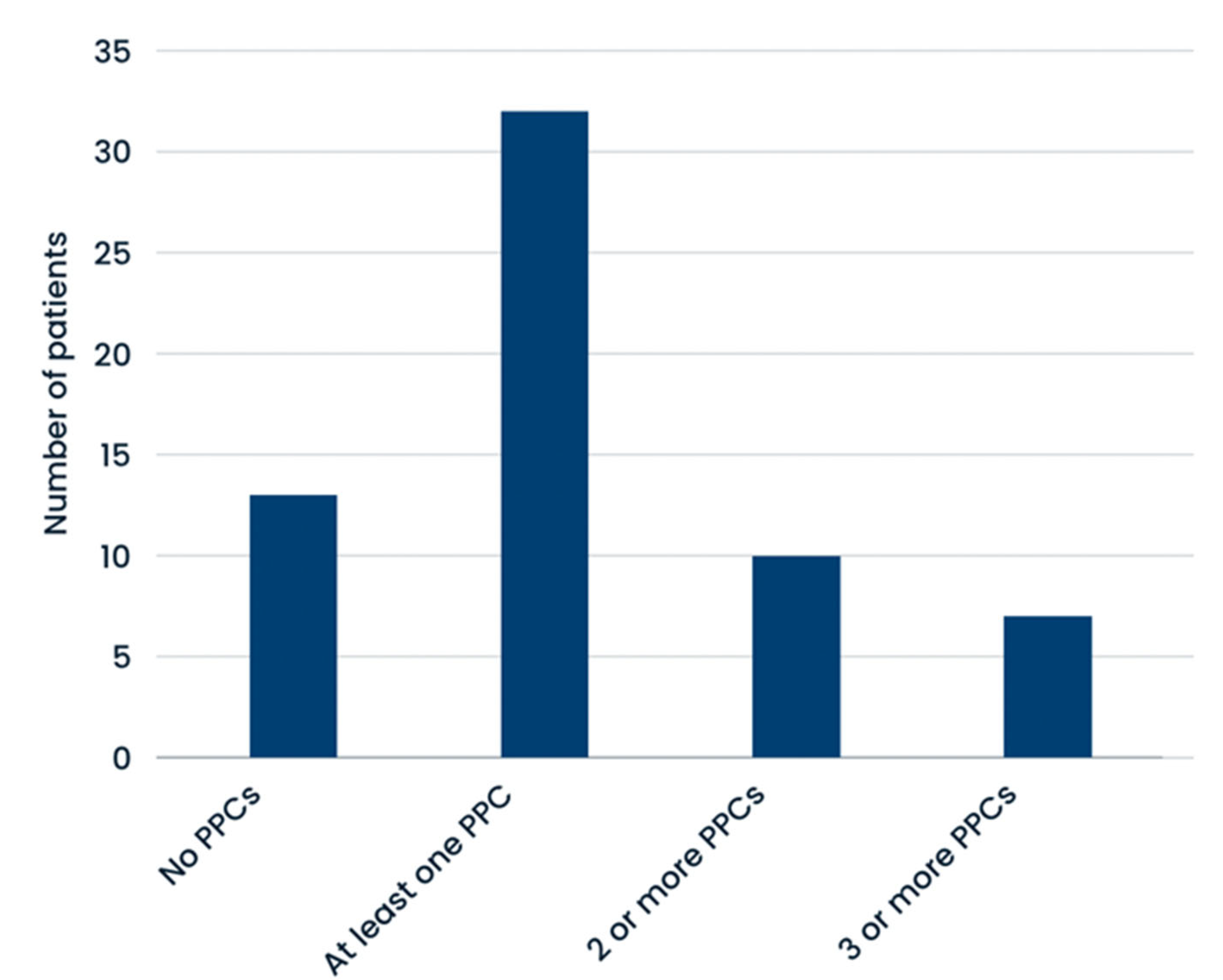
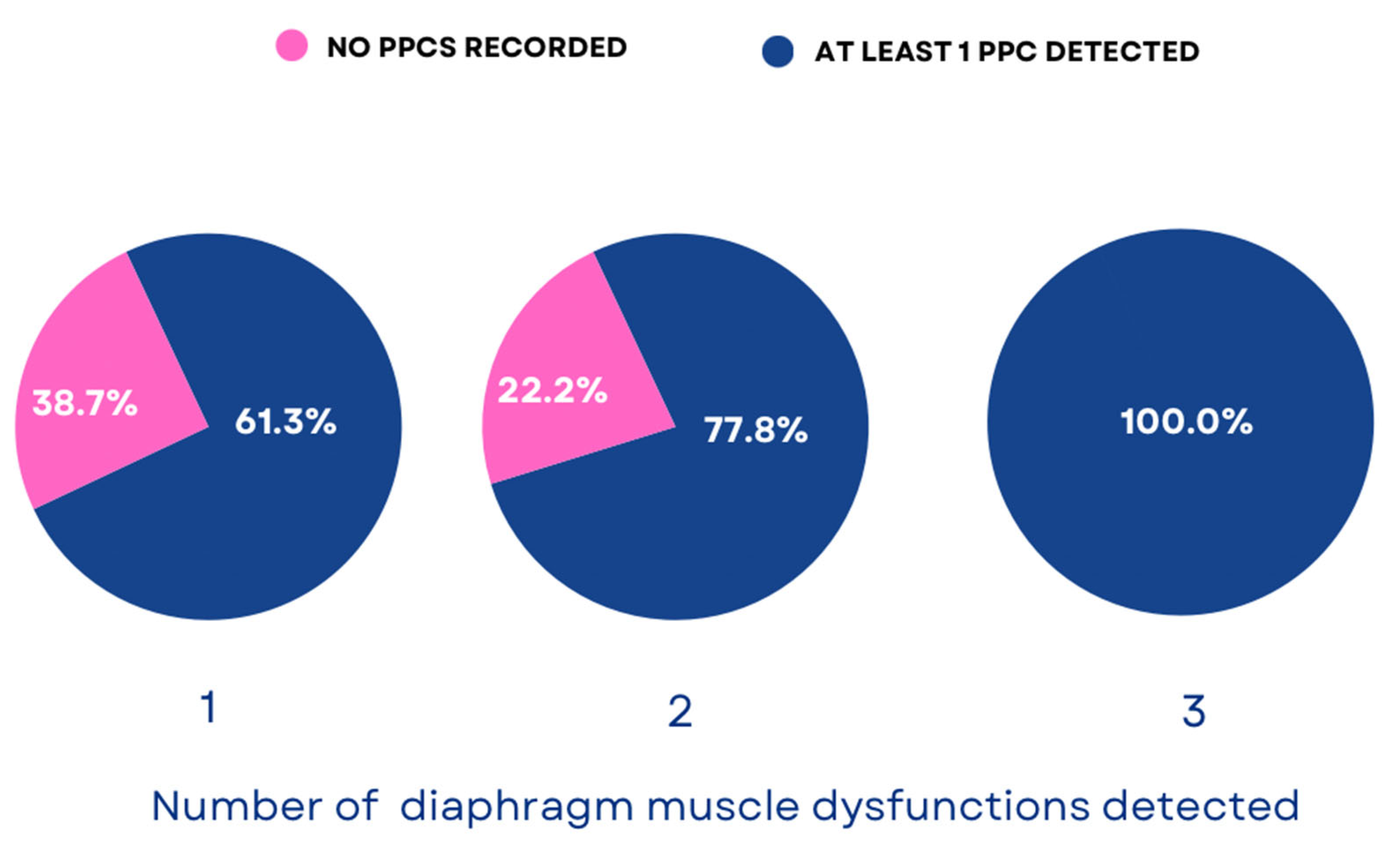
| No PPCs (n = 13) | 3 (15.8%) | 10 (37.0%) | 0.0486 | 1 (10%) | 12 (34.3%) | 0.1351 | 10 (32.3%) | 3 (21.3%) | 0.4581 |
| at least 1 PPC (n = 32) | 16 (84.2%) | 16 (61.5%) | 0.0486 | 9 (90%) | 23 (65.7%) | 0.1351 | 21 (67.7%) | 11 (78.1%) | 0.4581 |
| 2 or more PPCs (n = 10) | 6 (31.6%) | 4 (15.4%) | 0.1747 | 4 (40%) | 6 (17.4%) | 0.0730 | 7 (22.6%) | 3 (21.3%) | 0.5196 |
| 3 or more PPCs (n = 7) | 5 (26.3%) | 2 (7.7%) | 0.0787 | 3 (30%) | 4 (11.4%) | 0.0787 | 5 (16.1%) | 2 (14.2%) | 0.8995 |
| Individuals PPCs | |||||||||
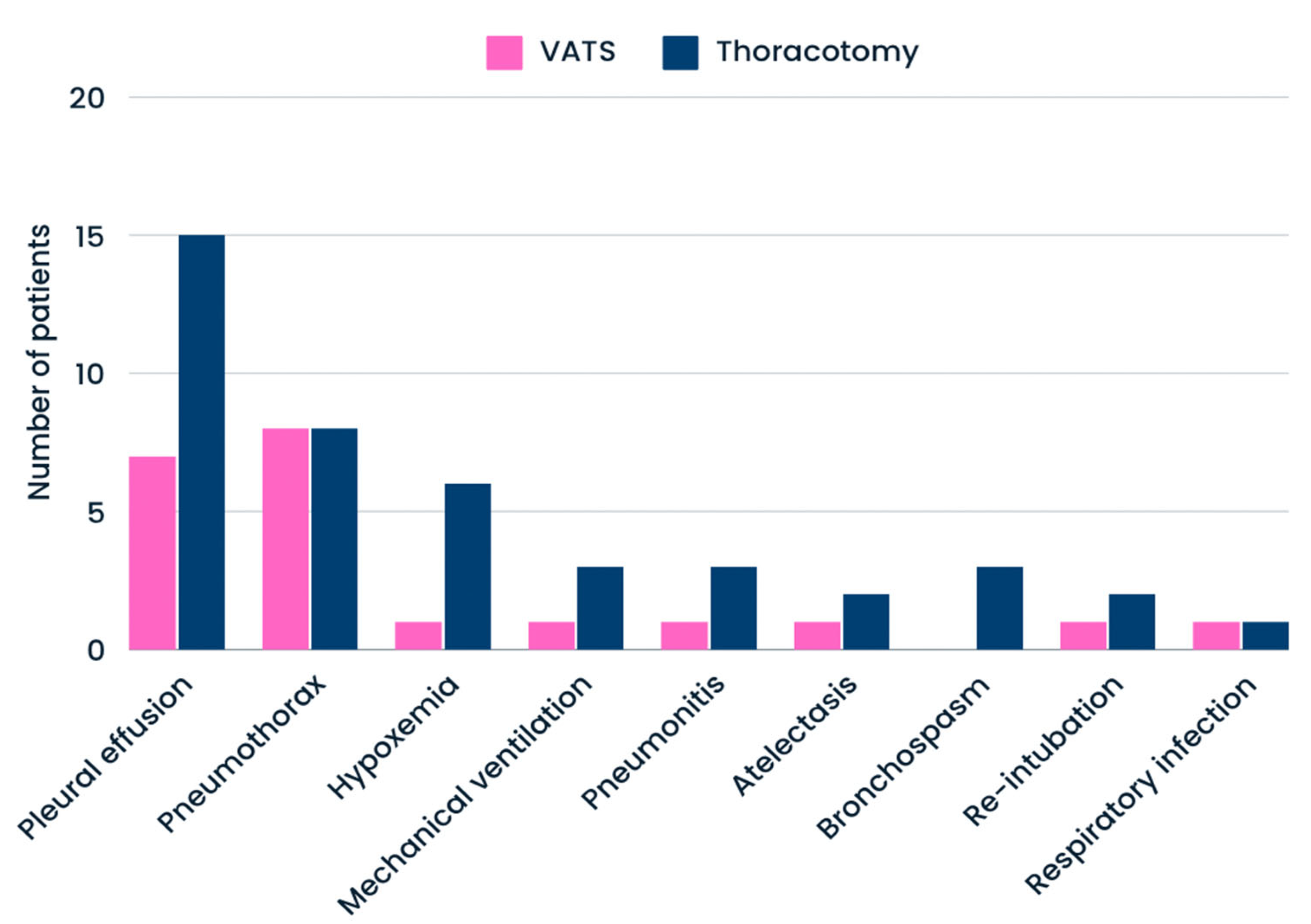
| Atelectasis (n = 3) | 3 (15.8%) | 0 (0.0%) | 0.0327 | 0 (0.0%) | 3 (7.9%) | 0.4416 | 3 (9.7%) | 0 (0.0%) | 0.2280 |
| pneumothorax (n = 16) | 7 (36.8% | 9 (33.3%) | 0.8057 | 3 (42.9%) | 13 (34.2%) | 0.6605 | 10 (32.3%) | 6 (42.6%) | 0.4971 |
| respiratory infection (n = 2) | 2 (10.5%) | 0 (0.0%) | 0.0847 | 0 (0.0%) | 2 (5.2%) | 0.5346 | 2 (6.4%) | 0 (0.0%) | 0.3300 |
| bronchospasm (n = 3) | 2 (10.5%) | 1 (3.7%) | 0.3561 | 1 (14.3%) | 2 (5.2%) | 0.3792 | 2 (6.4%) | 1 (7.1%) | 0.9311 |
| pleural effusion (n = 23) | 13 (68.4%) | 10 (37.0%) | 0.0457 | 3 (30.0) | 20 (57.1%) | 0.2963 | 14 (45.2%) | 9 (64.2%) | 0.3433 |
| mechanical ventilation (n = 4) | 3 (15.8%) | 1 (3.7%) | 0.1520 | 1 (14.3%) | 3 (7.9%) | 0.4416 | 3 (9.7%) | 1 (7.1%) | 0.7461 |
| pneumonitis (n = 4) | 3 (15.8%) | 1 (3.7%) | 0.1520 | 1 (14.3%) | 3 (7.9%) | 0.4416 | 3 (9.7%) | 1 (7.1%) | 0.7461 |
| re-intubation (n = 3) | 3 (15.8%) | 0 (0.0%) | 0.0327 | 0 (0.0%) | 3 (7.9%) | 0.4416 | 2 (6.4%) | 1 (7.1%) | 0.9311 |
| Hypoxemia (n = 7) | 5 (26.3%) | 2 (7.7%) | 0.0787 | 3 (30.0) | 4 (11.4%) | 0.0787 | 4 (12.8%) | 3 (21.3%) | 0.7995 |
| Variables | VATS | Thoracotomy | ||||
|---|---|---|---|---|---|---|
| DA (n = 7) | NDT (n = 12) | p | DA (n = 12) | NDT (n = 19) | p | |
| PPCs: | 0.8295 | 0.0620 | ||||
| yes | 5 (71.4%) | 8 (66.7%) | 11 (91.7%) | 9 (47.4%) | ||
| no | 2 (28.6% | 4 (33.3%) | 1 (8.3%) | 6 (31.6%) | ||
| 2 or more PPCs: | 0.6834 | 0.2205 | ||||
| yes | 1 (14.3%) | 1 (8.3%) | 5 (41.7%) | 3 (15.8%) | ||
| no | 6 (85.7%) | 11 (91.7%) | 7 (58.3%) | 12 (84.2%) | ||
| 3 or more PPCs: | 0.1786 | 0.2142 | ||||
| yes | 1 (14.3%) | 0 (0.0%) | 4 (33.3%) | 2 (10.5%) | ||
| no | 6 (85.7%) | 12 (100%) | 8 (66.7%) | 13 (68.4%) | ||
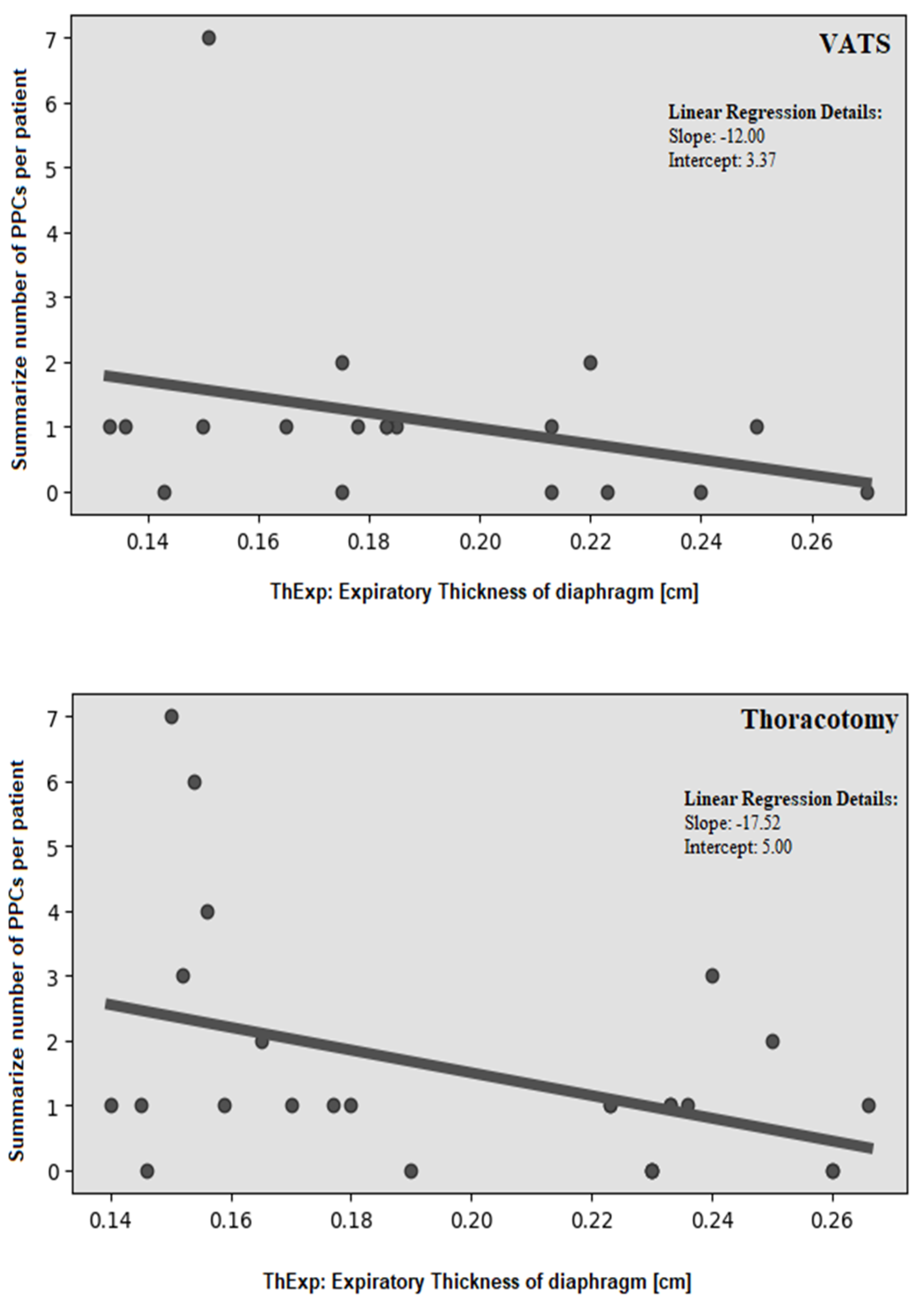
| Mean number of PPCs: | 1.71 ± 2.43 | 0.75 ± 0.62 | 0.0487 | 2.33 ± 2.22 | 1.00 ± 1.19 | 0.0435 |
| Variables | VATS | Thoracotomy | ||||
|---|---|---|---|---|---|---|
| DA (n = 7) | NDT (n = 12) | p | DA (n = 12) | NDT (n = 19) | p | |
| Length of hospital stay [days] | 10.6 ± 9.1 | 7.1 ± 3.3 | 0.2397 | 11.7 ± 7.4 | 8.2 ± 3.2 | 0.1056 |
| Drains [days] | 5.3 ± 3.4 | 4.0 ± 2.1 | 0.3221 | 7.9 ± 8.4 | 4.3 ± 2.5 | 0.1298 |
| Variables | Diaphragm Atrophy | Diaphragm Paralysis | Diaphragm Weakness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 19) | No (n = 26) | p | Yes (n = 10) | No (n = 35) | p | Yes (n = 31) | No (n = 14) | p | |
| Erythrocyte | 4.63 ± 0.41 | 4.40 ± 0.34 | 0.056 | 4.65 ± 0.24 | 4.50 + 0.43 | 0.313 | 4.58 ± 0.39 | 4.45 ± 0.43 | 0.321 |
| Hemoglobin | 13.79 ± 1.28 | 13.98 ± 1.41 | 0.641 | 14.04 ± 0.87 | 13.88 + 1.47 | 0.758 | 13.89 ± 1.40 | 13.99 ± 1.30 | 0.823 |
| Hematocrit | 40.75 ± 3.06 | 41.09 ± 3.71 | 0.754 | 41.57 ± 2.19 | 40.81 + 3.75 | 0.548 | 40.84 ± 3.54 | 41.27 ± 3.39 | 0.704 |
| Blood pH | 7.39 ± 0.05 | 7.40 ± 0.03 | 0.832 | 7.37 ± 0.04 | 7.41 + 0.04 | 0.278 | 7.39 ± 0.04 | 7.40 ± 0.04 | 0.581 |
| PaCO2 | 36.96 ± 5.56 | 38.76 ± 6.76 | 0.378 | 40.70 ± 6.00 | 36.64 + 5.85 | 0.071 | 37.52 ± 6.38 | 38.31 ± 5.49 | 0.735 |
| HCO3 | 23.08 ± 1.99 | 22.43 ± 2.70 | 0.430 | 23.03 ± 2.32 | 22.6 + 2.48 | 0.637 | 22.47 ± 2.54 | 23.36 ± 2.01 | 0.330 |
| BE | −1.4 ± 1.8 | −1.8 ± 2.17 | 0.582 | −2.02 ± 2.19 | −1.48 + 1.94 | 0.477 | −1.02 ± 1.67 | −1.85 ± 2.09 | 0.264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocjan, J.; Rydel, M.; Czyżewski, D.; Adamek, M. Preoperative Diaphragm Muscle Atrophy Increases the Likelihood of Postoperative Pulmonary Complications After Lung Cancer Resection: A Pilot Study. Cancers 2025, 17, 373. https://doi.org/10.3390/cancers17030373
Kocjan J, Rydel M, Czyżewski D, Adamek M. Preoperative Diaphragm Muscle Atrophy Increases the Likelihood of Postoperative Pulmonary Complications After Lung Cancer Resection: A Pilot Study. Cancers. 2025; 17(3):373. https://doi.org/10.3390/cancers17030373
Chicago/Turabian StyleKocjan, Janusz, Mateusz Rydel, Damian Czyżewski, and Mariusz Adamek. 2025. "Preoperative Diaphragm Muscle Atrophy Increases the Likelihood of Postoperative Pulmonary Complications After Lung Cancer Resection: A Pilot Study" Cancers 17, no. 3: 373. https://doi.org/10.3390/cancers17030373
APA StyleKocjan, J., Rydel, M., Czyżewski, D., & Adamek, M. (2025). Preoperative Diaphragm Muscle Atrophy Increases the Likelihood of Postoperative Pulmonary Complications After Lung Cancer Resection: A Pilot Study. Cancers, 17(3), 373. https://doi.org/10.3390/cancers17030373




