ONCOBREAST-TEST Is a Quick Diagnostic, Prognostic and Predictive Method of Response to Systemic Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
Inclusion and Exclusion Criteria
2.2. Core Needle Biopsy of the Breast
2.3. Immunohistochemical Staining
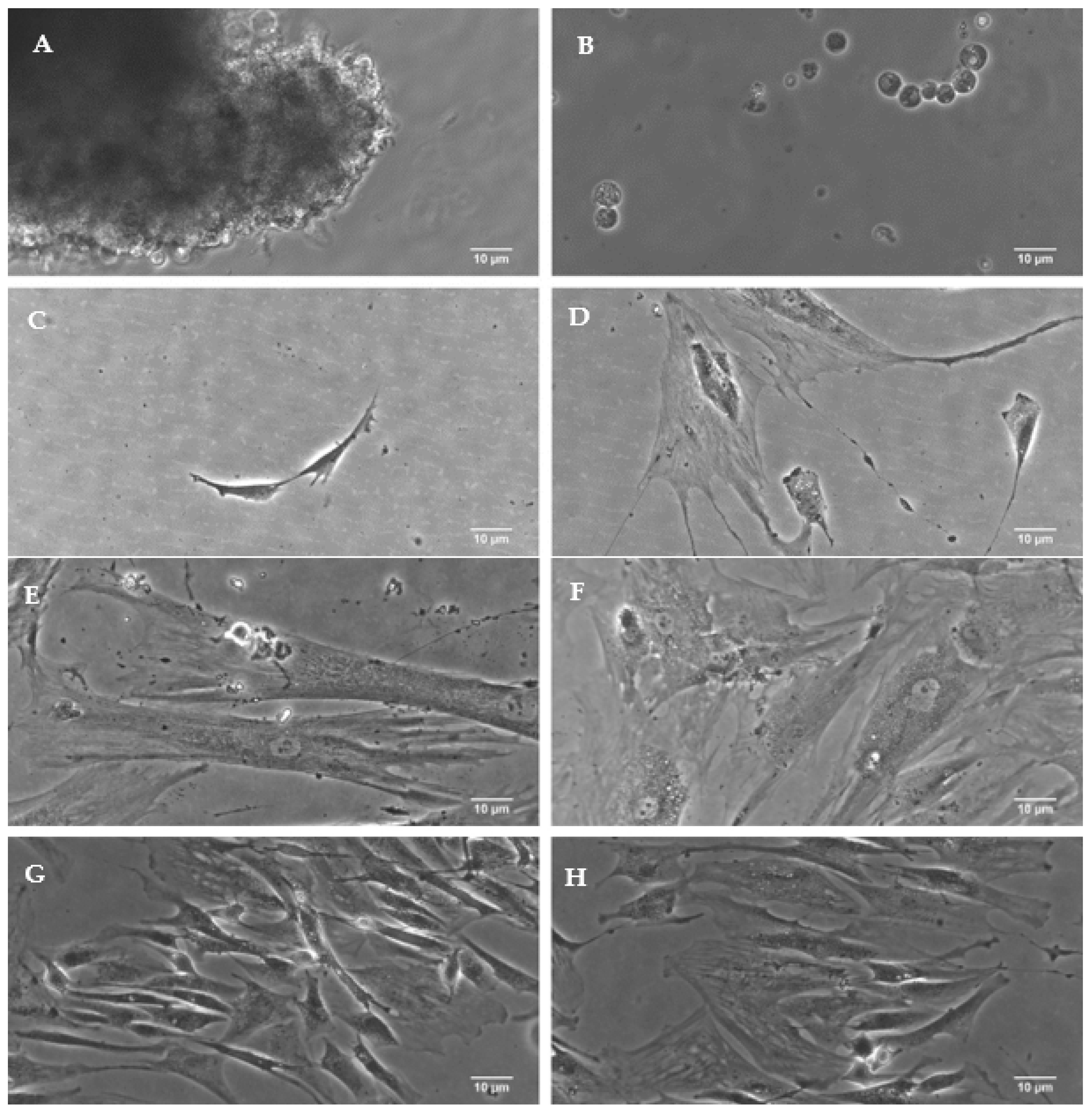
2.4. Obtaining Cancer Cells
2.5. Verification of Cancer Cells
2.6. Assessment of Neoplastic Cell Chemosensitivity
- Doxorubicin (4 μM) + 4-Hydroxycyclophosphamide (the active metabolite of cyclophosphamide) (1 μM)
- Cisplatin (20 μM)
- Paclitaxel (2 μM)
- Paclitaxel (2 μM) + Trastuzumab (0.7 μM)
- Docetaxel (1 μM)
- Docetaxel (1 μM) + Trastuzumab (0.7 μM)
Evaluation of Lactate Dehydrogenase (LDH) Activity in Culture Media
2.7. Statistics
3. Results
3.1. General Patient and Tumor Characteristics
3.2. Isolation of Neoplastic Cells from Tumor Stroma
3.3. Extensive Research Panel
3.3.1. Confirmation of Cellular Heterogeneity
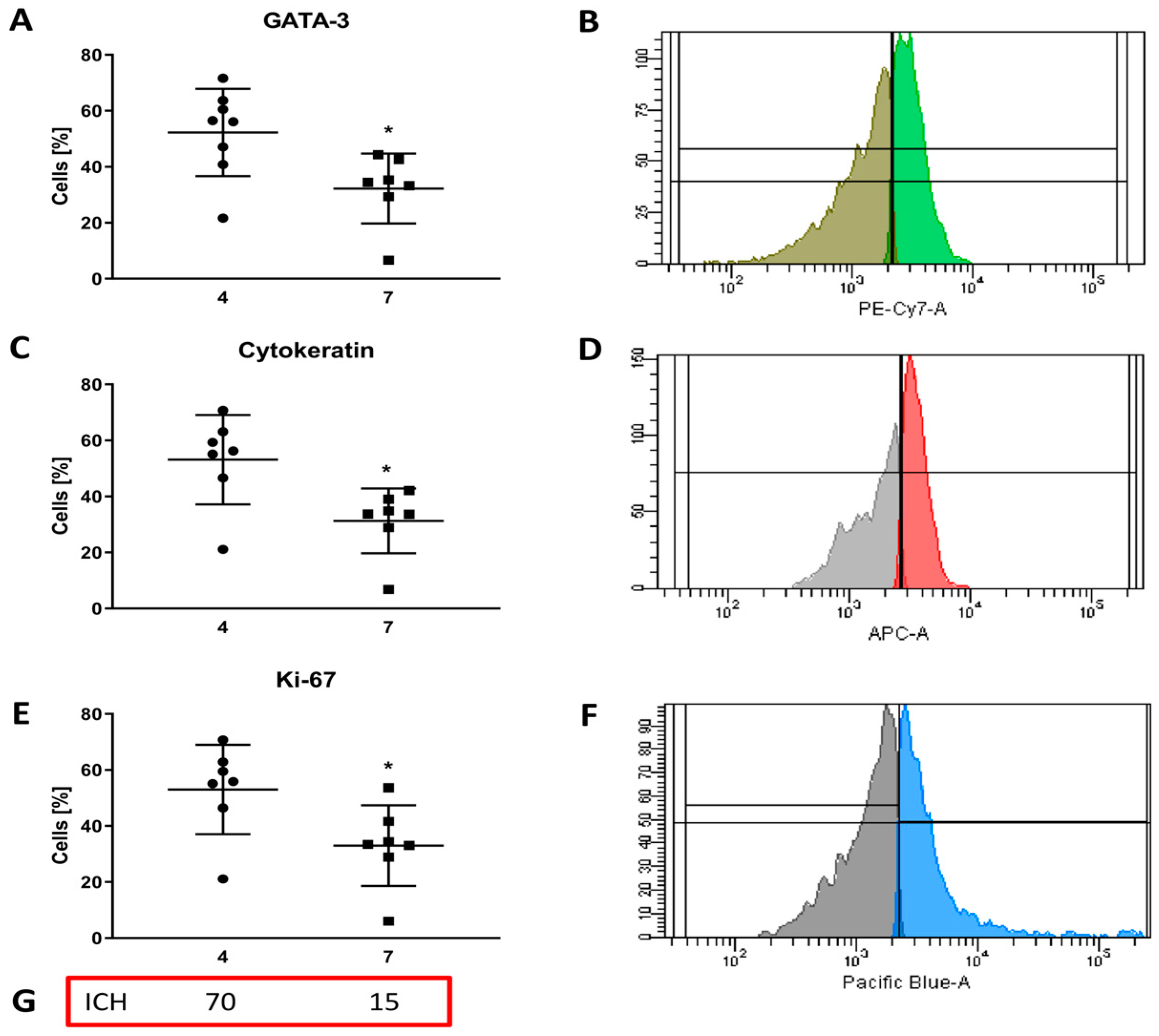
3.3.2. Evaluation of Extracellular Marker Expression
3.3.3. Evaluation of Intracellular Marker Expression
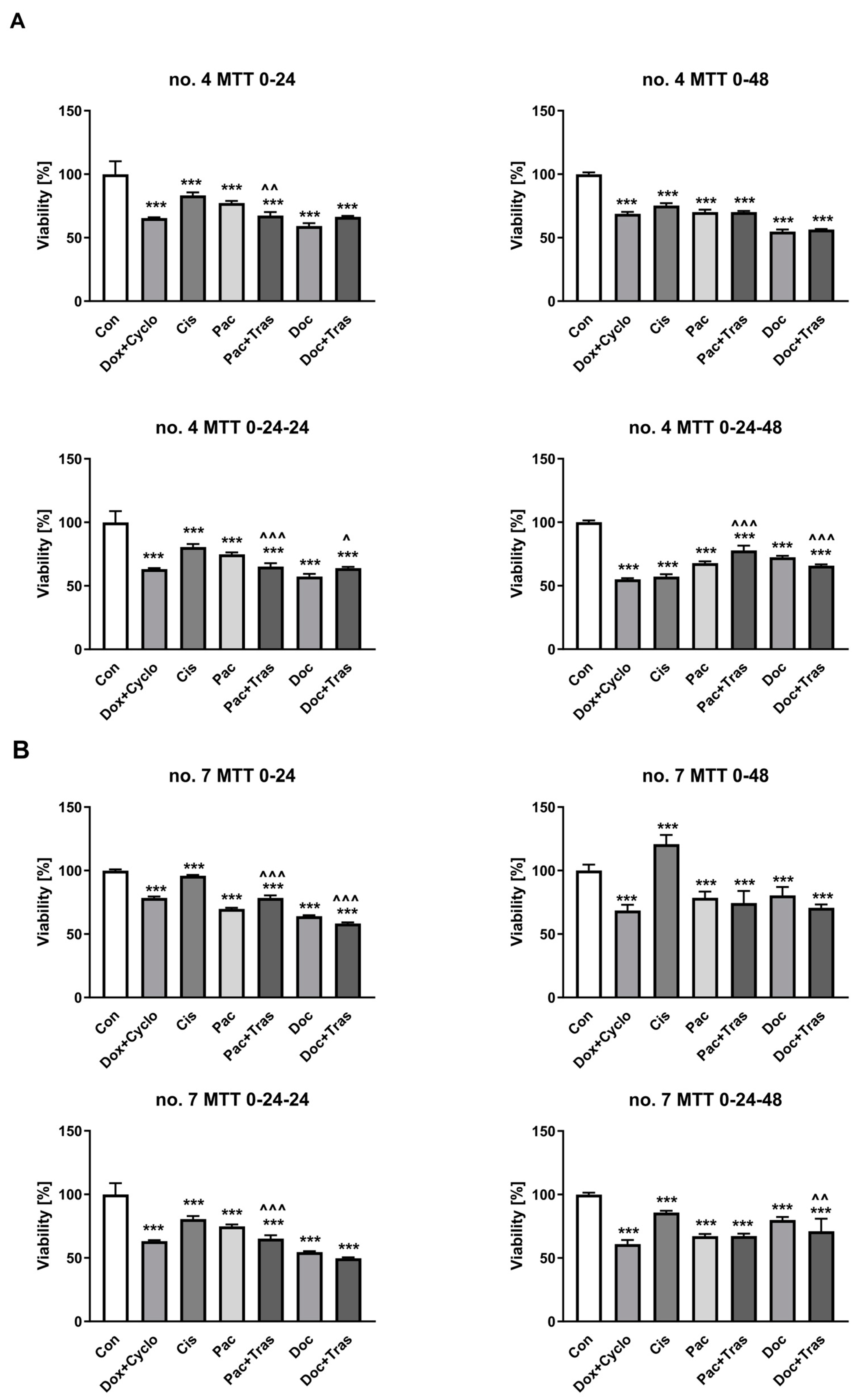
3.4. Evaluation of Cancer Cell Chemosensitivity
3.4.1. Cell Viability Testing
3.4.2. Assessing the Effects of Drugs on Cancer Cell Morphology
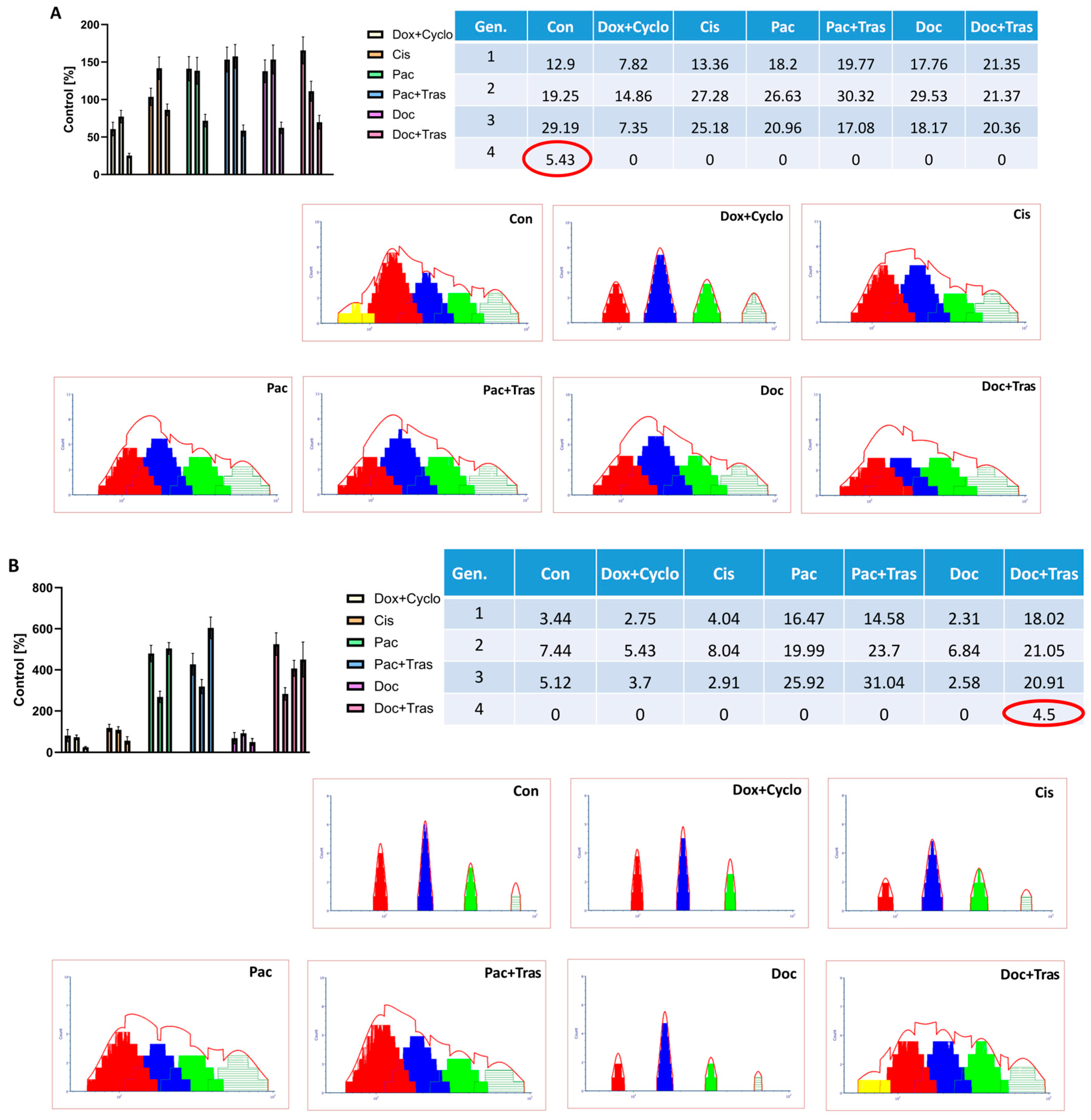
3.4.3. Assessment of Cell Proliferation
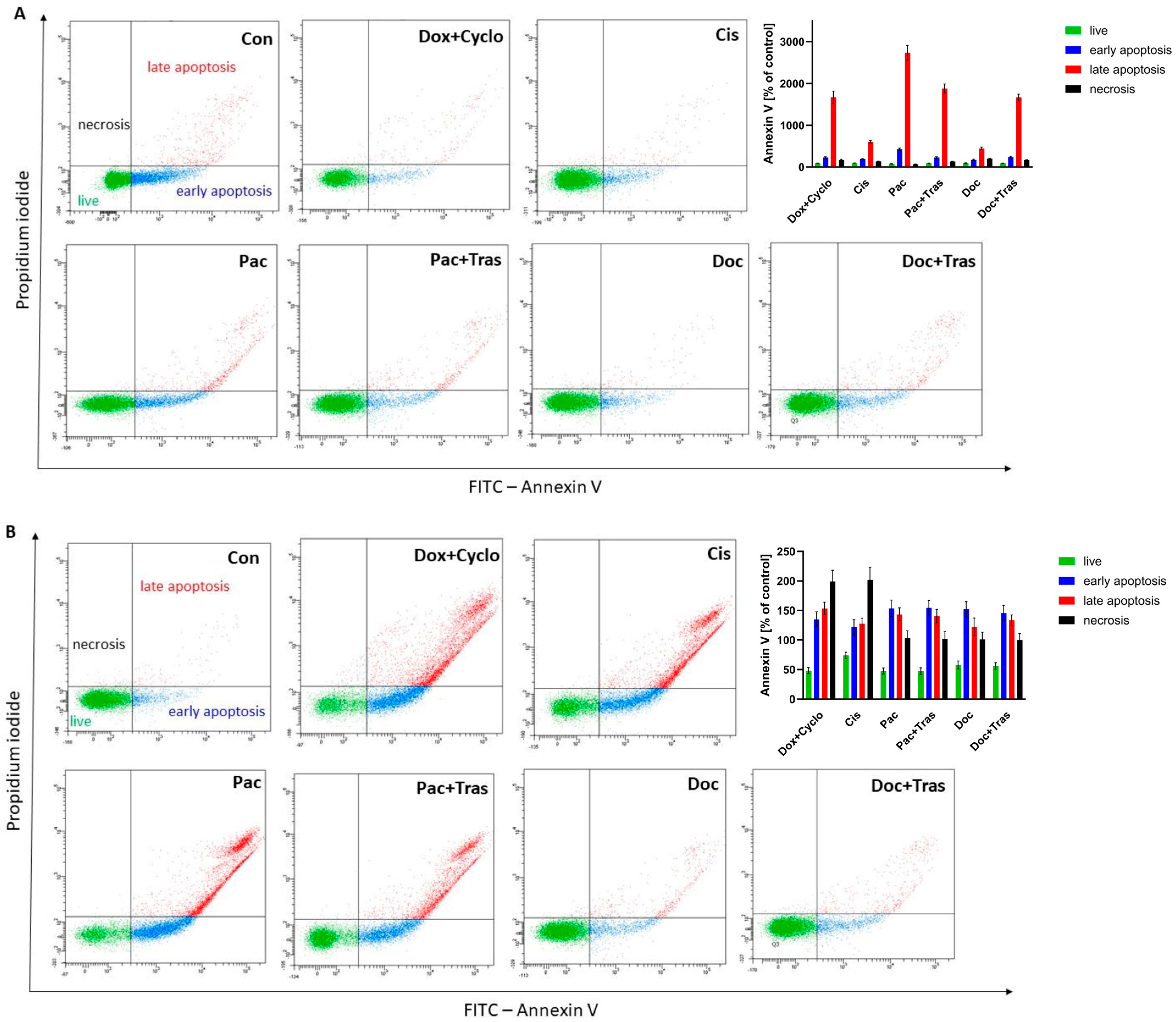
3.4.4. Evaluation of Apoptosis
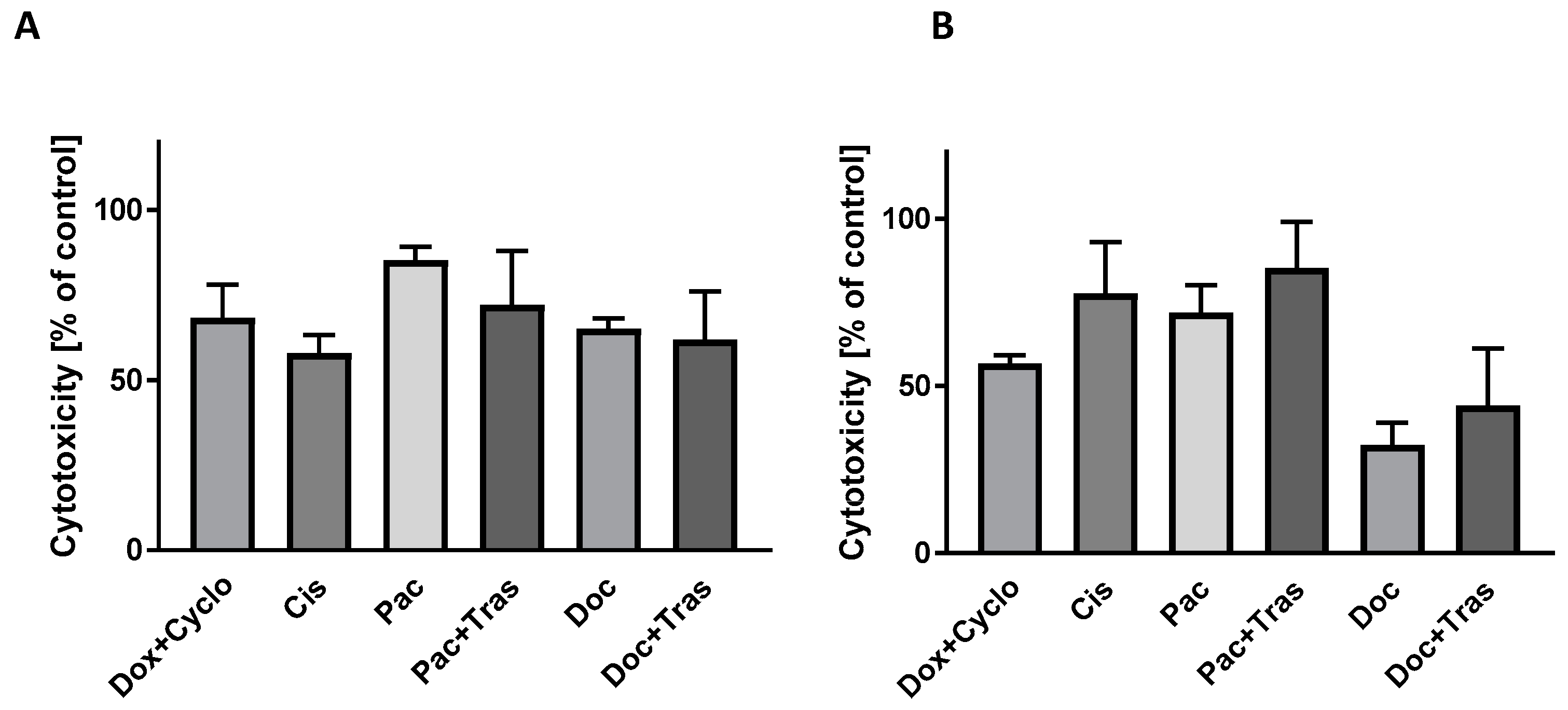
3.4.5. Evaluation of LDH Activity in Culture Medium
3.5. Proposed Procedure
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breast Cancer Facts and Statistics. Available online: https://www.breastcancer.org/facts-statistics (accessed on 18 October 2023).
- EuroeanCancer Information System. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 18 October 2023).
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 18 October 2023).[Green Version]
- Wang, X.; Chen, H.; Middha Kapoor, P.; Su, Y.-R.; Bolla, M.K.; Dennis, J.; Dunning, A.M.; Lush, M.; Wang, Q.; Michailidou, K.; et al. A Genome-Wide Gene-Based Gene-Environment Interaction Study of Breast Cancer in More than 90,000 Women. Cancer Res. Commun. 2022, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz-Kwedlo, A.; Pawlak, D.; Domaniewski, T.; Buczko, W. Effect of Erythropoietin, 5-Fluorouracil and SN-38 on the Growth of DLD-1 Cells. Pharmacol. Rep. 2010, 62, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Ronsmans, S.; Hoet, P. Differential Immunological Effects of Silica Nanoparticles on Peripheral Blood Mononuclear Cells of Silicosis Patients and Controls. Front. Immunol. 2022, 13, 1025028. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.-J.; Ahn, S.H.; Son, B.H.; Kim, S.B.; Ahn, J.H.; Noh, W.C.; Gong, G. Different Prognostic Significance of CD24 and CD44 Expression in Breast Cancer According to Hormone Receptor Status. Breast Edinb. Scotl. 2011, 20, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Voduc, D.; Cheang, M.; Nielsen, T. GATA-3 Expression in Breast Cancer Has a Strong Association with Estrogen Receptor but Lacks Independent Prognostic Value. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, S.; Xu, J.; Zhu, J. Prognostic Value and Clinicopathological Significance of Serum- and Tissue-Based Cytokeratin 18 Express Level in Breast Cancer: A Meta-Analysis. Biosci. Rep. 2018, 38, BSR20171145. [Google Scholar] [CrossRef] [PubMed]
- Soule, H.D.; Vazguez, J.; Long, A.; Albert, S.; Brennan, M. A Human Cell Line from a Pleural Effusion Derived from a Breast Carcinoma. J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Cailleau, R.; Young, R.; Olivé, M.; Reeves, W.J. Breast Tumor Cell Lines from Pleural Effusions. J. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Rederer, A.; Rose, V.; Krüger, R.; Schmittutz, L.; Swierzy, I.; Fischer, L.; Thievessen, I.; Bauer, J.; Friedrich, O.; Schiffer, M.; et al. Partner, Neighbor, Housekeeper and Dimension: 3D versus 2D Glomerular Co-Cultures Reveal Drawbacks of Currently Used Cell Culture Models. Int. J. Mol. Sci. 2023, 24, 10384. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.J.; Lin, A.F.; Arendt, L.M.; Klebba, I.; Jones, A.D.; Rudnick, J.A.; DiMeo, T.A.; Gilmore, H.; Jefferson, D.M.; Graham, R.A.; et al. Mapping the Cellular and Molecular Heterogeneity of Normal and Malignant Breast Tissues and Cultured Cell Lines. Breast Cancer Res. BCR 2010, 12, R87. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Boos, A.M.; Tasbihi, K.; Beier, J.P.; Dalton, P.D.; Schrauder, M.; Horch, R.E.; Beckmann, M.W.; Strissel, P.L.; Strick, R. Selective Isolation and Characterization of Primary Cells from Normal Breast and Tumors Reveal Plasticity of Adipose Derived Stem Cells. Breast Cancer Res. BCR 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, M.; Zhang, C.; Luo, L.; Qian, H. Establishment of a primary renal lymphoma model and its clinical relevance. Front. Oncol. 2023, 13, 1089187. [Google Scholar] [CrossRef] [PubMed]
- Pershina, O.; Ermakova, N.; Pakhomova, A.; Widera, D.; Pan, E.; Zhukova, M.; Slonimskaya, E.; Morozov, S.G.; Kubatiev, A.; Dygai, A.; et al. Cancer Stem Cells and Somatic Stem Cells as Potential New Drug Targets, Prognosis Markers, and Therapy Efficacy Predictors in Breast Cancer Treatment. Biomedicines 2021, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lei, C.; Yang, Y.; Bu, X.; Ma, H.; Gong, H.; Liu, J.; Fang, X.; Hu, Z.; Fang, Q. Abraxane, the Nanoparticle Formulation of Paclitaxel Can Induce Drug Resistance by Up-Regulation of P-gp. PLoS ONE 2015, 10, e0131429. [Google Scholar] [CrossRef] [PubMed]



| No. | Patient | Age | H-p | ER | PR | Ki-67 | HER2 |
|---|---|---|---|---|---|---|---|
| 1 | BM | 54 | NOS G3 | 0 | 5 | 60 | 0 |
| 2 | IG | 85 | NOS G3 | 100 | 100 | 70 | 3+ |
| 3 | AS | 53 | NOS G1 | 100 | 90 | 20 | 1+ |
| 4 | IH | 66 | NOS G3 | 90 | 5 | 70 | 3+ |
| 5 | DC | 74 | NOS G2 | 90 | 0 | 60 | 0 |
| 6 | WZ | 74 | NOS G2 | 90 | 20 | 70 | 1+ |
| 7 | CS | 80 | NOS G1 | 100 | 70 | 15 | 0 |
| 8 | MM | 49 | NOS G1 | 80 | 100 | 10 | 2+ |
| Patient No. | Con | Dox + Cyclo | Cis | Pac | Pac + Tras | Doc | Doc + Tras |
|---|---|---|---|---|---|---|---|
| 1 | 100 ± 2.34 | 89.00 ± 6.25 p = 0.0231 | 92.23 ± 4.60 p = 0.0288 | 82.50 ± 7.13 p = 0.0001 | 93.08 ± 2.51 | 86.13 ± 4.13 p = 0.0023 | 94.08 ± 3.48 |
| 2 | 100 ± 3.44 | 62.71 ± 5.24 p = 0.0001 | 28.00 ± 5.65 p = 0.0001 | 36.27 ± 9.79 p = 0.0001 | 21.06 ± 7.91 p = 0.0001 | 62.29 ± 10.64 p = 0.0001 | 64.58 ± 15.69 p = 0.0001 |
| 3 | 100 ± 8.43 | 73.88 ± 6.05 p < 0.0001 | 131.80 ± 12.14 p = 0.0259 | 113.15 ± 14.62 | 100.31 ± 35.36 | 105.81 ± 22.37 | 129.36 ± 13.47 p = 0.0447 |
| 5 | 100 ± 8.36 | 76.19 ± 1.45 p < 0.0001 | 102.18 ± 8.76 | 89.96 ± 6.71 | 91.09 ± 3.50 | 92.20 ± 3.38 | 80.06 ± 2.04 p < 0.0001 p = 0.0040 * |
| 6 | 100 ± 9.22 | 65.86 ± 6.09 p = 0.0001 | 91.67 ± 8.44 | 79.03 ± 15.61 p = 0.0001 | 84.07 ± 5.40 p = 0.0006 | 66.67 ± 7.32 p = 0.0001 | 75.60 ± 1.92 p = 0.0001 |
| 8 | 100 ± 6.50 | 76.17 ± 7.05 p = 0.0001 | 93.06 ± 7.20 | 98.66 ± 5.65 | 89.80 ± 7.57 p = 0.231 | 76.96 ± 6.50 p = 0.0001 | 89.65 ± 7.01 p = 0.0268 p = 0.0280 * |
| Con | Dox + Cyclo | Cis | Pac | Pac + Trans | Doc | Doc + Trans |
|---|---|---|---|---|---|---|
 | ||||||
 | ||||||
 | ||||||
 | ||||||
 | ||||||
 | ||||||
| Patient | Con | Dox + Cyclo | Cis | Pac | Pac + Tras | Doc | Doc + Tras |
|---|---|---|---|---|---|---|---|
| 1 | 3.53 ± 1.22 | 26.83 ± 5.04 p = 0.0410 | 10.13 ± 4.03 | 23.10 ± 5.40 | 20.93 ± 7.136 | 26.13 ± 8.07 | 22.93 ± 4.98 |
| 2 | 4.57 ± 0.65 | 41.8 ± 9.50 p = 0.0006 | 76.03 ± 6.89 p = 0.0001 | 52.27 ± 15.10 p = 0.0001 | 68.13 ± 6.82 p = 0.0001 | 56.73 ± 7.75 p = 0.0001 | 57.27 ± 6.87 p = 0.0001 |
| 3 | 3.60 ± 1.10 | 33.37 ± 12.22 p = 0.0001 | 6.90 ± 3.90 | 11.40 ± 2.86 | 11.13 ± 4.00 | 13.27 ± 4.99 | 6.80 ± 4.83 |
| 5 | 4.83 ± 0.91 | 34.10 ± 7.42 p = 0.0002 | 13.27 ± 7.20 | 17.17 ± 7.50 | 11.07 ± 5.48 | 14.80 ± 5.62 | 24.90 ± 5.83 |
| 6 | 3.46 ± 0.60 | 46.37 ± 6.79 p = 0.0001 | 14.67 ± 5.75 | 24.30 ± 6.85 p = 0.0033 | 26.63 ± 7.51 p = 0.0013 | 36.17 ± 6.25 p = 0.0001 | 19.63 ± 4.35 p = 0.0210 |
| 8 | 4.40 ± 0.60 | 39.13 ± 6.26 p = 0.0001 | 10.40 ± 1.91 | 11.87 ± 3.07 | 15.00 ± 5.85 | 28.80 ± 8.44 p = 0.0002 | 19.80 ± 4.37 p = 0.0103 |
| No. | MTT | Morphology | LDH | Suggested Chemotherapy |
|---|---|---|---|---|
| 1 | Dox + Cyclo, Cis, Pac, Doc | Dox + Cyclo, Cis, Pac | Dox + Cyclo | Dox + Cyclo |
| 2 | Dox + Cyclo, Cis, Pac, Pac + Tras, Doc, Doc + Tras | Dox + Cyclo, Cis, Pac, Pac + Tras, Doc, Doc + Tras | Dox + Cyclo, Cis, Pac, Pac + Tras, Doc, Doc + Tras | Dox + Cyclo, Cis, Pac, Pac + Tras, Doc, Doc + Tras |
| 3 | Dox + Cyclo | Dox + Cyclo | Dox + Cyclo | Dox + Cyclo |
| 4 | Doc, Doc + Tras, Dox + Cyclo | Pac, Pac + Tras, Doc, Doc + Tras | Pac, Pac + Tras, Doc + Cyclo, Doc + Tras | Doc + Tras |
| 5 | Dox + Cyclo | Dox + Cyclo, Cis | Dox + Cyclo | Dox + Cyclo |
| 6 | Dox + Cyclo, Pac, Pac + Tras, Doc, Doc + Tras | Dox + Cyclo, Pac, Pac + Tras | Dox + Cyclo, Pac, Pac + Tras, Doc, Doc + Tras | Dox + Cyclo, Pac, Pac + Tras |
| 7 | Dox + Cyclo, Pac, Pac + Tras, Doc + Tras | Pac, Pac + Trass, Doc, Doc + Tras | Pac, Pac + Tras, Cis | Pac, Pac + Tras |
| 8 | Dox + Cyclo, Pac + Tras, Doc, Doc + Tras | Dox + Cyclo, Doc, Doc + Tras | Dox + Cyclo, Doc, Doc + Tras | Dox + Cyclo, Doc, Doc + Tras |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tankiewicz-Kwedlo, A.; Lobacz, T.; Kozlowski, L.; Czartoryska-Arlukowicz, B.; Koda, M.; Pawlak, K.; Czarnomysy, R.; Borkowska, M.J.; Pawlak, D. ONCOBREAST-TEST Is a Quick Diagnostic, Prognostic and Predictive Method of Response to Systemic Treatment. Cancers 2024, 16, 120. https://doi.org/10.3390/cancers16010120
Tankiewicz-Kwedlo A, Lobacz T, Kozlowski L, Czartoryska-Arlukowicz B, Koda M, Pawlak K, Czarnomysy R, Borkowska MJ, Pawlak D. ONCOBREAST-TEST Is a Quick Diagnostic, Prognostic and Predictive Method of Response to Systemic Treatment. Cancers. 2024; 16(1):120. https://doi.org/10.3390/cancers16010120
Chicago/Turabian StyleTankiewicz-Kwedlo, Anna, Tomasz Lobacz, Leszek Kozlowski, Bogumila Czartoryska-Arlukowicz, Mariusz Koda, Krystyna Pawlak, Robert Czarnomysy, Magdalena Joanna Borkowska, and Dariusz Pawlak. 2024. "ONCOBREAST-TEST Is a Quick Diagnostic, Prognostic and Predictive Method of Response to Systemic Treatment" Cancers 16, no. 1: 120. https://doi.org/10.3390/cancers16010120
APA StyleTankiewicz-Kwedlo, A., Lobacz, T., Kozlowski, L., Czartoryska-Arlukowicz, B., Koda, M., Pawlak, K., Czarnomysy, R., Borkowska, M. J., & Pawlak, D. (2024). ONCOBREAST-TEST Is a Quick Diagnostic, Prognostic and Predictive Method of Response to Systemic Treatment. Cancers, 16(1), 120. https://doi.org/10.3390/cancers16010120








