Impact of Frailty on the Outcomes of Patients with Pancreatic Cancer Undergoing Neoadjuvant Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Study Design
2.2. Comprehensive Frailty Assessments
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Predictors of Resection Status and OS
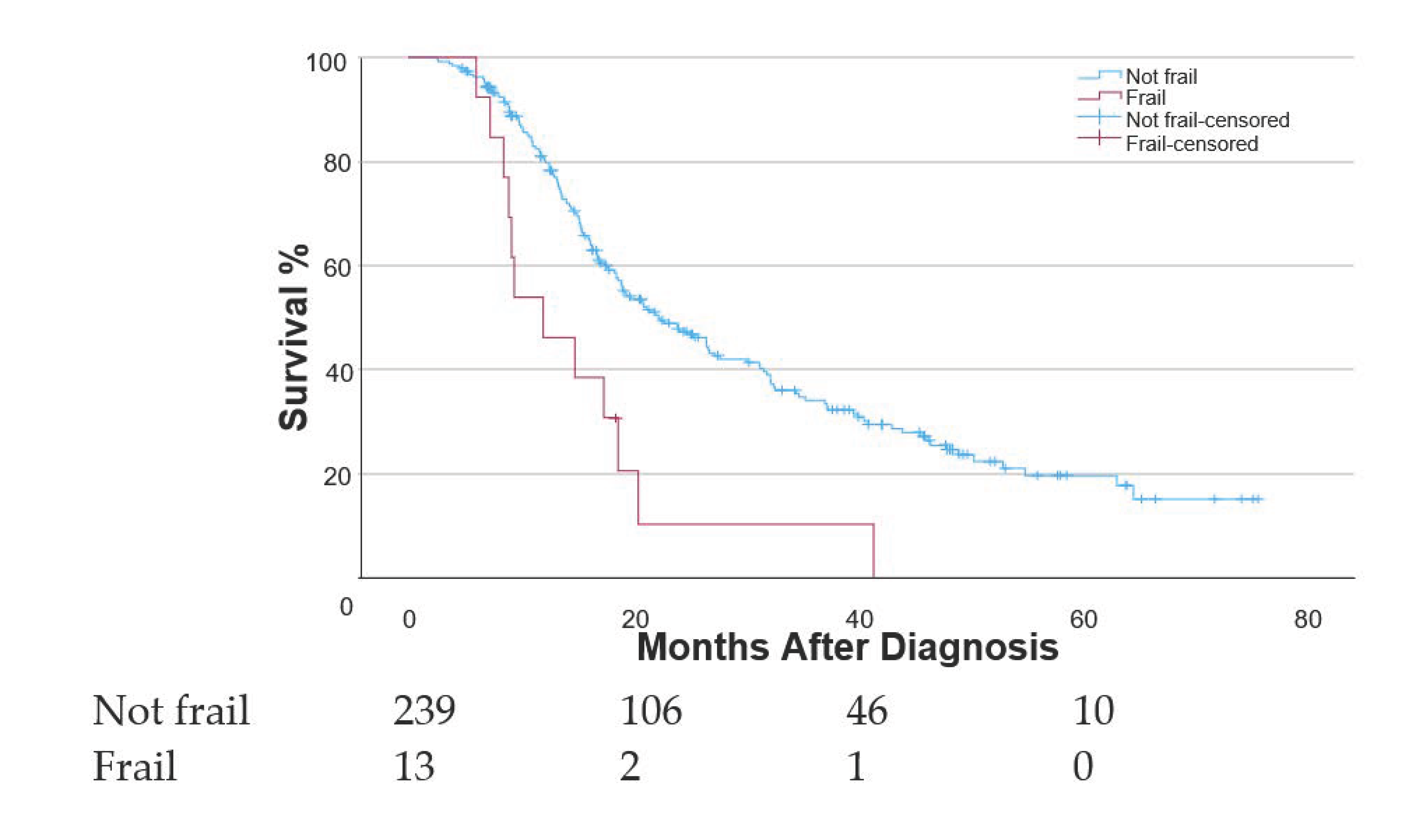
3.3. Functional Frailty Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Aquina, C.T.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. National Trends in the Use of Neoadjuvant Therapy Before Cancer Surgery in the US from 2004 to 2016. JAMA Netw. Open 2021, 4, e211031. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant Therapy for Pancreatic Cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef]
- Stoop, T.F.; Theijse, R.T.; Seelen, L.W.F.; Groot Koerkamp, B.; van Eijck, C.H.J.; Wolfgang, C.L.; van Tienhoven, G.; van Santvoort, H.C.; Molenaar, I.Q.; Wilmink, J.W.; et al. Preoperative Chemotherapy, Radiotherapy and Surgical Decision-Making in Patients with Borderline Resectable and Locally Advanced Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Vecchia, S.; Anselmi, E.; Toscani, I.; Guasconi, M.; Perrone, G.; Citterio, C.; Banchini, F.; Giuffrida, M. Neoadjuvant Treatment in Localized Pancreatic Cancer of the Elderly: A Systematic Review of the Current Literature. Cancers 2025, 17, 747. [Google Scholar] [CrossRef]
- Van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; Van Santvoort, H.C.; Van Tienhoven, G.; De Wilde, R.F.; Wilmink, J.W.; Van Eijck, C.H.J.; Groot Koerkamp, B. Neoadjuvant Therapy or Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomised Controlled Trials. Eur. J. Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef]
- Schreiber, L.; Zeh, R.; Monsour, C.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Miller, E.; Noonan, A.; Krishna, S.G.; Santry, H.; et al. Multi-Specialty Physician Perspectives on Barriers and Facilitators to the Use of Neoadjuvant Therapy for Pancreatic Ductal Adenocarcinoma. HPB 2022, 24, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Sarna, A.; Arango, M.J.; Bates, S.E.; Bhutani, M.S.; Bloomston, M.; Chung, V.; Dotan, E.; Ferrone, C.R.; Gambino, P.F.; et al. Best Practices for Delivering Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2025, 160, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.J.; Maxwell, J.E.; Katz, M.H.G.; Snyder, R.A. Surgical Considerations for Neoadjuvant Therapy for Pancreatic Adenocarcinoma. Cancers 2023, 15, 4174. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Colby, S.; Guthrie, K.A.; Lowy, A.M.; Chiorean, E.G.; Philip, P.; Sohal, D.; Ahmad, S. Failure to Undergo Resection Following Neoadjuvant Therapy for Resectable Pancreatic Cancer: A Secondary Analysis of SWOG S1505. J. Natl. Compr. Canc. Netw. 2024, 22, e237099. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty Defined by Deficit Accumulation and Geriatric Medicine Defined by Frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef]
- Ngo-Huang, A.; Holmes, H.M.; Des Bordes, J.K.A.; Parker, N.H.; Fogelman, D.; Petzel, M.Q.B.; Song, J.; Bruera, E.; Katz, M.H.G. Association between Frailty Syndrome and Survival in Patients with Pancreatic Adenocarcinoma. Cancer Med. 2019, 8, 2867–2876. [Google Scholar] [CrossRef]
- Giri, S.; Al-Obaidi, M.; Harmon, C.; Clark, D.; Ubersax, C.; Dai, C.; Young-Smith, C.; Outlaw, D.; Gbolahan, O.; Khushman, M.; et al. Patient-Reported Geriatric Assessment-Based Frailty Index among Older Adults with Gastrointestinal Malignancies. J. Am. Geriatr. Soc. 2023, 71, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The Prevalence and Outcomes of Frailty in Older Cancer Patients: A Systematic Review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and Cancer: Implications for Oncology Surgery, Medical Oncology, and Radiation Oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Velanovich, V.; Antoine, H.; Swartz, A.; Peters, D.; Rubinfeld, I. Accumulating Deficits Model of Frailty and Postoperative Mortality and Morbidity: Its Application to a National Database. J. Surg. Res. 2013, 183, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Cabot, J.; Buckner, J.; Field, A.; Pounds, L.; Quint, C. Increased Frailty Associated with Higher Long-Term Mortality after Major Lower Extremity Amputation. Ann. Vasc. Surg. 2022, 86, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A. Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Fried, L.P.; Borhani, N.O.; Enright, P.; Furberg, C.D.; Gardin, J.M.; Kronmal, R.A.; Kuller, L.H.; Manolio, T.A.; Mittelmark, M.B.; Newman, A.; et al. The Cardiovascular Health Study: Design and Rationale. Ann. Epidemiol. 1991, 1, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Javed, A.A.; Oba, A.; Koerkamp, B.G.; Seufferlein, T.; Wilmink, J.W.; Besselink, M.G. Pancreatic Cancer. Lancet 2025, 405, 1182–1202. [Google Scholar] [CrossRef]
- Davis, C.H.; Augustinus, S.; De Graaf, N.; Wellner, U.F.; Johansen, K.; Andersson, B.; Beane, J.D.; Björnsson, B.; Busch, O.R.; Gleeson, E.M.; et al. Impact of Neoadjuvant Therapy for Pancreatic Cancer: Transatlantic Trend and Postoperative Outcomes Analysis. J. Am. Coll. Surg. 2024, 238, 613–621. [Google Scholar] [CrossRef]
- Reni, M.; Macchini, M.; Orsi, G.; Procaccio, L.; Malleo, G.; Carconi, C.; Rapposelli, I.G.; Bencardino, K.; Scartozzi, M.; Balzano, G.; et al. Preoperative mFOLFIRINOX versus PAXG for Stage I–III Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma (PACT-21 CASSANDRA): Results of the First Randomisation Analysis of a Randomised, Open-Label, 2 × 2 Factorial Phase 3 Trial. Lancet 2025. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; Van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Duong, M.; Sohal, D.P.S.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2020, 272, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Fong, Z.V.; Verdugo, F.L.; Fernandez-del Castillo, C.; Ferrone, C.R.; Allen, J.N.; Blaszkowsky, L.S.; Clark, J.W.; Parikh, A.R.; Ryan, D.P.; Weekes, C.D.; et al. Tolerability, Attrition Rates, and Survival Outcomes of Neoadjuvant FOLFIRINOX for Nonmetastatic Pancreatic Adenocarcinoma: Intent-to-Treat Analysis. J. Am. Coll. Surg. 2023, 236, 1126–1136. [Google Scholar] [CrossRef]
- Dickey, E.M.; Min, L.; Dhawan, A.; Martos, M.; Franceschi, D.; Livingstone, A.S.; Hosein, P.; Terrero, G.; Datta, J.; Merchant, N.; et al. Factors Driving Attrition from Neoadjuvant Therapy to Pancreatectomy in Localized Pancreatic Cancer. Ann. Surg. Oncol. 2025. Epub ahead of printing. [Google Scholar] [CrossRef]
- Funamizu, N.; Mori, S.; Sakamoto, A.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Umeda, Y.; Aoki, T.; Takada, Y. Novel Modified Frailty Index Predicts Completion of Adjuvant Chemotherapy in Resectable Pancreatic Cancer in a Dual Center Study. Sci. Rep. 2025, 15, 17000. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kohada, Y.; Kobatake, K.; Tasaka, R.; Iwamoto, H.; Ueno, T.; Fujita, A.; Furutani, T.; Hashimoto, K.; Kurimura, Y.; et al. Impact of the Preoperative Modified 5-Item Frailty Index on the Efficacy of Neoadjuvant Chemotherapy in Patients with Muscle Invasive Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2025, 43, 695.e11–695.e19. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Li, X.; Lin, G.; Ma, Y.; Xiao, R.; Li, H.; Qiu, M.; Yang, F. Skeletal Muscle Wasting during Neoadjuvant Therapy as a Prognosticator in Patients with Esophageal and Esophagogastric Junction Cancer: A Systematic Review and Meta-Analysis. Int. J. Surg. 2022, 97, 106206. [Google Scholar] [CrossRef]
- Bruce, K.H.; Xu, C.; Shah, R.P.; McGree, M.E.; Fought, A.J.; Yadav, S.; Langstraat, C.L.; Cliby, W.A.; Kumar, A. Real-World Outcomes of Neoadjuvant Chemotherapy for Advanced Ovarian Cancer: What Happens to Patients Who Never Undergo Surgery? Gynecol. Oncol. 2025, 201, 137–143. [Google Scholar] [CrossRef]
- Ngo-Huang, A.T.; Parker, N.H.; Xiao, L.; Schadler, K.L.; Petzel, M.Q.B.; Prakash, L.R.; Kim, M.P.; Tzeng, C.-W.D.; Lee, J.E.; Ikoma, N.; et al. Effects of a Pragmatic Home-Based Exercise Program Concurrent with Neoadjuvant Therapy on Physical Function of Patients with Pancreatic Cancer: The PancFit Randomized Clinical Trial. Ann. Surg. 2023, 278, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.H.; Ngo-Huang, A.; Lee, R.E.; O’Connor, D.P.; Basen-Engquist, K.M.; Petzel, M.Q.B.; Wang, X.; Xiao, L.; Fogelman, D.R.; Schadler, K.L.; et al. Physical Activity and Exercise during Preoperative Pancreatic Cancer Treatment. Support. Care Cancer 2019, 27, 2275–2284. [Google Scholar] [CrossRef]
- Nemkov, T.; Cendali, F.; Dzieciatkowska, M.; Stephenson, D.; Hansen, K.C.; Jankowski, C.M.; D’Alessandro, A.; Marker, R.J. A Multiomics Assessment of Preoperative Exercise in Pancreatic Cancer Survivors Receiving Neoadjuvant Therapy: A Case Series. Pathophysiology 2024, 31, 166–182. [Google Scholar] [CrossRef]
- Farah, E.; Al Abbas, A.; Abreu, A.A.; Cheng, M.; Yopp, A.; Wang, S.; Mansour, J.; Porembka, M.; Zeh, H.J.; Polanco, P.M. Minimally Invasive Pancreaticoduodenectomy: A Favorable Approach for Frail Patients with Pancreatic Cancer. Surgery 2024, 175, 1168–1175. [Google Scholar] [CrossRef]
- Khalid, A.; Pasha, S.A.; Demyan, L.; Standring, O.; Newman, E.; King, D.A.; DePeralta, D.; Gholami, S.; Weiss, M.J.; Melis, M. Modified 5-Item Frailty Index (mFI-5) May Predict Postoperative Outcomes after Pancreatoduodenectomy for Pancreatic Cancer. Langenbecks Arch. Surg. 2024, 409, 286. [Google Scholar] [CrossRef]
- Dias-Santos, D.; Ferrone, C.R.; Zheng, H.; Lillemoe, K.D.; Fernández-del Castillo, C. The Charlson Age Comorbidity Index Predicts Early Mortality after Surgery for Pancreatic Cancer. Surgery 2015, 157, 881–887. [Google Scholar] [CrossRef]
| Characteristic | Not Frail, n = 239 | Frail, n = 13 | p Value | Missing, n |
|---|---|---|---|---|
| Sex, n (%) | 0.72 | |||
| Female | 104 (43.5) | 5 (38.5) | ||
| Male | 135 (56.5) | 8 (61.5) | ||
| Age, y, median (IQR) | 67 (13) | 63 (17) | 0.89 | |
| Race, n (%) | 0.34 a | |||
| White | 218 (91.2) | 11 (84.6) | ||
| Black/Unknown | 21 (8.8) | 2 (15.4) | ||
| BMI, kg/m2, median (IQR) | 25.16 (7.67) | 27.37 (11.71) | 0.11 | 3 |
| Charlson Comorbidity Index, median (IQR) | 5 (2) | 9 (3) | <0.001 | |
| Albumin, g/dL, median (IQR) | 4.0 (0.7) | 3.9 (0.9) | 0.23 | 6 |
| ECOG performance status, n (%) | <0.001 | 15 | ||
| 0 | 93 (38.9) | 3 (23.1) | ||
| 1 | 119 (49.8) | 4 (30.8) | ||
| ≥2 | 12 (5.0) | 6 (46.2) | ||
| Anatomic stage, n (%) | 0.80 | |||
| Potentially resectable | 119 (49.8) | 6 (46.2) | ||
| Borderline resectable | 120 (50.2) | 7 (53.8) | ||
| Initial neoadjuvant chemotherapy, n (%) | 0.03 a | |||
| FOLFIRINOX | 160 (66.9) | 6 (46.2) | ||
| Gemcitabine + nab-paclitaxel | 69 (28.9) | 4 (30.8) | ||
| Other | 10 (4.2) | 3 (23.1) | ||
| Days from diagnosis to NT, median (IQR) | 20 (13) | 18 (11) | 0.90 | 7 |
| Total days spent in NT, median (IQR) | 108 (89) | 166 (119) | 0.12 | 17 |
| ER visit or hospital admission during NT, n (%) | 0.06 a | 2 | ||
| No | 139 (58.2) | 3 (23.1) | ||
| Yes | 100 (41.8) | 8 (61.5) | ||
| Chemotherapy dose reduction, n (%) | 0.84 | 17 | ||
| No | 109 (45.6) | 5 (38.5) | ||
| Yes | 115 (48.1) | 6 (46.2) | ||
| Surgical resection, n (%) | <0.001 a | |||
| No | 83 (34.7) | 11 (84.6) | ||
| Yes | 156 (65.3) | 2 (15.4) | ||
| Pre-NT CA 19-9, U/mL, median (IQR) | 191.1 (753.8) | 1274.1 (2651.3) | 0.08 | 5 |
| Post-NT CA 19-9, U/mL, median (IQR) | 45.3 (206.8) | 82.8 (2166.7) | 0.18 | 14 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 0.54 (0.32–0.92) | 0.02 | 0.50 (0.29–0.89) | 0.02 |
| Age | 0.98 (0.96–1.01) | 0.25 | ||
| Race | ||||
| White | Ref | |||
| Black/Unknown | 0.62 (0.26–1.47) | 0.28 | ||
| BMI | 0.99 (0.95–1.04) | 0.76 | ||
| Modified 11-Item Frailty Index | ||||
| <0.55 | Ref | Ref | ||
| ≥0.55 | 0.10 (0.02–0.45) | 0.003 | 0.09 (0.02–0.44) | 0.003 |
| Albumin | 1.40 (0.87–2.23) | 0.17 | ||
| ECOG performance status | 0.21 | |||
| 0 | Ref | |||
| 1 | 0.68 (0.39–1.19) | 0.18 | ||
| ≥2 | 0.49 (0.19–1.30) | 0.15 | ||
| Anatomic stage | ||||
| Potentially resectable | Ref | Ref | ||
| Borderline resectable | 0.48 (0.29–0.81) | 0.01 | 0.42 (0.24–0.73) | 0.002 |
| Pre-NT CA 19-9 | 1.00 (0.99–1.00) | 0.28 | ||
| Initial neoadjuvant chemotherapy | 0.04 | 0.04 | ||
| FOLFIRINOX | Ref | Ref | ||
| Gemcitabine + nab-paclitaxel | 0.48 (0.28–0.85) | 0.01 | 0.43 (0.22–0.84) | 0.01 |
| Other | 0.75 (0.23–2.40) | 0.63 | 0.98 (0.24–4.04) | 0.98 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Sex | ||||
| Female | Ref | |||
| Male | 1.14 (0.83–1.55) | 0.42 | ||
| Age | 1.01 (1.00–1.03) | 0.13 | ||
| Race | ||||
| White | Ref | |||
| Black/Unknown | 1.43 (0.84–2.45) | 0.19 | ||
| BMI | 0.99 (0.97–1.02) | 0.49 | ||
| Modified 11-Item Frailty Index | ||||
| <0.55 | Ref | Ref | ||
| ≥0.55 | 2.64 (1.46–4.77) | 0.001 | 3.00 (1.46–6.20) | 0.003 |
| Albumin | 0.89 (0.67–1.15) | 0.34 | ||
| ECOG performance status | 0.06 | 0.02 | ||
| 0 | Ref | Ref | ||
| 1 | 1.44 (1.03–2.00) | 0.03 | 1.37 (0.98–1.91) | 0.06 |
| ≥2 | 0.91 (0.46–1.82) | 0.79 | 0.60 (0.27–1.34) | 0.21 |
| Anatomic stage | ||||
| Potentially resectable | Ref | |||
| Borderline resectable | 1.29 (0.95–1.76) | 0.10 | ||
| Pre-NT CA 19-9 | 1.00 (1.00–1.01) | 0.01 | 1.00 (1.00–1.01) | 0.02 |
| Initial neoadjuvant chemotherapy | <0.001 | <0.001 | ||
| FOLFIRINOX | Ref | Ref | ||
| Gemcitabine + nab-paclitaxel | 1.88 (1.35–2.63) | <0.001 | 2.19 (1.43–3.33) | <0.001 |
| Other | 1.47 (0.71–3.02) | 0.30 | 1.06 (0.45–2.49) | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, N.R.; Leuschner, T.; Walsh, A.K.; Gault, K.; Ingram, A.; Blair, A.B.; Tsai, S.; Pawlik, T.M.; Dillhoff, M.E.; Cloyd, J.M. Impact of Frailty on the Outcomes of Patients with Pancreatic Cancer Undergoing Neoadjuvant Therapy. Cancers 2025, 17, 4030. https://doi.org/10.3390/cancers17244030
Williams NR, Leuschner T, Walsh AK, Gault K, Ingram A, Blair AB, Tsai S, Pawlik TM, Dillhoff ME, Cloyd JM. Impact of Frailty on the Outcomes of Patients with Pancreatic Cancer Undergoing Neoadjuvant Therapy. Cancers. 2025; 17(24):4030. https://doi.org/10.3390/cancers17244030
Chicago/Turabian StyleWilliams, Nicholas R., Thomas Leuschner, Amanda K. Walsh, Kayla Gault, Amber Ingram, Alex B. Blair, Susan Tsai, Timothy M. Pawlik, Mary E. Dillhoff, and Jordan M. Cloyd. 2025. "Impact of Frailty on the Outcomes of Patients with Pancreatic Cancer Undergoing Neoadjuvant Therapy" Cancers 17, no. 24: 4030. https://doi.org/10.3390/cancers17244030
APA StyleWilliams, N. R., Leuschner, T., Walsh, A. K., Gault, K., Ingram, A., Blair, A. B., Tsai, S., Pawlik, T. M., Dillhoff, M. E., & Cloyd, J. M. (2025). Impact of Frailty on the Outcomes of Patients with Pancreatic Cancer Undergoing Neoadjuvant Therapy. Cancers, 17(24), 4030. https://doi.org/10.3390/cancers17244030







