Implementation Rates and Predictors of Compliance with Enhanced Recovery After Surgery Protocols in Gynecologic Oncology: A Prospective Multi-Institutional Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Protocol Registration
2.2. Data Collection and Measured Variables
2.3. ERAS Protocol Implementation
2.4. Outcomes
2.5. Sample Size Calculation—Decision Point of Interim Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Principal Findings
4.2. Comparison with Existing Literature
4.3. Strengths and Limitations
5. Conclusions and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fearon, K.C.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin. Nutr. 2005, 24, 466–477. [Google Scholar] [CrossRef]
- Noh, J.J.; Kim, M.-S.; Lee, Y.-Y. The implementation of enhanced recovery after surgery protocols in ovarian malignancy surgery. Gland. Surg. 2020, 10, 1182–1194. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Carli, F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: Implications of the stress response. Can. J. Anaesth. 2015, 62, 110–119. [Google Scholar] [CrossRef]
- Lassen, K.; Coolsen, M.M.; Slim, K.; Carli, F.; de Aguilar-Nascimento, J.E.; Schäfer, M.; Parks, R.W.; Fearon, K.C.; Lobo, D.N.; Demartines, N.; et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, T.W.; Gill, M.; McDonald, D.A.; Middleton, R.G.; Reed, M.; Sahota, O.; Yates, P.; Ljungqvist, O. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020, 91, 3–19. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Ore, A.S.; Shear, M.A.; Liu, F.W.; Dalrymple, J.L.; Awtrey, C.S.; Garrett, L.; Stack-Dunnbier, H.; Hacker, M.R.; Esselen, K.M. Adoption of enhanced recovery after laparotomy in gynecologic oncology. Int. J. Gynecol. Cancer 2020, 30, 122–127. [Google Scholar] [CrossRef]
- Sauro, K.M.; Smith, C.; Ibadin, S.; Thomas, A.; Ganshorn, H.; Bakunda, L.; Bajgain, B.; Bisch, S.P.; Nelson, G. Enhanced Recovery After Surgery Guidelines and Hospital Length of Stay, Readmission, Complications, and Mortality: A Meta-Analysis of Randomized Clinical Trials. JAMA Netw. Open 2024, 7, e2417310. [Google Scholar] [CrossRef] [PubMed]
- Margioula-Siarkou, C.; Almperis, A.; Almperi, E.A.; Margioula-Siarkou, G.; Flindris, S.; Daponte, N.; Daponte, A.; Dinas, K.; Petousis, S. Prophylactic and Therapeutic Usage of Drains in Gynecologic Oncology Procedures: A Comprehensive Review. J. Pers. Med. 2025, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Pache, B.; Jurt, J.; Grass, F.; Hübner, M.; Demartines, N.; Mathevet, P.; Achtari, C. Compliance with enhanced recovery after surgery program in gynecology: Are all items of equal importance? Int. J. Gynecol. Cancer 2019, 29, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Rasilainen, S.; Tiainen, T.; Pakarinen, M.; Bumblyte, V.; Scheinin, T.; Schramko, A. ERAS failure and major complications in elective colon surgery: Common risk factors. Surg. Pract. Sci. 2022, 10, 100080. [Google Scholar] [CrossRef]
- Ritter, A.S.; Welsch, T.; Brodersen, F.; Auinger, J.; Moll-Khosrawi, P.; Goetz, M.R.; Bardenhagen, J.; Nitschke, C.; Schneider, T.; Wellge, B.; et al. Impact of Enhanced Recovery After Surgery Protocol Compliance on Outcome After Pancreatic Surgery: Results From a Certified ERAS Center. Ann. Surg. Open 2024, 5, e501. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Gramlich, L.M.; Sheppard, C.E.; Wasylak, T.; Gilmour, L.E.; Ljungqvist, O.; Basualdo-Hammond, C.; Nelson, G. Implementation of Enhanced Recovery After Surgery: A strategy to transform surgical care across a health system. Implement. Sci. 2017, 12, 67. [Google Scholar] [CrossRef]
- Millan, M. Enhanced recovery after surgery in elderly and high-risk patients. Ann. Laparosc. Endosc. Surg. 2020, 5, 39. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, F.; Min, S.; Wu, G.; Jin, J.; Gong, Y.; Yu, J.; Qin, P.; Zhang, Y. Impact of enhanced recovery after surgery protocol compliance on patients’ outcome in benign hysterectomy and establishment of a predictive nomogram model. BMC Anesthesiol. 2021, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Wijk, L.; Udumyan, R.; Pache, B.; Altman, A.D.; Williams, L.L.; Elias, K.M.; McGee, J.; Wells, T.; Gramlich, L.; Holcomb, K.; et al. International validation of Enhanced Recovery After Surgery Society guidelines on enhanced recovery for gynecologic surgery. Am. J. Obstet. Gynecol. 2019, 221, 237.e1–237.e11. [Google Scholar] [CrossRef]
- Pandraklakis, A.; Liakou, C.; La Russa, M.; Ochoa-Ferraro, R.; Stearns, A.; Burbos, N. Implementation of enhanced recovery protocols in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for metastatic ovarian cancer following neoadjuvant chemotherapy. A feasibility study. Gynecol. Oncol. Rep. 2024, 56, 101536. [Google Scholar] [CrossRef]
- Iniesta, M.D.; Lasala, J.; Mena, G.; Rodriguez-Restrepo, A.; Salvo, G.; Pitcher, B.; Washington, L.D.; Harris, M.; Meyer, L.A.; Ramirez, P.T. Impact of compliance with an enhanced recovery after surgery pathway on patient outcomes in open gynecologic surgery. Int. J. Gynecol. Cancer 2019, 29, 1417–1424. [Google Scholar] [CrossRef]
- Bisch, S.P.; Wells, T.; Gramlich, L.; Faris, P.; Wang, X.; Tran, D.T.; Thanh, N.X.; Glaze, S.; Chu, P.; Ghatage, P.; et al. Enhanced Recovery After Surgery (ERAS) in gynecologic oncology: System-wide implementation and audit leads to improved value and patient outcomes. Gynecol. Oncol. 2018, 151, 117–123. [Google Scholar] [CrossRef]
- Hayek, J.; Zorrilla-Vaca, A.; Meyer, L.A.; Mena, G.; Lasala, J.; Iniesta, M.D.; Suki, T.; Huepenbecker, S.; Cain, K.; Garcia-Lopez, J.; et al. Patient outcomes and adherence to an enhanced recovery pathway for open gynecologic surgery: A 6-year single-center experience. Int. J. Gynecol. Cancer 2022, 32, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hidalgo, N.R.; Pletnev, A.; Razumova, Z.; Bizzarri, N.; Selcuk, I.; Theofanakis, C.; Zalewski, K.; Nikolova, T.; Lanner, M.; Kacperczyk-Bartnik, J.; et al. European Enhanced Recovery After Surgery (ERAS) gynecologic oncology survey: Status of ERAS protocol implementation across Europe. Int. J. Gynecol. Obstet. 2023, 160, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Chen, Y.; Ren, L.; Lv, F.; Hu, J.; Liu, C.; Wang, C.; An, R.; Shen, Y. Adherence to the enhanced recovery after surgery protocol and its influencing factors among patients in Southwestern China: A multicenter cross-sectional study. Front. Med. 2025, 12, 1660083. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Wang, X.; Nelson, A.; Faris, P.; Lagendyk, L.; Wasylak, T.; Bathe, O.F.; Bigam, D.; Bruce, E.; Buie, W.D.; et al. Evaluation of the Implementation of Multiple Enhanced Recovery After Surgery Pathways Across a Provincial Health Care System in Alberta, Canada. JAMA Netw. Open 2021, 4, e2119769. [Google Scholar] [CrossRef]
- Lu, P.W.; Fields, A.C.; Shabat, G.; Bleday, R.; Goldberg, J.E.; Irani, J.; Stopfkuchen-Evans, M.; Melnitchouk, N. Cytoreductive Surgery and HIPEC in an Enhanced Recovery After Surgery Program: A Feasibility Study. J. Surg. Res. 2020, 247, 59–65. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, J.L.; Carbonell-Socias, M.; Pérez-Benavente, M.A.; Monreal Clua, S.; Manrique-Muñoz, S.; García Gorriz, M.; Burgos-Peláez, R.; Segurola Gurrutxaga, H.; Pamies Serrano, M.; Pilar Gutiérrez-Barceló, M.D.; et al. PROFAST: A randomised trial implementing enhanced recovery after surgery for highcomplexity advanced ovarian cancer surgery. Eur. J. Cancer 2020, 136, 149–158. [Google Scholar] [CrossRef]
| Phase | ERAS Component | Definition of Compliance |
|---|---|---|
| Preoperative | Patient counseling | Written and verbal preoperative information provided, including expectations of early mobilization, oral intake, and discharge criteria. |
| Fasting interval | Solids discontinued <6 h and clear fluids allowed up to 2 h preoperatively. | |
| Carbohydrate loading | Administration of carbohydrate-rich drink 2–3 h before anesthesia induction. | |
| No mechanical bowel preparation | Absence of preoperative mechanical bowel preparation unless required by procedure (e.g., bowel resection). | |
| Thromboprophylaxis | Pharmacologic and mechanical prophylaxis according to institutional protocol. | |
| Antibiotic prophylaxis | Administration within 60 min prior to incision and discontinuation within 24 h postoperatively. | |
| Intraoperative | Multimodal analgesia | Use of regional anesthesia, paracetamol, or NSAIDs in combination with minimized opioid use. |
| Maintenance of normothermia | Core body temperature maintained >36 °C intraoperatively. | |
| Goal-directed fluid therapy | Intraoperative fluids are tailored considering patient’s hemodynamic status. | |
| No routine use of nasogastric tube | Absence of nasogastric tube beyond recovery period. | |
| Avoidance of peritoneal drains | No prophylactic intraperitoneal drains used unless clinically indicated. | |
| Antiemetic prophylaxis | Administration of dual antiemetic regimen (5-HT3 antagonist + dexamethasone or equivalent). | |
| Postoperative | Early oral intake | Initiation of clear fluids within 6 h post-surgery. |
| Early mobilization | Patient ambulating or sitting out of bed within 24 h postoperatively. | |
| Early urinary catheter removal | Catheter removed within 24 h following surgery. | |
| Multimodal postoperative analgesia | Use of opioid-sparing analgesic regimen, avoiding patient-controlled opioids unless necessary. | |
| Early resumption of normal diet | Soft or regular diet tolerated within 24 h of surgery. | |
| Glycemic control | Postoperative blood glucose maintained <180 mg/dL in non-diabetic and diabetic patients alike. | |
| Fluid balance optimization | Cessation of intravenous fluids within 24 h postoperatively. | |
| Discharge criteria | Achievement of tolerance to diet, independent ambulation, controlled pain with oral medications, and stable vital signs. |
| ERAS Variable | Alexandra Hosp. (n = 158) | St.Savvas Hosp. (n = 32) | Papanikolaou Hosp. (n = 26) | Aristotle Univ. (n = 20) | Univ. of Thessaly (n = 64) | Total (n = 300) | p-Value |
|---|---|---|---|---|---|---|---|
| Preoperative ERAS components | |||||||
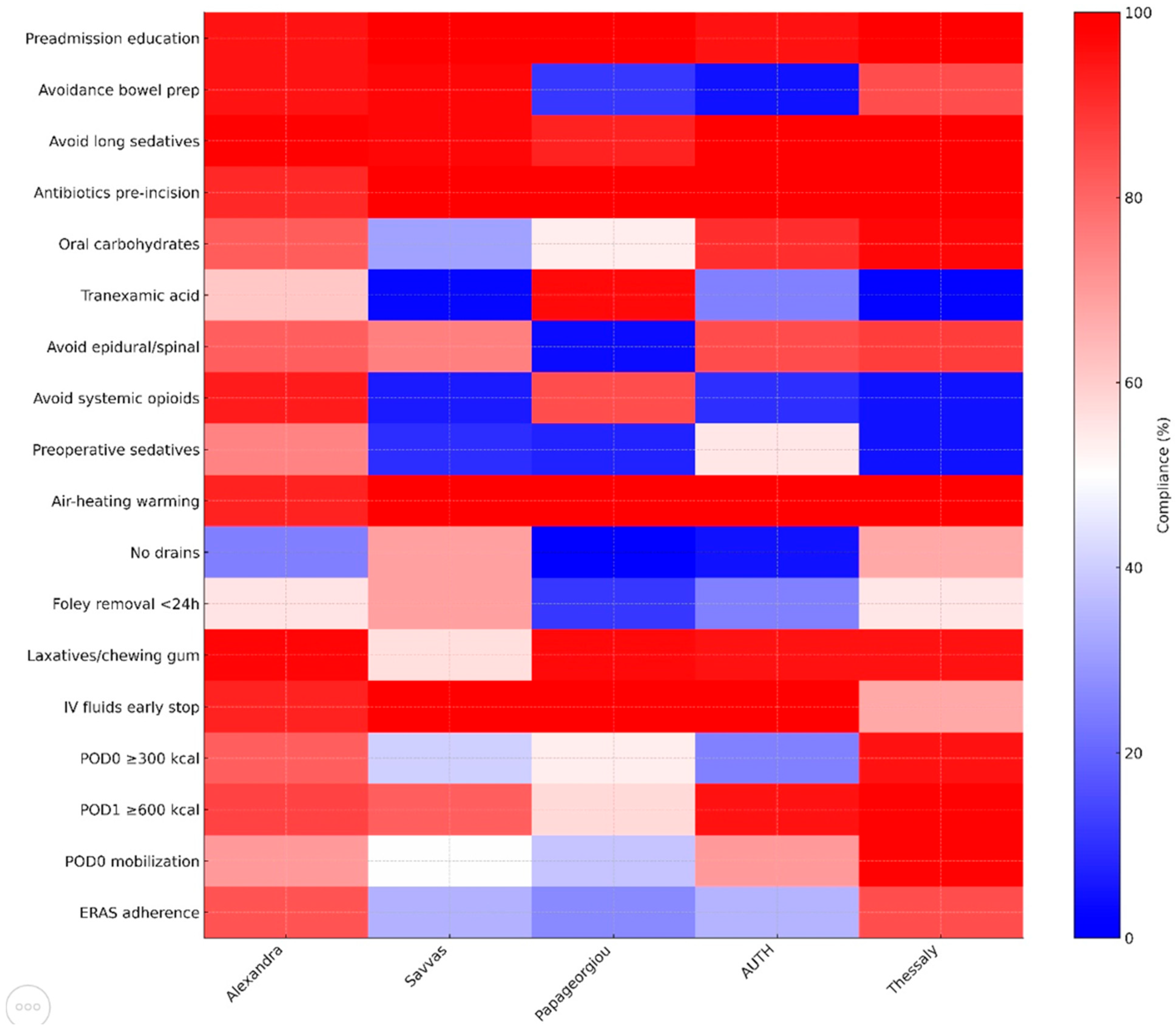
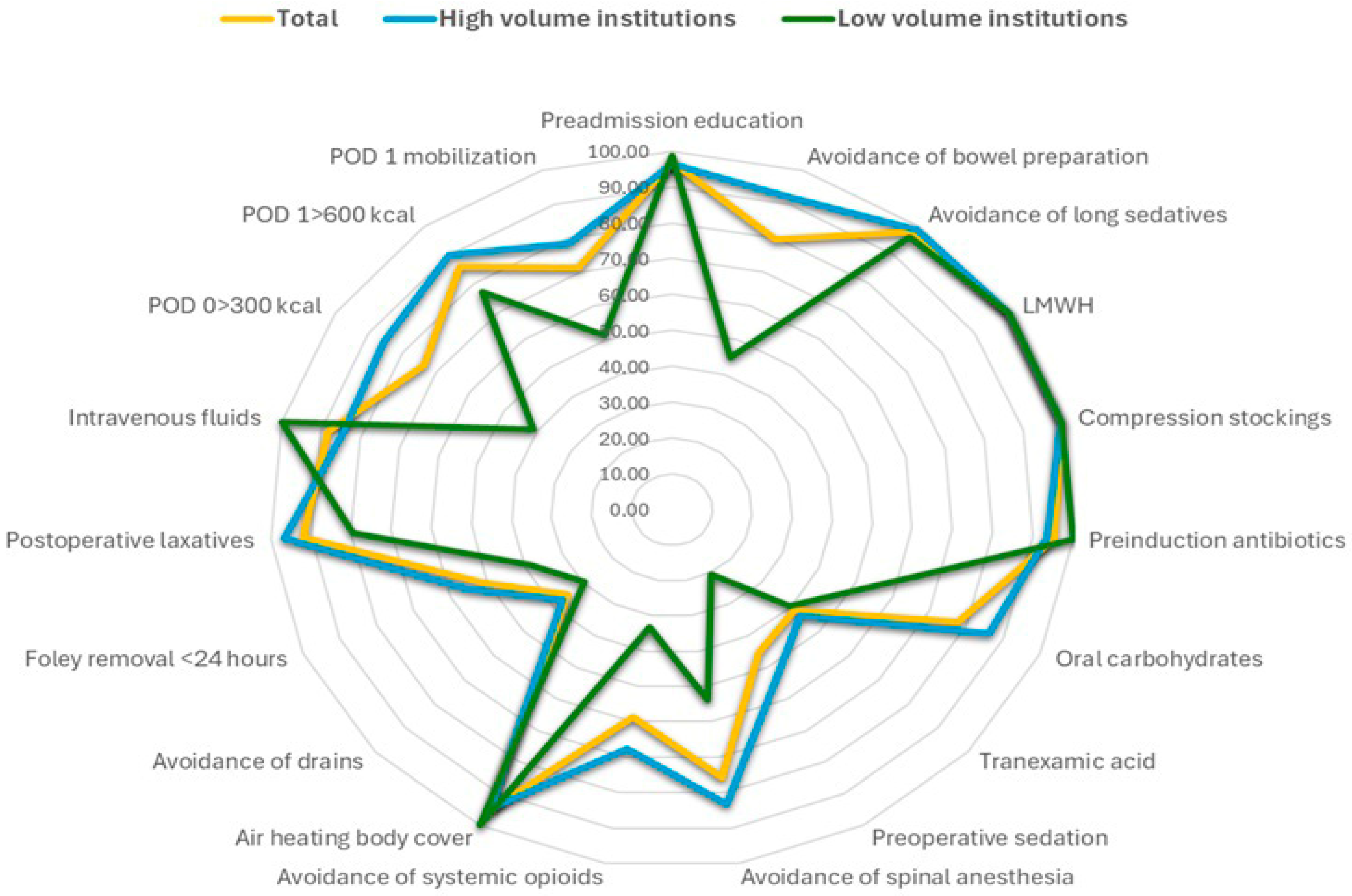
| Preadmission education | 150 (94.9%) | 32 (100%) | 26 (100%) | 19 (95%) | 64 (100%) | 291 (97.0%) | 0.174 |
| Avoidance bowel prep | 150 (94.9%) | 31 (96.9%) | 3 (11.5%) | 1 (5%) | 54 (84.4%) | 239 (79.7%) | <0.001 |
| Avoid long sedatives | 156 (98.7%) | 31 (96.9%) | 24 (92.3%) | 20 (100%) | 64 (100%) | 295 (98.3%) | 0.101 |
| Antibiotics pre-incision | 144 (91.1%) | 32 (100%) | 26 (100%) | 20 (100%) | 64 (100%) | 286 (95.3%) | 0.010 |
| Oral carbohydrates | 129 (81.7%) | 10 (31.3%) | 14 (53.8%) | 18 (90%) | 62 (96.9%) | 233 (77.7%) | <0.001 |
| Tranexamic acid | 92 (61.3%) | 1 (3.1%) | 25 (96.2%) | 5 (25%) | 1 (1.6%) | 124 (57.1%) | <0.001 |
| LMWH prophylaxis | 158 (100%) | 32 (100%) | 26 (100%) | 20 (100%) | 64 (100%) | 300 (100%) | – |
| Compression stockings | 158 (100%) | 32 (100%) | 26 (100%) | 20 (100%) | 64 (100%) | 300 (100%) | – |
| Intraoperative ERAS components | |||||||
| Avoid epidural/spinal | 129 (81.6%) | 24 (75%) | 1 (3.8%) | 17 (85%) | 56 (87.5%) | 227 (75.7%) | <0.001 |
| Avoid systemic opioids | 147 (93.0%) | 2 (6.3%) | 22 (84.6%) | 2 (10%) | 3 (4.7%) | 176 (58.7%) | <0.001 |
| Preoperative sedatives | 117 (74.5%) | 3 (9.4%) | 2 (7.7%) | 11 (55%) | 3 (4.7%) | 136 (54.5%) | <0.001 |
| Air-heating warming | 145 (92.4%) | 32 (100%) | 26 (100%) | 20 (100%) | 64 (100%) | 287 (96.0%) | 0.023 |
| No drains | 39 (24.7%) | 22 (68.8%) | 0 (0%) | 1 (5%) | 43 (67.2%) | 105 (35.0%) | <0.001 |
| Postoperative ERAS components | |||||||
| Foley removal < 24 h | 88 (55.7%) | 22 (68.8%) | 3 (11.5%) | 5 (25%) | 35 (54.7%) | 153 (51.0%) | <0.001 |
| Laxatives/chewing gum | 153 (97.5%) | 18 (56.3%) | 25 (96.2%) | 19 (95%) | 61 (95.3%) | 276 (92.3%) | <0.001 |
| IV fluids early stop | 144 (92.3%) | 32 (100%) | 26 (100%) | 20 (100%) | 43 (67.2%) | 265 (88.6%) | <0.001 |
| POD 0 ≥ 300 kcal | 128 (81.5%) | 13 (40.6%) | 14 (53.8%) | 5 (25%) | 61 (95.3%) | 221 (73.9%) | <0.001 |
| POD 1 ≥ 600 kcal | 134 (86.5%) | 26 (81.3%) | 15 (57.7%) | 19 (95%) | 63 (98.4%) | 257 (86.5%) | <0.001 |
| POD 0 mobilization | 111 (70.3%) | 16 (50%) | 10 (38.5%) | 14 (70%) | 63 (98.4%) | 214 (71.3%) | <0.001 |
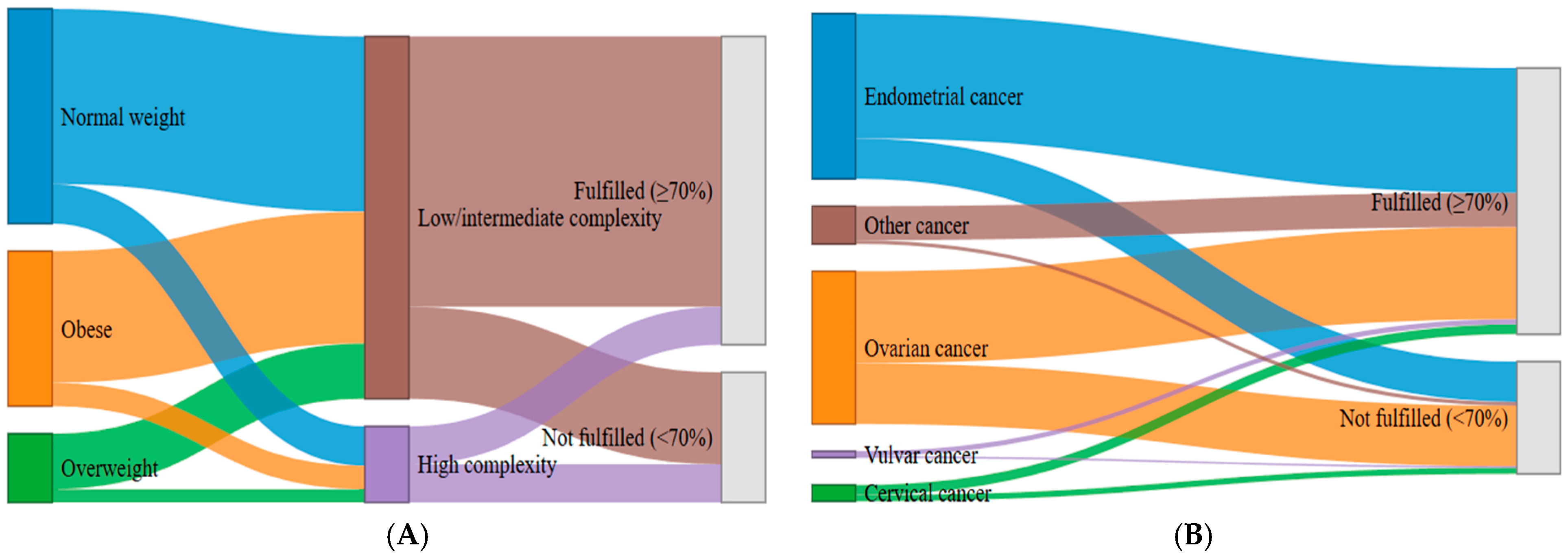
| ERAS adherence (%) | 132 (83.5%) | 11 (34.4%) | 7 (26.9%) | 7 (35.0%) | 54 (84.4%) | 211 (70.3%) | |
| Predictor | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Surgical complexity | |||
| • Intermediate vs. Low | 0.263 | 0.128–0.540 | <0.001 |
| • High vs. Low | 0.000 | 0.000 | 0.999 |
| BMI status | |||
| • Overweight vs. normal | 0.857 | 0.377–1.946 | 0.712 |
| • Obese vs. normal | 1.007 | 0.404–2.510 | 0.988 |
| • Severly obese vs. normal | 1.248 | 0.542–2.877 | 0.603 |
| Age | 1.019 | 0.992–1.047 | 0.172 |
| Smoking status | 0.864 | 0.441–1.692 | 0.669 |
| Diabetes mellitus | 0.934 | 0.635–1.375 | 0.731 |
| Tumor type | |||
| • Ovarian vs. endometrial | 0.786 | 0.393–1.572 | 0.496 |
| • Vulvar vs. endometrial | 1.327 | 0.130–13.528 | 0.811 |
| • Cervical vs. endometrial | 0.455 | 0.125–1.652 | 0.231 |
| • Sarcoma vs. endometrial | 0.263 | 0.053–1.308 | 0.103 |
| ECOG performance status | |||
| • ECOG 2 vs. 0–1 | 0.437 | 0.211–0.906 | 0.026 |
| • ECOG 3 vs. 0–1 | 0.032 | 0.003–0.292 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergialiotis, V.; Haidopoulos, D.; Daponte, A.; Tsolakidis, D.; Petousis, S.; Kalogiannidis, I.; Vlachos, D.E.; Lygizos, V.; Fanaki, M.; Delinasios, G.; et al. Implementation Rates and Predictors of Compliance with Enhanced Recovery After Surgery Protocols in Gynecologic Oncology: A Prospective Multi-Institutional Cohort Study. Cancers 2025, 17, 3991. https://doi.org/10.3390/cancers17243991
Pergialiotis V, Haidopoulos D, Daponte A, Tsolakidis D, Petousis S, Kalogiannidis I, Vlachos DE, Lygizos V, Fanaki M, Delinasios G, et al. Implementation Rates and Predictors of Compliance with Enhanced Recovery After Surgery Protocols in Gynecologic Oncology: A Prospective Multi-Institutional Cohort Study. Cancers. 2025; 17(24):3991. https://doi.org/10.3390/cancers17243991
Chicago/Turabian StylePergialiotis, Vasilios, Dimitrios Haidopoulos, Alexandros Daponte, Dimitrios Tsolakidis, Stamatios Petousis, Ioannis Kalogiannidis, Dimitrios Efthymios Vlachos, Vasilios Lygizos, Maria Fanaki, George Delinasios, and et al. 2025. "Implementation Rates and Predictors of Compliance with Enhanced Recovery After Surgery Protocols in Gynecologic Oncology: A Prospective Multi-Institutional Cohort Study" Cancers 17, no. 24: 3991. https://doi.org/10.3390/cancers17243991
APA StylePergialiotis, V., Haidopoulos, D., Daponte, A., Tsolakidis, D., Petousis, S., Kalogiannidis, I., Vlachos, D. E., Lygizos, V., Fanaki, M., Delinasios, G., Tzitzis, P., Ntailianas, P., Theodoulidis, V., Margioula Siarkou, G., Daponte, N., & Thomakos, N. (2025). Implementation Rates and Predictors of Compliance with Enhanced Recovery After Surgery Protocols in Gynecologic Oncology: A Prospective Multi-Institutional Cohort Study. Cancers, 17(24), 3991. https://doi.org/10.3390/cancers17243991







