Can TP53, TMB and TME Expand the Immunotherapy Benefit in Metastatic Colorectal Cancer?
Simple Summary
Abstract
1. Introduction
1.1. Rationale for Focusing on TP53
1.2. The Current Immunotherapy Baseline
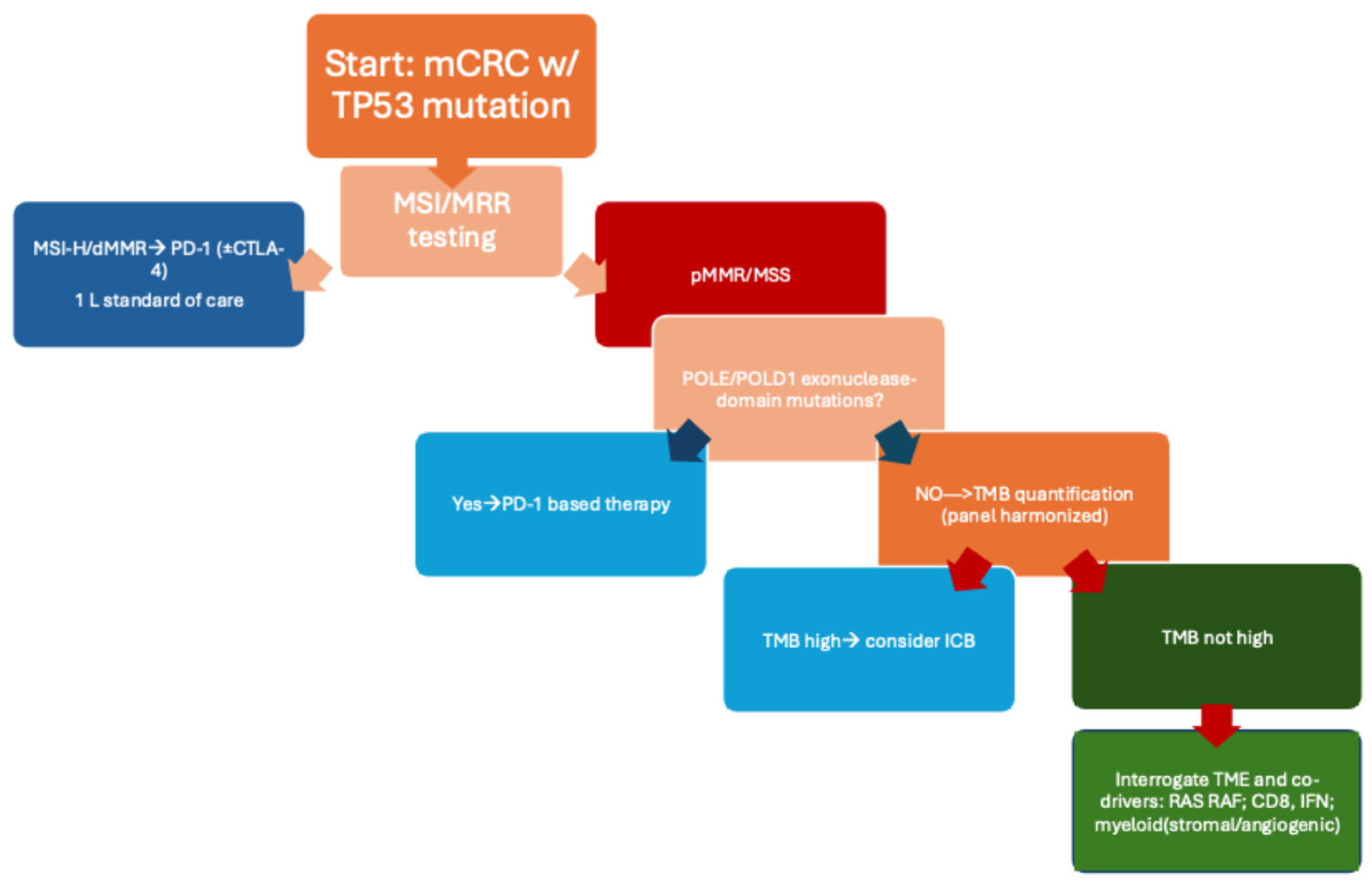
1.3. Centrality of Tumor Mutational Burden (TMB)
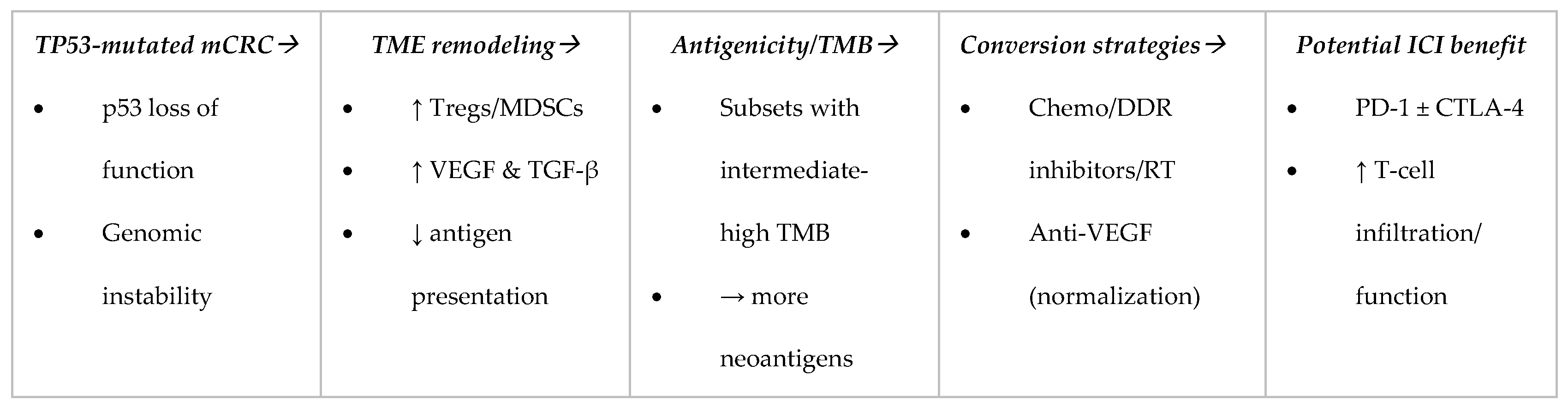
1.4. How TP53 Reshapes the TME
1.5. Signals from Combination Studies
1.6. Where We Are Going and Why It Matters for Patients
1.7. Organotropism and ctDNA
2. Methods
2.1. Design and Quality Framework
2.2. Data Sources and Scope
2.3. Search Strategy and Selection
2.4. Data Extraction and Synthesis
2.5. Bias and Limitations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer. |
| MSI-H | microsatellite instability-high. |
| dMMR | deficient mismatch repair. |
| pMMR | proficient mismatch repair. |
| MSS | microsatellite stable. |
| ICB | immune checkpoint blockade. |
| PD-1 | programmed cell death protein 1. |
| CTLA-4 | cytotoxic T-lymphocyte–associated protein 4. |
| TMB | tumor mutational burden. |
| TME | tumor microenvironment. |
| VEGF | vascular endothelial growth factor. |
| POLE | DNA polymerase epsilon. |
| POLD1 | DNA polymerase delta 1. |
| ctDNA | circulating tumor DNA. |
| CIN | chromosomal instability. |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 11 October 2025).
- Cremolini, C.; Antoniotti, C.; Stein, A.; Bendell, J.; Gruenberger, T.; Rossini, D.; Masi, G.; Ongaro, E.; Hurwitz, H.; Falcone, A.; et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. 2020, 38, 3314–3324. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Muller, P.A.J.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fan, Y. Combined KRAS and TP53 mutation in patients with colorectal cancer enhance chemoresistance and promote postoperative recurrence and metastasis. BMC Cancer 2024, 24, 1155. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; Alcaide-Garcia, J.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy in MSI-H/dMMR metastatic colorectal cancer: 5-year follow-up of KEYNOTE-177. Ann. Oncol. 2025, 36, 277–284. [Google Scholar] [CrossRef]
- André, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus low-dose ipilimumab in previously treated MSI-H/dMMR metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. Version 3. 2025. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 11 October 2025).
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden as a predictive biomarker. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor mutational burden as a predictor of immunotherapy response: Pitfalls and promises. Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Woodhouse, R.; Li, M.; Hughes, J.; Delfosse, D.; Skoletsky, J.; Ma, P.; Meng, W.; Dewal, N.; Milbury, C.; Clark, T.; et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS ONE 2020, 15, e0237802. [Google Scholar] [CrossRef]
- Lanman, R.B.; Mortimer, S.A.; Zill, O.A.; Sebisanovic, D.; Lopez, R.; Blau, S.; Collisson, E.A.; Divers, S.G.; Hoon, D.S.; Kopetz, E.S.; et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS ONE 2015, 10, e0140712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blagih, J.; Buck, M.D.; Vousden, K.H. p53, cancer and the immune response. J Cell Sci. 2020, 133, jcs237453. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Tumor microenvironment and immunotherapy. Nat. Rev. Clin. Oncol. 2018, 15, 150–165. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Lenz, H.J.; Parikh, A.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.; Elez, E.; Shao, S.H.; Deming, D.; Holdridge, R.; et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: Phase 2 results from the CheckMate 9X8 randomized clinical trial. J. Immunother. Cancer 2024, 12, e008409. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, A.; Xu, R.H.; García-Alfonso, P.; Passhak, M.; Teng, H.W.; Shergill, A.; Gumus, M.; Qvortrup, C.; Stintzing, S.; Towns, K.; et al. Lenvatinib plus pembrolizumab versus standard of care for previously treated pMMR/MSS metastatic colorectal cancer: Final analysis of the randomized, open-label, phase III LEAP-017 study. J. Clin. Oncol. 2024, 42, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Curigliano, G.; Biffoni, M.; Lonardi, S.; Scagnoli, S.; Fornaro, L.; Guarneri, V.; De Giorgi, U.; Ascierto, P.A.; Blandino, G.; et al. Genomically matched therapy in advanced solid tumors: The randomized phase 2 ROME trial. Nat. Med. 2025, 31, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K.J.; Carroll, P.; Martin, C.A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A.; et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017, 548, 461–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Lopes, L.; Lee, S.; Riano, I.; Saeed, A. The prognostic and predictive impact of BRAF mutations in deficient mismatch repair/microsatellite instability-high colorectal cancer: Systematic review/meta-analysis. Future Oncol. 2021, 17, 4221–4231. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade–based immunotherapy. Science 2018, 362, 6411. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, J.; Zhou, W.; Zhu, K.; Chen, X. Differential regulation of cellular target genes by p53 devoid of the PXXP motifs with impaired apoptotic activity. Oncogene 1999, 18, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Niu, D.; Lai, L.; Ren, E.C. p53 increases MHC class I expression by upregulating the endoplasmic reticulum aminopeptidase ERAP1. Nat. Commun. 2013, 4, 2359. [Google Scholar] [CrossRef]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2015, 108, djv303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manca, P.; Mazzoli, G.; Miceli, R.; Germani, M.M.; Bergamo, F.; Ambrosini, M.; Cristarella, E.; Cerantola, R.; Boccaccio, C.; Ricagno, G.; et al. Tumour mutational burden as a biomarker in patients with mismatch repair deficient/microsatellite instability-high metastatic colorectal cancer treated with immune checkpoint inhibitors. Eur. J. Cancer 2023, 187, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Kawazoe, A.; Kawazu, M.; Suzuki, T.; Nakamura, Y.; Nakatsura, T.; Kuwata, T.; Ueno, T.; Kuboki, Y.; Kotani, D.; et al. A Low Tumor Mutational Burden and PTEN Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clin. Cancer Res. 2021, 27, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiu, J.; Farrell, A.P.; Battaglin, F.; Arai, H.; Millstein, J.; Soni, S.; Zhang, W.; Shields, A.F.; Grothey, A.; et al. Genomic landscapes to characterize mismatch-repair deficiency (dMMR)/microsatellite instability-high (MSI-H) gastrointestinal (GI) cancers stratified by tumor mutation burden (TMB). J. Clin. Oncol. 2023, 41 (Suppl. 16), 2618. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Huang, Y.; Huang, M.; Li, S.; Zhai, X.; Zhao, J.; Gao, C.; Xie, W.; Qin, H.; et al. A next-generation sequencing-based strategy combining microsatellite instability and tumor mutation burden for comprehensive molecular diagnosis of advanced colorectal cancer. BMC Cancer 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Élez, E.; Mulet-Margalef, N.; Sanso, M.; Ruiz-Pace, F.; Mancuso, F.M.; Comas, R.; Ros, J.; Argilés, G.; Martini, G.; Sanz-Garcia, E.; et al. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int. J. Mol. Sci. 2022, 24, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. DYNAMIC Investigators. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tie, J.; Wang, Y.; Lo, S.N.; Lahouel, K.; Cohen, J.D.; Wong, R.; Shapiro, J.D.; Harris, S.J.; Khattak, A.; Burge, M.E.; et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: 5-year outcomes of the randomized DYNAMIC trial. Nat. Med. 2025, 31, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Greuter, M.J.E.; Schraa, S.J.; Vink, G.R.; Phallen, J.; Velculescu, V.E.; Meijer, G.A.; van den Broek, D.; Koopman, M.; Roodhart, J.M.L.; et al. Early evaluation of the effectiveness and cost-effectiveness of ctDNA-guided selection for adjuvant chemotherapy in stage II colon cancer. Ther. Adv. Med. Oncol. 2024, 16, 17588359241266164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aziz, Z.; Wagner, S.; Agyekum, A.; Pumpalova, Y.S.; Prest, M.; Lim, F.; Rustgi, S.; Kastrinos, F.; Grady, W.M.; Hur, C. Cost-Effectiveness of Liquid Biopsy for Colorectal Cancer Screening in Patients Who Are Unscreened. JAMA Netw. Open 2023, 6, e2343392. [Google Scholar] [CrossRef] [PubMed]
| Trial/Study | Population | MSI/MMR Status | TP53 Info | TMB Range | TME Features | ICI/Treatment Arms | Key Outcomes | Key References |
|---|---|---|---|---|---|---|---|---|
| KEYNOTE-177 | First-line mCRC | MSI-H/dMMR | Not stratified | High TMB (>23 mut/Mb typical) | Inflamed phenotype, high CD8 | Pembrolizumab vs. chemotherapy | Improved PFS & OS; durable responses | [7,27] |
| CheckMate-142 | Pre-treated mCRC | MSI-H/dMMR | Not stratified | High TMB | Inflamed TME | Nivolumab ± Ipilimumab | High ORR (65%), long-term PFS/OS | [8,29] |
| AtezoTRIBE | First-line mCRC | MSS/pMMR (majority) | Not reported | Low–intermediate TMB | Partial TME conversion with priming | FOLFOXIRI+Bev ± Atezolizumab | PFS improvement in unselected MSS subset | [19] |
| CheckMate-9X8 | First-line mCRC | MSS/pMMR | Not reported | Low–intermediate TMB | Non-inflamed MSS TME | mFOLFOX6+Bev ± Nivolumab | No significant PFS benefit | [20] |
| LEAP-017 | Pre-treated mCRC | MSS/pMMR | Not stratified | Low–intermediate TMB | Myeloid-inflamed, ICI-resistant | Pembrolizumab + Lenvatinib | No OS benefit vs. SOC | [21] |
| ROME Trial | Advanced solid tumors (CRC subset) | Mixed MSI & MSS | TP53 via NGS | TMB-high vs. TMB-low | Genomic clusters (not full TME) | Genomically matched therapy incl. ICI | Improved PFS; benefit in TMB-high MSS | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Specchia, M.; Drittone, D.; Mazzotti, E.; Mazzuca, F. Can TP53, TMB and TME Expand the Immunotherapy Benefit in Metastatic Colorectal Cancer? Cancers 2025, 17, 3984. https://doi.org/10.3390/cancers17243984
Specchia M, Drittone D, Mazzotti E, Mazzuca F. Can TP53, TMB and TME Expand the Immunotherapy Benefit in Metastatic Colorectal Cancer? Cancers. 2025; 17(24):3984. https://doi.org/10.3390/cancers17243984
Chicago/Turabian StyleSpecchia, Monia, Denise Drittone, Eva Mazzotti, and Federica Mazzuca. 2025. "Can TP53, TMB and TME Expand the Immunotherapy Benefit in Metastatic Colorectal Cancer?" Cancers 17, no. 24: 3984. https://doi.org/10.3390/cancers17243984
APA StyleSpecchia, M., Drittone, D., Mazzotti, E., & Mazzuca, F. (2025). Can TP53, TMB and TME Expand the Immunotherapy Benefit in Metastatic Colorectal Cancer? Cancers, 17(24), 3984. https://doi.org/10.3390/cancers17243984





