Sex Differences in the Response to Lung Cancer and Its Relation to Programmed Cell Death Protein-1/Programmed Death-Ligand-1 Checkpoint Therapies
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
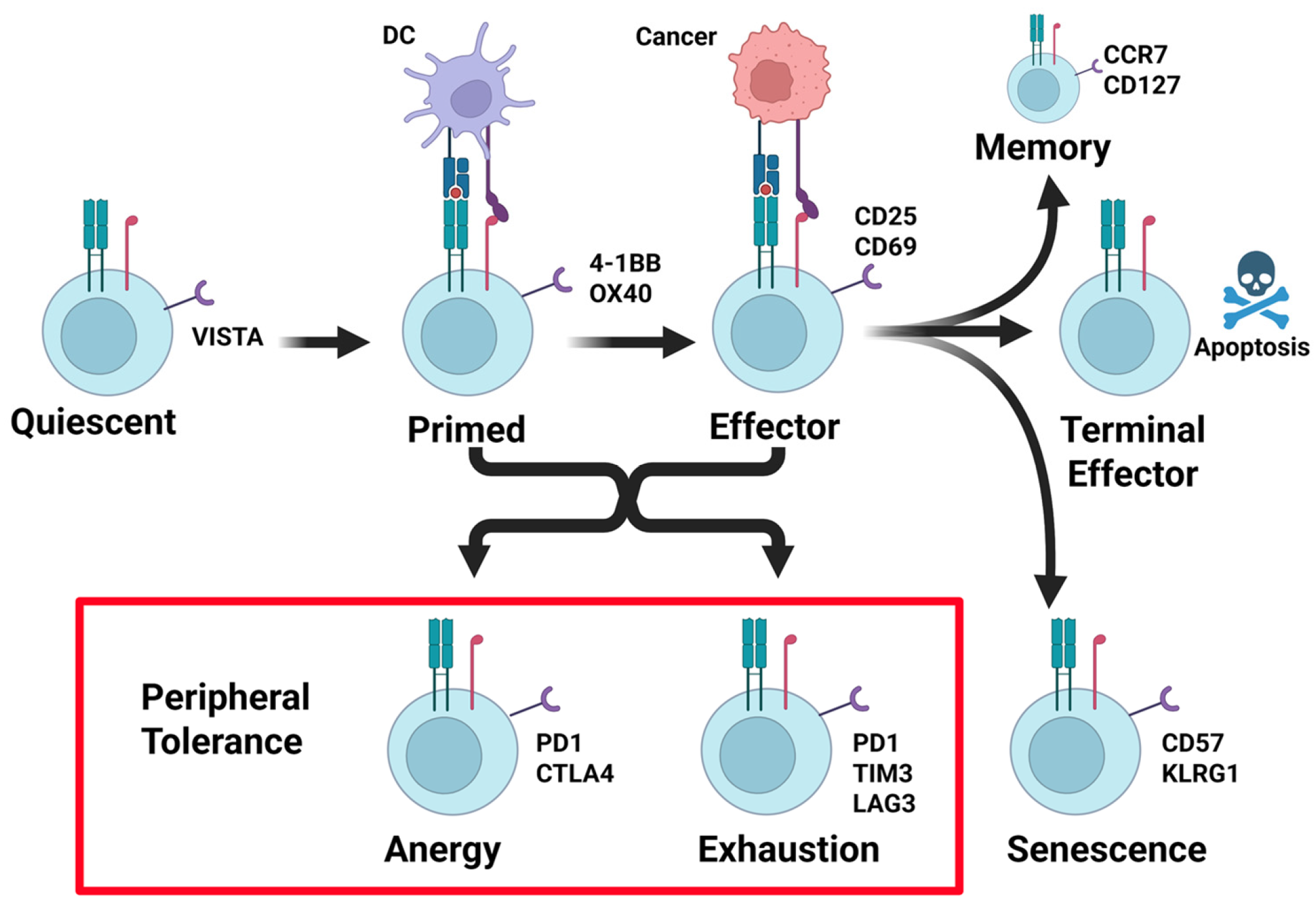
3.1. States of T Cell Activity, Its Relation to PD-1/PD-L1, and the Antitumor Immune Response
3.2. T Cell Activation
3.3. Tumor Microenvironment and T Cell Activity
3.4. PD-1/PD-L1 Pathway
3.5. PD-1/PD-L1 in Lung Cancer
3.6. T-Cell Exhaustion by PD-L1
3.7. Therapeutic Options
3.8. Sex Difference in Treatment Response PD-1/PD-L1 Immunotherapy
3.9. Sex Differences in PD-L1 Expression in Lung Cancer
3.10. General Differences Between Male and Female Hormone Physiology
3.11. Role of Sex Hormones in the Immune System
3.11.1. Estrogen Regulation of the Immune System
3.11.2. Progesterone Regulation of the Immune System
3.11.3. Testosterone Regulation of the Immune System
3.11.4. Follicle-Stimulating Hormone Regulation of the Immune System
3.11.5. Leptin Regulation of the Immune System
3.11.6. Prolactin Regulation of the Immune System
3.11.7. Luteinizing Hormone Regulation of the Immune System
3.11.8. Activin A and Inhibin Regulation of the Immune System
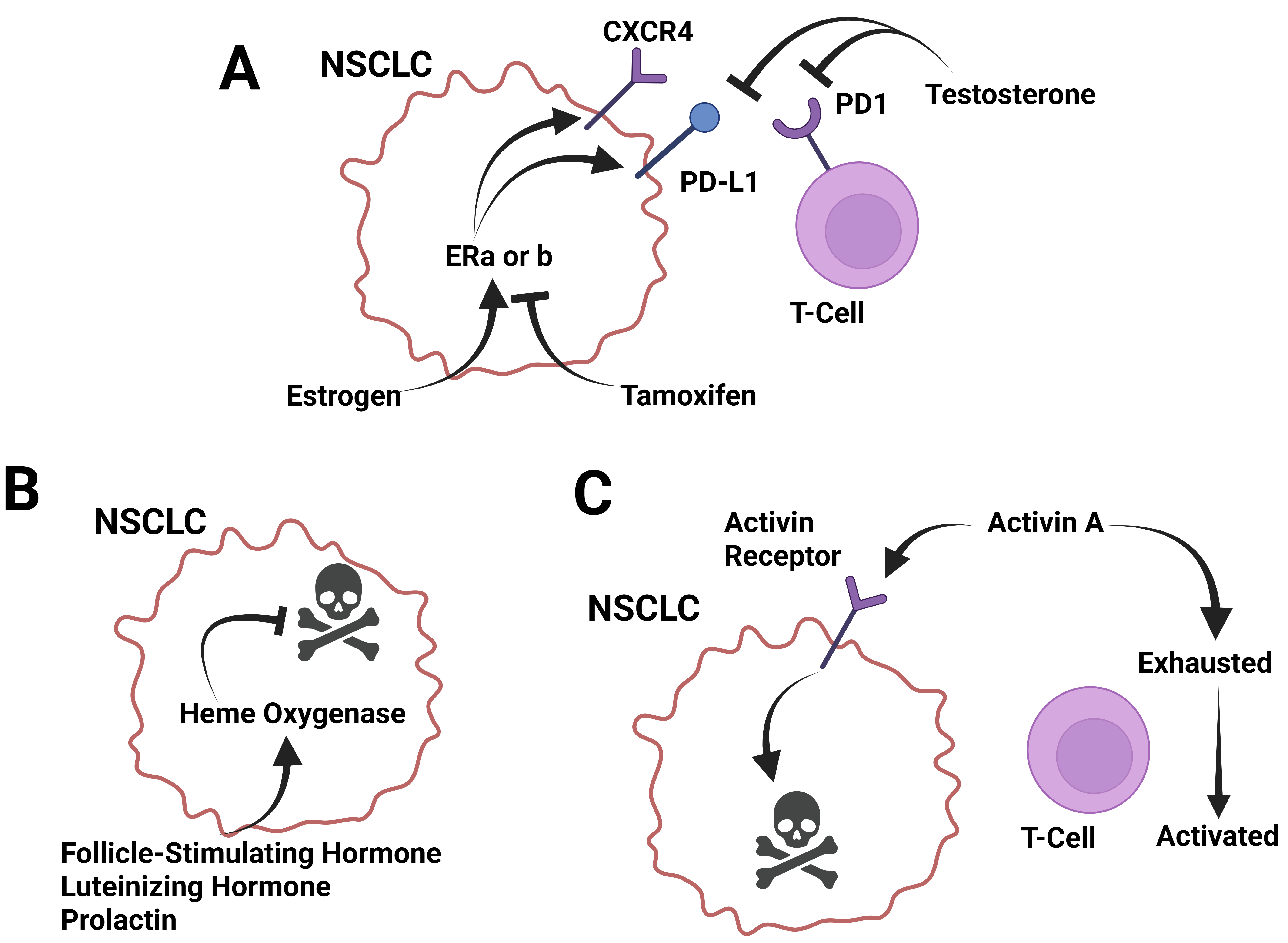
3.12. Hormones in the Immune Response to Lung Cancer
3.12.1. Estrogen and Estradiol in the Response to Lung Cancer
3.12.2. Progesterone in the Response to Lung Cancer
3.12.3. Testosterone in the Response to Lung Cancer
3.12.4. Follicle-Stimulating Hormone and Luteinizing Hormone in the Response to Lung Cancer
3.12.5. Prolactin in the Response to Lung Cancer
3.12.6. Leptin in the Response to Lung Cancer
3.12.7. Activin A and Inhibin in the Response to Lung Cancer
| Molecule | Abundance | Effects on Immune System | Effects on Lung Cancer |
|---|---|---|---|
| Estrogen | Highest in females, with increased abundance during the follicular and leuteal phases, and decreased abundance at menstruation. Low after menopause. |
| |
| Progesterone | Highest in females, with increased abundance during the leuteal phase, and decreased abundance at menstruation. Absent after menopause. |
| |
| Testosterone | Highest in males, with decreasing abundance after 50 years old. |
|
|
| Follicular-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) | Highest in females, with increased abundance during ovulation. High after menopause. |
| |
| Leptin | Generally higher in females. | ||
| Prolactin | Generally higher in females. |
| |
| Activin/Inhibin | Activin—similar between females and males. Inhibin—highest in males and in females in the follicular phase. Low after menopause. |
3.13. Role of Sex Hormones in the Response to PD1/PDL1 Therapies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolarz, B.; Lukasiewicz, H.; Samulak, D.; Piekarska, E.; Kolacinski, R.; Romanowicz, H. Lung Cancer-Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). Int. J. Mol. Sci. 2025, 26, 2049. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Stapelfeld, C.; Dammann, C.; Maser, E. Sex-specificity in lung cancer risk. Int. J. Cancer 2020, 146, 2376–2382. [Google Scholar] [CrossRef]
- Silveyra, P.; Babayev, M.; Ekpruke, C.D. Sex, hormones, and lung health. Physiol. Rev. 2026, 106, 53–86. [Google Scholar] [CrossRef]
- May, L.; Shows, K.; Nana-Sinkam, P.; Li, H.; Landry, J.W. Sex Differences in Lung Cancer. Cancers 2023, 15, 3111. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H.; Else, H.; Warren, M. Cancer immunologists scoop medicine Nobel prize. Nature 2018, 562, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Arafat Hossain, M. A comprehensive review of immune checkpoint inhibitors for cancer treatment. Int. Immunopharmacol. 2024, 143 Pt 2, 113365. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhang, J.B.; Zheng, Y.; Zhang, W.D.; Guo, H.X.; Cong, S.; Ding, Y.; Yuan, B. Comprehensive analysis of differences in N6-methyladenosine RNA methylomes in the rat adenohypophysis after GnRH treatment. FASEB J. 2022, 36, e22204. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, M.; Swalduz, A.; Chouaid, C.; Bylicki, O. Correction to: First-Line Treatment of Advanced Non-Small-Cell Lung Cancer with Immune-Checkpoint Inhibitors: New Combinations and Long-Term Data. BioDrugs 2023, 37, 121. [Google Scholar] [CrossRef]
- Xiao, T.; Lee, J.; Gauntner, T.D.; Velegraki, M.; Lathia, J.D.; Li, Z. Hallmarks of sex bias in immuno-oncology: Mechanisms and therapeutic implications. Nat. Rev. Cancer 2024, 24, 338–355. [Google Scholar] [CrossRef]
- Sprent, J. Central tolerance of T cells. Int. Rev. Immunol. 1995, 13, 95–105. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Noelle, R.J. Rethinking peripheral T cell tolerance: Checkpoints across a T cell’s journey. Nat. Rev. Immunol. 2021, 21, 257–267. [Google Scholar] [CrossRef]
- Corthay, A. A three-cell model for activation of naive T helper cells. Scand. J. Immunol. 2006, 64, 93–96. [Google Scholar]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.H. T cell anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.; Whitehead, T.; Fellermeyer, M.; Davis, S.J.; Sharma, S. The current state and future of T-cell exhaustion research. Oxf. Open Immunol. 2023, 4, iqad006. [Google Scholar] [CrossRef]
- Okazaki, T.; Honjo, T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006, 27, 195–201. [Google Scholar]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology 2016, 5, e1091146. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef]
- Oyer, J.L.; Gitto, S.B.; Altomare, D.A.; Copik, A.J. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology 2018, 7, e1509819. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Marasco, M.; Berteotti, A.; Weyershaeuser, J.; Thorausch, N.; Sikorska, J.; Krausze, J.; Brandt, H.J.; Kirkpatrick, J.; Rios, P.; Schamel, W.W.; et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci. Adv. 2020, 6, eaay4458. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Arasanz, H.; Gato-Canas, M.; Zuazo, M.; Ibanez-Vea, M.; Breckpot, K.; Kochan, G.; Escors, D. PD1 signal transduction pathways in T cells. Oncotarget 2017, 8, 51936–51945. [Google Scholar] [CrossRef]
- Muenst, S.; Soysal, S.D.; Tzankov, A.; Hoeller, S. The PD-1/PD-L1 pathway: Biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin. Ther. Targets 2015, 19, 201–211. [Google Scholar] [CrossRef]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J.; Osinska, I.; Hoser, G. Mechanisms of immune response regulation in lung cancer. Transl. Lung Cancer Res. 2014, 3, 15–22. [Google Scholar]
- Domagala-Kulawik, J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl. Lung Cancer Res. 2015, 4, 177–190. [Google Scholar]
- Ullah, A.; Pulliam, S.; Karki, N.R.; Khan, J.; Jogezai, S.; Sultan, S.; Muhammad, L.; Khan, M.; Jamil, N.; Waheed, A.; et al. PD-L1 Over-Expression Varies in Different Subtypes of Lung Cancer: Will This Affect Future Therapies? Clin. Pract. 2022, 12, 653–671. [Google Scholar] [CrossRef]
- Pawelczyk, K.; Piotrowska, A.; Ciesielska, U.; Jablonska, K.; Gletzel-Plucinska, N.; Grzegrzolka, J.; Podhorska-Okolow, M.; Dziegiel, P.; Nowinska, K. Role of PD-L1 Expression in Non-Small Cell Lung Cancer and Their Prognostic Significance according to Clinicopathological Factors and Diagnostic Markers. Int. J. Mol. Sci. 2019, 20, 824. [Google Scholar] [CrossRef]
- Dhawan, V.; Chandra, S.; Kala, M.; Khanduri, S. Expression of PD-L1 in Lung Carcinoma and Its Correlation with Histopathological Grade, Stage, and Survival of Patients. J. Lab. Physicians 2023, 15, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.; Singh, H.; Larkins, E.; Drezner, N.; Ricciuti, B.; Mishra-Kalyani, P.; Tang, S.; Beaver, J.A.; Awad, M.M. Impact of Increasing PD-L1 Levels on Outcomes to PD-1/PD-L1 Inhibition in Patients with NSCLC: A Pooled Analysis of 11 Prospective Clinical Trials. Oncologist 2024, 29, 422–430. [Google Scholar] [CrossRef]
- Sanchez-Magraner, L.; Gumuzio, J.; Miles, J.; Quimi, N.; Martinez Del Prado, P.; Abad-Villar, M.T.; Pikabea, F.; Ortega, L.; Etxezarraga, C.; Martin-Algarra, S.; et al. Functional Engagement of the PD-1/PD-L1 Complex But Not PD-L1 Expression Is Highly Predictive of Patient Response to Immunotherapy in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Luo, S.; Ye, P.; Hwang, W.L.; Cha, J.H.; Yan, X.; Yang, W.H. T-cell exhaustion and stemness in antitumor immunity: Characteristics, mechanisms, and implications. Front. Immunol. 2023, 14, 1104771. [Google Scholar] [CrossRef]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK cell exhaustion in the tumor microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef]
- Budimir, N.; Thomas, G.D.; Dolina, J.S.; Salek-Ardakani, S. Reversing T-cell Exhaustion in Cancer: Lessons Learned from PD-1/PD-L1 Immune Checkpoint Blockade. Cancer Immunol. Res. 2022, 10, 146–153. [Google Scholar] [CrossRef]
- Catalano, M.; Shabani, S.; Venturini, J.; Ottanelli, C.; Voltolini, L.; Roviello, G. Lung Cancer Immunotherapy: Beyond Common Immune Checkpoints Inhibitors. Cancers 2022, 14, 6145. [Google Scholar] [CrossRef]
- Dang, T.O.; Ogunniyi, A.; Barbee, M.S.; Drilon, A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 13–20. [Google Scholar]
- Silva, V.; Matos, C. Recent updates in the therapeutic uses of Pembrolizumab: A brief narrative review. Clin. Transl. Oncol. 2024, 26, 2431–2443. [Google Scholar] [CrossRef]
- Palumbo, G.; Carillio, G.; Manzo, A.; Montanino, A.; Sforza, V.; Costanzo, R.; Sandomenico, C.; Manna, C.; Luca, G.; Piccirillo, M.C.; et al. Pembrolizumab in lung cancer: Current evidence and future perspectives. Future Oncol. 2019, 15, 3327–3336. [Google Scholar] [CrossRef]
- Mager, L.; Gardeen, S.; Carr, D.R.; Shahwan, K.T. Cemiplimab for the Treatment of Advanced Cutaneous Squamous Cell Carcinoma: Appropriate Patient Selection and Perspectives. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Tanvetyanon, T.; Creelan, B.C.; Antonia, S.J. The safety and efficacy of nivolumab in advanced (metastatic) non-small cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 903–910. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes with Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar]
- Rajan, A.; Kim, C.; Heery, C.R.; Guha, U.; Gulley, J.L. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum. Vaccines Immunother. 2016, 12, 2219–2231. [Google Scholar] [CrossRef]
- Ozguroglu, M.; Kilickap, S.; Sezer, A.; Gumus, M.; Bondarenko, I.; Gogishvili, M.; Nechaeva, M.; Schenker, M.; Cicin, I.; Ho, G.F.; et al. First-line cemiplimab monotherapy and continued cemiplimab beyond progression plus chemotherapy for advanced non-small-cell lung cancer with PD-L1 50% or more (EMPOWER-Lung 1): 35-month follow-up from a mutlicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gumus, M.; Bondarenko, I.; Ozguroglu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial. Nat. Med. 2022, 28, 2374–2380. [Google Scholar]
- Makharadze, T.; Quek, R.G.W.; Melkadze, T.; Gogishvili, M.; Ivanescu, C.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; et al. Quality of life with cemiplimab plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: Patient-reported outcomes from phase 3 EMPOWER-Lung 3. Cancer 2023, 129, 2256–2265. [Google Scholar]
- Rajasekaran, N.; Wang, X.; Ravindranathan, S.; Chin, D.J.; Tseng, S.Y.; Klakamp, S.L.; Widmann, K.; Kapoor, V.N.; Vexler, V.; Keegan, P.; et al. Toripalimab, a therapeutic monoclonal anti-PD-1 antibody with high binding affinity to PD-1 and enhanced potency to activate human T cells. Cancer Immunol. Immunother. 2024, 73, 60. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Jimeno, A. Atezolizumab: A novel PD-L1 inhibitor in cancer therapy with a focus in bladder and non-small cell lung cancers. Drugs Today 2017, 53, 217–237. [Google Scholar]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csoszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Durvalumab: A Review in Extensive-Stage SCLC. Target. Oncol. 2021, 16, 857–864. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [PubMed]
- Lee, C.K.; Man, J.; Lord, S.; Cooper, W.; Links, M.; Gebski, V.; Herbst, R.S.; Gralla, R.J.; Mok, T.; Yang, J.C. Clinical and Molecular Characteristics Associated with Survival Among Patients Treated with Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 210–216. [Google Scholar]
- Wu, B.; Sun, C.; Sun, X.; Li, X. The effect of gender on the clinical outcome of PD-1/PD-L1 inhibitor in advanced lung cancer patients. Medicine 2023, 102, e34849. [Google Scholar]
- Wang, C.; Qiao, W.; Jiang, Y.; Zhu, M.; Shao, J.; Ren, P.; Liu, D.; Li, W. Effect of sex on the efficacy of patients receiving immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer Med. 2019, 8, 4023–4031. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Corti, C.; Bagnardi, V.; Queirolo, P.; Catania, C.; De Pas, T.; Giaccone, G. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100251. [Google Scholar] [CrossRef]
- Yu, X.; You, Z.; Liu, Y.; Fang, J.; Zhao, Q.; Sun, Z.; Song, Y.; Liu, J.; Sun, C. Sex-based immune microenvironmental feature heterogeneity in response to PD-1 blockade in combination with chemotherapy for patients with untreated advanced non-small-cell lung cancer. Cancer Med. 2024, 13, e7423. [Google Scholar] [CrossRef]
- Sarova, P.; Mosleh, B.; Zehetmayer, S.; Oberndorfer, F.; Widder, J.; Prosch, H.; Aigner, C.; Idzko, M.; Hoda, M.A.; Gompelmann, D. PD-L1 expression in patients with non-small-cell lung cancer is associated with sex and genetic alterations: A retrospective study within the Caucasian population. Thorac. Cancer 2024, 15, 1598–1606. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, S.E.; Kim, C.H.; Lee, Y.J. Revisiting the impact of clinicopathologic characteristics in PD-L1 profile in a large cohort of non-small cell lung cancer. Transl. Lung Cancer Res. 2024, 13, 475–490. [Google Scholar] [CrossRef]
- Li, J.; Ge, S.; Sang, S.; Hu, C.; Deng, S. Evaluation of PD-L1 Expression Level in Patients with Non-Small Cell Lung Cancer by (18)F-FDG PET/CT Radiomics and Clinicopathological Characteristics. Front. Oncol. 2021, 11, 789014. [Google Scholar] [CrossRef]
- Fu, F.; Deng, C.; Sun, W.; Zheng, Q.; Jin, Y.; Li, Y.; Zhang, Y.; Chen, H. Distribution and concordance of PD-L1 expression by routine 22C3 assays in East-Asian patients with non-small cell lung cancer. Respir. Res. 2022, 23, 302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, A.; Li, X.; Wang, S.; Fang, S.; Mo, Y. A retrospective analysis of eleven gene mutations, PD-L1 expression and clinicopathological characteristics in non-small cell lung cancer patients. Asian J. Surg. 2022, 45, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Fan, X.; Zhu, W.; Huang, C.; Zhuang, W.; Xu, H.; Lin, X.; Hu, D.; Huang, Y.; Jiang, K.; et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017, 8, 83986–83994. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Tang, Y.Y.; Wan, J.X.; Zou, J.Y.; Lu, C.G.; Zhu, H.S.; Sheng, S.Y.; Wang, Y.F.; Liu, H.C.; Yang, J.; et al. Sex difference in the expression of PD-1 of non-small cell lung cancer. Front. Immunol. 2022, 13, 1026214. [Google Scholar] [CrossRef]
- Bain, J. The many faces of testosterone. Clin. Interv. Aging 2007, 2, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Gilmore, A.; Roudebush, W.E. Clinical Utilities of Anti-Mullerian Hormone. J. Clin. Med. 2022, 11, 7209. [Google Scholar] [CrossRef] [PubMed]
- Lauretta, R.; Sansone, M.; Sansone, A.; Romanelli, F.; Appetecchia, M. Gender in Endocrine Diseases: Role of Sex Gonadal Hormones. Int. J. Endocrinol. 2018, 2018, 4847376. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Szekeres-Bartho, J.; Kovacs, G.L.; Sulyok, E.; Farkas, B.; Varnagy, A.; Vertes, V.; Kovacs, K.; Bodis, J. Key to Life: Physiological Role and Clinical Implications of Progesterone. Int. J. Mol. Sci. 2021, 22, 11039. [Google Scholar] [CrossRef]
- Catenaccio, E.; Mu, W.; Lipton, M.L. Estrogen- and progesterone-mediated structural neuroplasticity in women: Evidence from neuroimaging. Brain Struct. Funct. 2016, 221, 3845–3867. [Google Scholar] [CrossRef]
- Laven, J.S.; Fauser, B.C. What role of estrogens in ovarian stimulation. Maturitas 2006, 54, 356–362. [Google Scholar] [CrossRef]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. Revisiting the role of testosterone: Are we missing something? Rev. Urol. 2017, 19, 16–24. [Google Scholar]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 77 (Suppl. 4), S3–S5. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Binart, N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef]
- Sawin, C.T.; Carlson, H.E.; Geller, A.; Castelli, W.P.; Bacharach, P. Serum prolactin and aging: Basal values and changes with estrogen use and hypothyroidism. J. Gerontol. 1989, 44, M131–M135. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, A.; Luisi, S.; Reis, F.M.; Petraglia, F. Inhibins as diagnostic markers in human reproduction. Adv. Clin. Chem. 2008, 45, 1–29. [Google Scholar] [PubMed]
- Morianos, I.; Papadopoulou, G.; Semitekolou, M.; Xanthou, G. Activin-A in the regulation of immunity in health and disease. J. Autoimmun. 2019, 104, 102314. [Google Scholar] [CrossRef] [PubMed]
- Funaba, M.; Murata, T.; Fujimura, H.; Murata, E.; Abe, M.; Takahashi, M.; Torii, K. Unique recognition of activin and inhibin by polyclonal antibodies to inhibin subunits. J. Biochem. 1996, 119, 953–960. [Google Scholar] [CrossRef]
- Luisi, S.; Florio, P.; Reis, F.M.; Petraglia, F. Inhibins in female and male reproductive physiology: Role in gametogenesis, conception, implantation and early pregnancy. Hum. Reprod. Update 2005, 11, 123–135. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Robertson, D.M. The isolation and physiology of inhibin and related proteins. Biol. Reprod. 1989, 40, 33–47. [Google Scholar] [CrossRef]
- Elsholz, D.D.; Padmanabhan, V.; Rosenfield, R.L.; Olton, P.R.; Phillips, D.J.; Foster, C.M. GnRH agonist stimulation of the pituitary-gonadal axis in children: Age and sex differences in circulating inhibin-B and activin-A. Hum. Reprod. 2004, 19, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.M.; Olton, P.R.; Racine, M.S.; Phillips, D.J.; Padmanabhan, V. Sex differences in FSH-regulatory peptides in pubertal age boys and girls and effects of sex steroid treatment. Hum. Reprod. 2004, 19, 1668–1676. [Google Scholar] [CrossRef]
- Nicks, K.M.; Perrien, D.S.; Akel, N.S.; Suva, L.J.; Gaddy, D. Regulation of osteoblastogenesis and osteoclastogenesis by the other reproductive hormones, Activin and Inhibin. Mol. Cell. Endocrinol. 2009, 310, 11–20. [Google Scholar] [CrossRef]
- Zheng, W.; Sung, C.J.; Hanna, I.; DePetris, G.; Lambert-Messerlian, G.; Steinhoff, M.; Lauchlan, S.C. Alpha and beta subunits of inhibin/activin as sex cord-stromal differentiation markers. Int. J. Gynecol. Pathol. 1997, 16, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shikone, T.; Matzuk, M.M.; Perlas, E.; Finegold, M.J.; Lewis, K.A.; Vale, W.; Bradley, A.; Hsueh, A.J. Characterization of gonadal sex cord-stromal tumor cell lines from inhibin-alpha and p53-deficient mice: The role of activin as an autocrine growth factor. Mol. Endocrinol. 1994, 8, 983–995. [Google Scholar] [PubMed]
- Tribulo, P.; Jumatayeva, G.; Lehloenya, K.; Moss, J.I.; Negron-Perez, V.M.; Hansen, P.J. Effects of sex on response of the bovine preimplantation embryo to insulin-like growth factor 1, activin A, and WNT7A. BMC Dev. Biol. 2018, 18, 16. [Google Scholar] [CrossRef]
- Nakaya, M.; Tachibana, H.; Yamada, K. Effect of estrogens on the interferon-gamma producing cell population of mouse splenocytes. Biosci. Biotechnol. Biochem. 2006, 70, 47–53. [Google Scholar] [CrossRef]
- Hao, S.; Zhao, J.; Zhou, J.; Zhao, S.; Hu, Y.; Hou, Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int. Immunopharmacol. 2007, 7, 1765–1775. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005, 97, 107–113. [Google Scholar] [CrossRef]
- Dunn, S.E.; Ousman, S.S.; Sobel, R.A.; Zuniga, L.; Baranzini, S.E.; Youssef, S.; Crowell, A.; Loh, J.; Oksenberg, J.; Steinman, L. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J. Exp. Med. 2007, 204, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Wang, J.; Jin, H.; Song, X.; Yan, J.; Kang, Y.; Zhao, L.; An, X.; Du, X.; Chen, X.; et al. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2008, 214, 456–464. [Google Scholar] [CrossRef]
- Hewagama, A.; Patel, D.; Yarlagadda, S.; Strickland, F.M.; Richardson, B.C. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009, 10, 509–516. [Google Scholar] [CrossRef]
- Bengtsson, A.K.; Ryan, E.J.; Giordano, D.; Magaletti, D.M.; Clark, E.A. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 2004, 104, 1404–1410. [Google Scholar]
- Hannah, M.F.; Bajic, V.B.; Klein, S.L. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav. Immun. 2008, 22, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Care, A.; Bellenghi, M.; Matarrese, P.; Gabriele, L.; Salvioli, S.; Malorni, W. Sex disparity in cancer: Roles of microRNAs and related functional players. Cell Death Differ. 2018, 25, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Abramenko, N.; Vellieux, F.; Vesela, K.; Kejik, Z.; Hajduch, J.; Masarik, M.; Babula, P.; Hoskovec, D.; Pacak, K.; Martasek, P.; et al. Investigation of the potential effects of estrogen receptor modulators on immune checkpoint molecules. Sci. Rep. 2024, 14, 3043. [Google Scholar] [CrossRef]
- Butts, C.L.; Shukair, S.A.; Duncan, K.M.; Bowers, E.; Horn, C.; Belyavskaya, E.; Tonelli, L.; Sternberg, E.M. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int. Immunol. 2007, 19, 287–296. [Google Scholar] [CrossRef]
- Arruvito, L.; Giulianelli, S.; Flores, A.C.; Paladino, N.; Barboza, M.; Lanari, C.; Fainboim, L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008, 180, 5746–5753. [Google Scholar] [CrossRef]
- Menzies, F.M.; Henriquez, F.L.; Alexander, J.; Roberts, C.W. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology 2011, 134, 281–291. [Google Scholar] [CrossRef]
- Su, S.; Hua, D.; Li, J.P.; Zhang, X.N.; Bai, L.; Cao, L.B.; Guo, Y.; Zhang, M.; Dong, J.Z.; Liang, X.W.; et al. Modulation of innate immune response to viruses including SARS-CoV-2 by progesterone. Signal Transduct. Target. Ther. 2022, 7, 137. [Google Scholar]
- Hierweger, A.M.; Engler, J.B.; Friese, M.A.; Reichardt, H.M.; Lydon, J.; DeMayo, F.; Mittrucker, H.W.; Arck, P.C. Progesterone modulates the T-cell response via glucocorticoid receptor-dependent pathways. Am. J. Reprod. Immunol. 2019, 81, e13084. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, W.F. Effect of sex hormones on NK and ADCC activity of mice. Int. J. Immunopharmacol. 1988, 10, 15–22. [Google Scholar]
- D’Agostino, P.; Milano, S.; Barbera, C.; Di Bella, G.; La Rosa, M.; Ferlazzo, V.; Farruggio, R.; Miceli, D.M.; Miele, M.; Castagnetta, L.; et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann. N. Y. Acad. Sci. 1999, 876, 426–429. [Google Scholar] [CrossRef]
- Kanda, N.; Tsuchida, T.; Tamaki, K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin. Exp. Immunol. 1996, 106, 410–415. [Google Scholar] [CrossRef]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet-Hudson, Y.M.; Marriott, I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008, 78, 432–437. [Google Scholar] [CrossRef]
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004, 173, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanth, T.; Consiglio, C.; Sardh, F.; Forlin, R.; Wang, J.; Tan, Z.; Barcenilla, H.; Rodriguez, L.; Sugrue, J.; Noori, P.; et al. Immune system adaptation during gender-affirming testosterone treatment. Nature 2024, 633, 155–164. [Google Scholar] [CrossRef]
- Walecki, M.; Eisel, F.; Klug, J.; Baal, N.; Paradowska-Dogan, A.; Wahle, E.; Hackstein, H.; Meinhardt, A.; Fijak, M. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol. Biol. Cell 2015, 26, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Damm, L.J.; Wenzel, J.P.; Aslani, F.; Walecki, M.; Wahle, E.; Eisel, F.; Bhushan, S.; Hackstein, H.; Baal, N.; et al. Influence of Testosterone on Inflammatory Response in Testicular Cells and Expression of Transcription Factor Foxp3 in T Cells. Am. J. Reprod. Immunol. 2015, 74, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Grunhagel, B.; Borggrewe, M.; Hagen, S.H.; Ziegler, S.M.; Henseling, F.; Glau, L.; Thiele, R.J.; Pujantell, M.; Sivayoganathan, V.; Padoan, B.; et al. Reduction of IFN-I responses by plasmacytoid dendritic cells in a longitudinal trans men cohort. iScience 2023, 26, 108209. [Google Scholar] [CrossRef]
- Corrales, J.J.; Almeida, M.; Cordero, M.; Martin-Martin, L.; Mendez, C.; Miralles, J.M.; Orfao, A. Enhanced immunological response by dendritic cells in male hypogonadism. Eur. J. Clin. Investig. 2012, 42, 1205–1212. [Google Scholar] [CrossRef]
- Iqbal, J.; Sun, L.; Kumar, T.R.; Blair, H.C.; Zaidi, M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc. Natl. Acad. Sci. USA 2006, 103, 14925–14930. [Google Scholar] [CrossRef]
- Spaziani, M.; Carlomagno, F.; Tenuta, M.; Sesti, F.; Angelini, F.; Bonaventura, I.; Ferrari, D.; Tarantino, C.; Fiore, M.; Petrella, C.; et al. Extra-Gonadal and Non-Canonical Effects of FSH in Males. Pharmaceuticals 2023, 16, 813. [Google Scholar] [CrossRef] [PubMed]
- Belenska-Todorova, L.; Zhivkova, R.; Markova, M.; Ivanovska, N. Follicle stimulating hormone and estradiol alter immune response in osteoarthritic mice in an opposite manner. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211016198. [Google Scholar] [CrossRef] [PubMed]
- He, Y.B.; Zhang, L.; Zhou, L.L.; Chen, Y.M.; Lu, J.H.; Chen, J.; Liu, Y.L. Effect of human follicle-stimulating hormone on immunomodulatory function of decidual mesenchymal stem cells by reducing interleukin-6 levels. J. Ovarian Res. 2022, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Fernandez-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martin-Romero, C.; Perez-Perez, A.; Gonzalez-Yanes, C.; Sanchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef]
- Mattioli, B.; Straface, E.; Quaranta, M.G.; Giordani, L.; Viora, M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 2005, 174, 6820–6828. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, R.; You, L.; Gao, C.; Tian, Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem. Biophys. Res. Commun. 2003, 300, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Legorreta-Haquet, M.V.; Santana-Sanchez, P.; Chavez-Sanchez, L.; Chavez-Rueda, A.K. The effect of prolactin on immune cell subsets involved in SLE pathogenesis. Front. Immunol. 2022, 13, 1016427. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and Autoimmunity. Front. Immunol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yu, H.; Hao, X.; Dong, W.; Yin, X.; Lin, M.; Zheng, J.; Zhou, B.O. Luteinizing hormone signaling restricts hematopoietic stem cell expansion during puberty. EMBO J. 2018, 37, e98984. [Google Scholar] [CrossRef]
- Schumacher, A.; Poloski, E.; Sporke, D.; Zenclussen, A.C. Luteinizing hormone contributes to fetal tolerance by regulating adaptive immune responses. Am. J. Reprod. Immunol. 2014, 71, 434–440. [Google Scholar] [CrossRef]
- Phillips, D.J.; Jones, K.L.; Scheerlinck, J.Y.; Hedger, M.P.; de Kretser, D.M. Evidence for activin A and follistatin involvement in the systemic inflammatory response. Mol. Cell. Endocrinol. 2001, 180, 155–162. [Google Scholar] [CrossRef]
- Petraglia, F.; Sacerdote, P.; Cossarizza, A.; Angioni, S.; Genazzani, A.D.; Franceschi, C.; Muscettola, M.; Grasso, G. Inhibin and activin modulate human monocyte chemotaxis and human lymphocyte interferon-gamma production. J. Clin. Endocrinol. Metab. 1991, 72, 496–502. [Google Scholar] [CrossRef]
- Segerer, S.E.; Muller, N.; Brandt, J.; Kapp, M.; Dietl, J.; Reichardt, H.M.; Rieger, L.; Kammerer, U. The glycoprotein-hormones activin A and inhibin A interfere with dendritic cell maturation. Reprod. Biol. Endocrinol. 2008, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Winnall, W.R. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol. Cell. Endocrinol. 2012, 359, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Indumathy, S.; Pueschl, D.; Klein, B.; Fietz, D.; Bergmann, M.; Schuppe, H.C.; Da Silva, N.; Loveland, B.E.; Hickey, M.J.; Hedger, M.P.; et al. Testicular immune cell populations and macrophage polarisation in adult male mice and the influence of altered activin A levels. J. Reprod. Immunol. 2020, 142, 103204. [Google Scholar] [CrossRef] [PubMed]
- Pinjusic, K.; Dubey, O.A.; Egorova, O.; Nassiri, S.; Meylan, E.; Faget, J.; Constam, D.B. Activin-A impairs CD8 T cell-mediated immunity and immune checkpoint therapy response in melanoma. J. Immunother. Cancer 2022, 10, e004533. [Google Scholar] [CrossRef]
- Niikawa, H.; Suzuki, T.; Miki, Y.; Suzuki, S.; Nagasaki, S.; Akahira, J.; Honma, S.; Evans, D.B.; Hayashi, S.; Kondo, T.; et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin. Cancer Res. 2008, 14, 4417–4426. [Google Scholar]
- Marquez-Garban, D.C.; Chen, H.W.; Goodglick, L.; Fishbein, M.C.; Pietras, R.J. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 194–205. [Google Scholar] [CrossRef]
- Singhal, N.; Vatandoust, S.; Brown, M.P. Phase II study evaluating efficacy and safety of everolimus with letrozole for management of advanced (unresectable or metastatic) non-small cell lung cancer after failure of platinum-based treatment: A preliminary analysis of toxicity. Cancer Chemother. Pharmacol. 2015, 75, 325–331. [Google Scholar] [CrossRef]
- Young, P.A.; Marquez-Garban, D.C.; Noor, Z.S.; Moatamed, N.; Elashoff, D.; Grogan, T.; Romero, T.; Sasano, H.; Saito, R.; Rausch, R.; et al. Investigation of Combination Treatment with an Aromatase Inhibitor Exemestane and Carboplatin-Based Therapy for Postmenopausal Women with Advanced NSCLC. JTO Clin. Res. Rep. 2021, 2, 100150. [Google Scholar] [CrossRef]
- Stabile, L.P.; Davis, A.L.; Gubish, C.T.; Hopkins, T.M.; Luketich, J.D.; Christie, N.; Finkelstein, S.; Siegfried, J.M. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002, 62, 2141–2150. [Google Scholar]
- Mollerup, S.; Jorgensen, K.; Berge, G.; Haugen, A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer 2002, 37, 153–159. [Google Scholar] [CrossRef]
- Rodriguez-Lara, V.; Ignacio, G.S.; Cerbon Cervantes, M.A. Estrogen induces CXCR4 overexpression and CXCR4/CXL12 pathway activation in lung adenocarcinoma cells in vitro. Endocr. Res. 2017, 42, 219–231. [Google Scholar] [PubMed]
- Liu, S.; Hu, C.; Li, M.; An, J.; Zhou, W.; Guo, J.; Xiao, Y. Estrogen receptor beta promotes lung cancer invasion via increasing CXCR4 expression. Cell Death Dis. 2022, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Anker, J.F.; Chae, Y.K. High gene expression of estrogen and progesterone receptors is associated with decreased t cell infiltration in patients with NSCLC. Cancer Treat. Res. Commun. 2021, 27, 100317. [Google Scholar] [CrossRef]
- He, M.; Yu, W.; Chang, C.; Miyamoto, H.; Liu, X.; Jiang, K.; Yeh, S. Estrogen receptor alpha promotes lung cancer cell invasion via increase of and cross-talk with infiltrated macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling pathways. Mol. Oncol. 2020, 14, 1779–1799. [Google Scholar] [PubMed]
- Rodriguez-Lara, V.; Pena-Mirabal, E.; Baez-Saldana, R.; Esparza-Silva, A.L.; Garcia-Zepeda, E.; Cerbon Cervantes, M.A.; Diaz, D.; Fortoul, T.I. Estrogen receptor beta and CXCR4/CXCL12 expression: Differences by sex and hormonal status in lung adenocarcinoma. Arch. Med. Res. 2014, 45, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Young, M.J.; Chang, H.P.; Liu, C.Y.; Lee, C.C.; Tseng, Y.L.; Wang, Y.C.; Chang, W.C.; Hung, J.J. Estradiol-mediated inhibition of DNMT1 decreases p53 expression to induce M2-macrophage polarization in lung cancer progression. Oncogenesis 2022, 11, 25. [Google Scholar]
- Kadzhoian, A.V.; Shevchenko, A.I. Effect of high dose tamoxifen therapy in conservative treatment of patients with III-IV stages of non-small cell lung cancer. Likars’ Ka Sprav 2014, 1–2, 103–110. [Google Scholar]
- Wen, S.; Fu, X.; Li, G.; He, L.; Zhao, C.; Hu, X.; Pan, R.; Guo, C.; Zhang, X.; Hu, X. Efficacy of tamoxifen in combination with docetaxel in patients with advanced non-small-cell lung cancer pretreated with platinum-based chemotherapy. Anticancer Drugs 2016, 27, 447–456. [Google Scholar] [CrossRef]
- Bertolaccini, L.; Pardolesi, A.; Forti Parri, S.N.; Bonfanti, B.; Brandolini, J.; Solli, P. Surgical approaches in patients with oligometastatic non-small cell lung cancer. J. Thorac. Dis. 2018, 10, 498–502. [Google Scholar] [CrossRef]
- Deng, S.; Ramos-Castaneda, M.; Velasco, W.V.; Clowers, M.J.; Gutierrez, B.A.; Noble, O.; Dong, Y.; Zarghooni, M.; Alvarado, L.; Caetano, M.S.; et al. Interplay between estrogen and Stat3/NF-kappaB-driven immunomodulation in lung cancer. Carcinogenesis 2020, 41, 1529–1542. [Google Scholar]
- Huo, N.; Smith, C.Y.; Gazzuola Rocca, L.; Rocca, W.A.; Mielke, M.M. Risk of de novo cancer after premenopausal bilateral oophorectomy. Am. J. Obstet. Gynecol. 2022, 226, 539.e1–539.e16. [Google Scholar] [CrossRef]
- Caetano, M.S.; Hassane, M.; Van, H.T.; Bugarin, E.; Cumpian, A.M.; McDowell, C.L.; Cavazos, C.G.; Zhang, H.; Deng, S.; Diao, L.; et al. Sex specific function of epithelial STAT3 signaling in pathogenesis of K-ras mutant lung cancer. Nat. Commun. 2018, 9, 4589. [Google Scholar] [CrossRef]
- Anobile, D.P.; Salaroglio, I.C.; Tabbo, F.; La Vecchia, S.; Akman, M.; Napoli, F.; Bungaro, M.; Benso, F.; Aldieri, E.; Bironzo, P.; et al. Autocrine 17-beta-Estradiol/Estrogen Receptor-alpha Loop Determines the Response to Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 3958–3973. [Google Scholar] [CrossRef]
- Song, S.; Tang, H.; Quan, W.; Shang, A.; Ling, C. Estradiol initiates the immune escape of non-small cell lung cancer cells via ERbeta/SIRT1/FOXO3a/PD-L1 axis. Int. Immunopharmacol. 2022, 107, 108629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, X.; Lu, X.; Xie, M.; He, B.; He, S.; You, S.; Chen, Q. Progesterone/Org inhibits lung adenocarcinoma cell growth via membrane progesterone receptor alpha. Thorac. Cancer 2020, 11, 2209–2223. [Google Scholar] [CrossRef]
- Skjefstad, K.; Richardsen, E.; Donnem, T.; Andersen, S.; Kiselev, Y.; Grindstad, T.; Hald, S.M.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T.; et al. The prognostic role of progesterone receptor expression in non-small cell lung cancer patients: Gender-related impacts and correlation with disease-specific survival. Steroids 2015, 98, 29–36. [Google Scholar] [PubMed]
- Nazha, B.; Zhang, C.; Chen, Z.; Ragin, C.; Owonikoko, T.K. Concurrent Androgen Deprivation Therapy for Prostate Cancer Improves Survival for Synchronous or Metachronous Non-Small Cell Lung Cancer: A SEER-Medicare Database Analysis. Cancers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset-Ismail, A.; Pedziwiatr, D.; Schneider, G.; Niklinski, J.; Charkiewicz, R.; Moniuszko, M.; Kucia, M.; Ratajczak, M.Z. Pituitary sex hormones enhance the pro-metastatic potential of human lung cancer cells by downregulating the intracellular expression of heme oxygenase-1. Int. J. Oncol. 2017, 50, 317–328. [Google Scholar] [CrossRef]
- Le Bescont, A.; Vitte, A.L.; Debernardi, A.; Curtet, S.; Buchou, T.; Vayr, J.; de Reynies, A.; Ito, A.; Guardiola, P.; Brambilla, C.; et al. Receptor-Independent Ectopic Activity of Prolactin Predicts Aggressive Lung Tumors and Indicates HDACi-Based Therapeutic Strategies. Antioxid. Redox Signal. 2015, 23, 1–14. [Google Scholar] [CrossRef]
- Caponnetto, S.; Iannantuono, G.M.; Barchiesi, G.; Magri, V.; Gelibter, A.; Cortesi, E. Prolactin as a Potential Early Predictive Factor in Metastatic Non-Small Cell Lung Cancer Patients Treated with Nivolumab. Oncology 2017, 93, 62–66. [Google Scholar] [CrossRef]
- Frak, M.; Grenda, A.; Krawczyk, P.; Kuznar-Kaminska, B.; Pazdrowski, P.; Kedra, K.; Chmielewska, I.; Milanowski, J. The influence of nutritional status, lipid profile, leptin concentration and polymorphism of genes encoding leptin and neuropeptide Y on the effectiveness of immunotherapy in advanced NSCLC patients. BMC Cancer 2024, 24, 937. [Google Scholar] [CrossRef]
- Li, F.; Zhao, S.; Guo, T.; Li, J.; Gu, C. The Nutritional Cytokine Leptin Promotes NSCLC by Activating the PI3K/AKT and MAPK/ERK Pathways in NSCLC Cells in a Paracrine Manner. BioMed Res. Int. 2019, 2019, 2585743. [Google Scholar]
- Du, J.; Han, J.C.; Zhang, Y.J.; Qi, G.B.; Zhang, Y.; Li, H.B. Relationship between serum leptin levels and non-small cell lung carcinoma: A meta-analysis. Genet. Mol. Res. 2015, 14, 13699–13708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar]
- Barany, N.; Rozsas, A.; Megyesfalvi, Z.; Grusch, M.; Hegedus, B.; Lang, C.; Boettiger, K.; Schwendenwein, A.; Tisza, A.; Renyi-Vamos, F.; et al. Clinical relevance of circulating activin A and follistatin in small cell lung cancer. Lung Cancer 2021, 161, 128–135. [Google Scholar] [CrossRef]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Circulating Activin A predicts survival in cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 768–777. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kataoka, Y.; Okamoto, K.; Yoden, M.; Shiratori, T.; Kaku, R.; Ueda, K.; Ohshio, Y.; Terashima, T.; Hanaoka, J. Activin A is deeply involved in the progression of sarcopenia and leads to poor prognosis in lung cancer patients. Sci. Rep. 2025, 15, 13641. [Google Scholar] [CrossRef]
- Taniguchi, S.; Matsui, T.; Kimura, K.; Funaki, S.; Miyamoto, Y.; Uchida, Y.; Sudo, T.; Kikuta, J.; Hara, T.; Motooka, D.; et al. In vivo induction of activin A-producing alveolar macrophages supports the progression of lung cell carcinoma. Nat. Commun. 2023, 14, 143. [Google Scholar] [CrossRef]
- Li, F.L.; Gu, L.H.; Tong, Y.L.; Chen, R.Q.; Chen, S.Y.; Yu, X.L.; Liu, N.; Lu, J.L.; Si, Y.; Sun, J.H.; et al. INHBA promotes tumor growth and induces resistance to PD-L1 blockade by suppressing IFN-gamma signaling. Acta Pharmacol. Sin. 2025, 46, 448–461. [Google Scholar] [CrossRef]
- Morianos, I.; Tsitsopoulou, A.; Potaris, K.; Valakos, D.; Fari, O.; Vatsellas, G.; Bostantzoglou, C.; Photiades, A.; Gaga, M.; Xanthou, G.; et al. Activin-A impedes the establishment of CD4+ T cell exhaustion and enhances anti-tumor immunity in the lung. J. Exp. Clin. Cancer Res. 2021, 40, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, S.; Li, Y.; Zhang, J.; Luo, Y.; Li, P.; Yang, Y.; Li, Y.; Huang, Y.; Wang, E. Inhibin betaA is an independent prognostic factor that promotes invasion via Hippo signaling in non-small cell lung cancer. Mol. Med. Rep. 2021, 24, 789. [Google Scholar] [CrossRef] [PubMed]
- Wamsley, J.J.; Kumar, M.; Allison, D.F.; Clift, S.H.; Holzknecht, C.M.; Szymura, S.J.; Hoang, S.A.; Xu, X.; Moskaluk, C.A.; Jones, D.R.; et al. Activin upregulation by NF-kappaB is required to maintain mesenchymal features of cancer stem-like cells in non-small cell lung cancer. Cancer Res. 2015, 75, 426–435. [Google Scholar]
- Shan, Y.; Li, S. Expression of Cripto-1 gene protein and Activin-A in human lung adenocarcinoma tissue. Pak. J. Pharm. Sci. 2015, 28 (Suppl. 2), 739–743. [Google Scholar] [PubMed]
- Seder, C.W.; Hartojo, W.; Lin, L.; Silvers, A.L.; Wang, Z.; Thomas, D.G.; Giordano, T.J.; Chen, G.; Chang, A.C.; Orringer, M.B.; et al. Upregulated INHBA expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia 2009, 11, 388–396. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Yin, H.; Wufuer, G.; Wang, L.; Abuduaini, S.; Chang, X. Knockdown of Inhibin Beta A Reversed the Epithelial Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance and Enhanced the Therapeutic Effect of Radiotherapy in Non-Small Cell Lung Cancer. Biochem. Genet. 2025, 63, 4723–4739. [Google Scholar]
- Zhang, F.; Qi, Y.; Li, J.; Liu, B.; Liu, Z.; Cui, X. Activin A induces apoptosis of human lung adenocarcinoma A549 cells through endoplasmic reticulum stress pathway. Oncol. Rep. 2024, 51, 29. [Google Scholar]
- Weinberg, O.K.; Marquez-Garban, D.C.; Fishbein, M.C.; Goodglick, L.; Garban, H.J.; Dubinett, S.M.; Pietras, R.J. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005, 65, 11287–11291. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lara, V.; Cortes-Ramirez, G.; Angeles-Torres, I.A.; Rodriguez-Cid, J.; Pedraza-Reyes, S.M.L.; Avila-Costa, M.R.; Ordonez-Librado, J.L.; Cerbon, M. Expression patterns of estrogen and androgen receptors in NSCLC patients according to the PD-L1 profile. Front. Immunol. 2025, 16, 1602579. [Google Scholar] [CrossRef]
- Oyanagi, J.; Koh, Y.; Sato, K.; Mori, K.; Teraoka, S.; Akamatsu, H.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer 2019, 132, 107–113. [Google Scholar] [CrossRef]
- Jackson, S.S.; Marks, M.A.; Katki, H.A.; Cook, M.B.; Hyun, N.; Freedman, N.D.; Kahle, L.L.; Castle, P.E.; Graubard, B.I.; Chaturvedi, A.K. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer 2022, 128, 3531–3540. [Google Scholar] [CrossRef]
- Greenblatt, R.B.; Oettinger, M.; Bohler, C.S. Estrogen-androgen levels in aging men and women: Therapeutic considerations. J. Am. Geriatr. Soc. 1976, 24, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.; Eberhart, R.; Chevailler, M.C.; Quinn, F.A.; Bischof, P.; Stricker, R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin. Chem. Lab. Med. 2006, 44, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Pagan, E.; Bagnardi, V.; De Pas, T.; Queirolo, P.; Pennacchioli, E.; Catania, C.; Cocorocchio, E.; Ferrucci, P.F.; et al. Sex-Based Dimorphism of Anticancer Immune Response and Molecular Mechanisms of Immune Evasion. Clin. Cancer Res. 2021, 27, 4311–4324. [Google Scholar] [CrossRef]
- Scott, S.C.; Shao, X.M.; Niknafs, N.; Balan, A.; Pereira, G.; Marrone, K.A.; Lam, V.K.; Murray, J.C.; Feliciano, J.L.; Levy, B.P.; et al. Sex-specific differences in immunogenomic features of response to immune checkpoint blockade. Front. Oncol. 2022, 12, 945798. [Google Scholar] [CrossRef]
- Li, C.H.; Prokopec, S.D.; Sun, R.X.; Yousif, F.; Schmitz, N.; Subtypes, P.T.; Clinical, T.; Boutros, P.C.; Consortium, P. Sex differences in oncogenic mutational processes. Nat. Commun. 2020, 11, 4330. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Haider, S.; Shiah, Y.J.; Thai, K.; Boutros, P.C. Sex Differences in Cancer Driver Genes and Biomarkers. Cancer Res. 2018, 78, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. Sex-differential responses to immune checkpoint inhibitors across the disease continuum unified by tumor mutational burden. Semin. Oncol. 2025, 52, 152414. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Liu, J.A.; Seddu, K.; Klein, S.L. Sex hormone signaling and regulation of immune function. Immunity 2023, 56, 2472–2491. [Google Scholar] [CrossRef]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef]
- Kwon, H.; Schafer, J.M.; Song, N.J.; Kaneko, S.; Li, A.; Xiao, T.; Ma, A.; Allen, C.; Das, K.; Zhou, L.; et al. Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 2022, 7, eabq2630. [Google Scholar] [CrossRef]
- Yang, C.; Jin, J.; Yang, Y.; Sun, H.; Wu, L.; Shen, M.; Hong, X.; Li, W.; Lu, L.; Cao, D.; et al. Androgen receptor-mediated CD8+ T cell stemness programs drive sex differences in antitumor immunity. Immunity 2022, 55, 1268–1283. [Google Scholar] [PubMed]
- May, L.; Hu, B.; Jerajani, P.; Jagdeesh, A.; Alhawiti, O.; Cai, L.; Semenova, N.; Guo, C.; Isbell, M.; Deng, X.; et al. The Innate Immune System and the TRAIL-Bcl-XL Axis Mediate a Sex Bias in Lung Cancer and Confer a Therapeutic Vulnerability in Females. Cancer Res. 2024, 84, 4140–4155. [Google Scholar]
- Vanguri, R.S.; Luo, J.; Aukerman, A.T.; Egger, J.V.; Fong, C.J.; Horvat, N.; Pagano, A.; Araujo-Filho, J.A.B.; Geneslaw, L.; Rizvi, H.; et al. Multimodal integration of radiology, pathology and genomics for prediction of response to PD-(L)1 blockade in patients with non-small cell lung cancer. Nat. Cancer 2022, 3, 1151–1164. [Google Scholar] [CrossRef]
| Drug | Antibody Type | Lung Cancer Type | Current Stage | Clinical Trials |
|---|---|---|---|---|
| Pembrolizumab | Humanized IgG4 PD-1 antibody | PD-L1+ or metastatic NSCLC | Phase IV | KEYNOTE 010, 024, 042, 189, 407 |
| Nivolumab | Humanized IgG4 PD-1 antibody | NSCLC | Phase IV | Checkmate 227 |
| Cemiplimab | Humanized IgG4 PD-1 antibody | NSCLC | Phase IV | EMPOWER-Lung1 |
| Toripalimab | Humanized PD-1 antibody | Advanced NSCLC | Phase III | CHOICE-01 CHOICE 03 Neotorch |
| Atezolizumab | Humanized IgG1 PD-L1 antibody | Platinum-resistant NSCLC | Phase IV | OAK IMpower010 |
| Durvalumab | Humanized IgG1 PD-L1 antibody | ES-SCLC NSCLC | Phase IV | CASPIAN PACIFIC |
| Meta Analysis | # Clinical Trials | Therapies | # Patients | Sex Difference Lung Cancer |
|---|---|---|---|---|
| Lee et al. [59] | CheckMate 017, CheckMate 057, KEYNOTE 010 OAK POPLAR | nivolumab, pembrolizumab, atezolizumab | 3025 | No |
| Wu et al. [60] | CheckMate 017, CheckMate 026, CheckMate 057, TASUKI-52, KEYNOTE 024, KEYNOTE 042, KEYNOTE 189, KEYNOTE 407, EMPOWER-Lung1, EMPOWER-Lung3, CameL-sq, CameL, RATIONALE 303, RATIONALE 304, RATIONALE 307, ORIENT-3, ORIENT-11, ORIENT-12, CHOICE-01, ASTRUM-005, IMpower110, IMpower130, IMpower 131, IMpower 132, IMpower 150, OAK JAVELIN Lung 200, PACIFIC MYSTIC POSEIDON CAPSTONE-1, GEMSTONE-301, GEMSTONE-302 | nivolumab, pembrolizumab, cemiplimab, tislelizumab, sintilimab, toripalimab, serplulimab, atezolizumab, avelumab, durvalumab, adebrelimab, sugemalimab | 11,883 | Yes, cemiplimab only |
| Wang et al. [61] | CheckMate 227, IMpower 131, IMpower 132, JAVELIN Lung 200, KEYNOTE 042, KEYNOTE 189, KEYNOTE 407, PACAFIC CA184-104, CheckMate 026, OAK KEYNOTE 010, KEYNOTE 024, CheckMate 057, CheckMate 017 | nivolumab, atezolizumab, avelumab, pembrolizumab, durvalumab, ipilimumab | 9583 | Yes |
| Conforti et al. [62] | KEYNOTE-042, KEYNOTE-024, IMpower110, EMPOWER-Lung 1 | pembrolizumab, atezolizumab, cemiplimab | 1672 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglisi, M.; May, L.; Gardiyehewa, T.; Landry, J.W. Sex Differences in the Response to Lung Cancer and Its Relation to Programmed Cell Death Protein-1/Programmed Death-Ligand-1 Checkpoint Therapies. Cancers 2025, 17, 3953. https://doi.org/10.3390/cancers17243953
Puglisi M, May L, Gardiyehewa T, Landry JW. Sex Differences in the Response to Lung Cancer and Its Relation to Programmed Cell Death Protein-1/Programmed Death-Ligand-1 Checkpoint Therapies. Cancers. 2025; 17(24):3953. https://doi.org/10.3390/cancers17243953
Chicago/Turabian StylePuglisi, Morgan, Lauren May, Thusna Gardiyehewa, and Joseph W. Landry. 2025. "Sex Differences in the Response to Lung Cancer and Its Relation to Programmed Cell Death Protein-1/Programmed Death-Ligand-1 Checkpoint Therapies" Cancers 17, no. 24: 3953. https://doi.org/10.3390/cancers17243953
APA StylePuglisi, M., May, L., Gardiyehewa, T., & Landry, J. W. (2025). Sex Differences in the Response to Lung Cancer and Its Relation to Programmed Cell Death Protein-1/Programmed Death-Ligand-1 Checkpoint Therapies. Cancers, 17(24), 3953. https://doi.org/10.3390/cancers17243953






