1. Introduction
Anaplastic Thyroid Cancer (ATC) is one of the most lethal and fast-growing metastatic malignancies [
1]. A key challenge in ATC research is that it arises from many of the same driver mutations (e.g.,
BRAFV600E) found in papillary thyroid cancer (PTC), a slow-growing and highly treatable carcinoma [
2]. Both ATC and PTC arise from thyroid follicular cells; however, the rapid progression of ATC is supported by its loss of follicular cell differentiation and acquisition of a high mutational burden—an oncogenic process not typically exhibited by PTC [
3]. Therefore, mutational studies alone are insufficient because ATC and PTC share similar cellular origins and many of the same mutations [
4]. The use of small molecule inhibitor therapy to treat PTC is successful in its curative efforts, whereas in ATC, these treatment modalities are relatively futile [
5]. Further, there are significant hurdles to be overcome when diagnosing and treating ATC—challenges that are not present in PTC. The identification of biomarkers that can contribute to early detection or serve as a molecular target to aid in therapeutic successes is pertinent.
Emerging evidence suggests that the aggressive and metastatic nature of ATC is driven, in part, by aberrant epigenetic mechanisms [
6]. The identification of differentially expressed genes (DEGs) that arise and drive feed-forward disease progression surpasses the forthcomings of mutational study [
7,
8]. The consideration that this aggressive carcinoma is driven by wild-type DEGs allows for a full-range molecular analysis of ATC’s unique cellular processes [
8]. A central focus of epigenetic processes in cancer has led to the discovery of long non-coding RNA (lncRNA) molecules [
9]. These molecules have extensive DNA-, RNA-, and protein-binding domains that enable both broad and fine-tuned impacts on the transformation of cancer cells and the orchestration of cancer phenotypes [
10,
11].
The differential expression patterns of lncRNAs have enabled the elucidation of unknown molecular mechanisms that drive the various hallmarks of cancer (i.e., immortality, invasive potential, migratory propensity, clonogenic capacity, etc.) [
12]. Using publicly available National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) datasets, we aimed to identify differentially expressed lncRNAs for further molecular evaluation. Double Homeobox A Pseudogene 10 (DUXAP10) was the top statistically significant differentially expressed gene in ATC compared to normal thyroid tissue (GSE33630) and has been previously established as an overexpressed oncogene in various carcinomas [
13]. However, its role in ATC has not yet been explored. DUXAP10, a large lncRNA molecule, has been described for its ability to regulate a broad spectrum of cellular processes, making this molecule indispensable for evaluation. In the present study, we aimed to examine the effects of its overexpression on ATC’s phenotypic landscape, and we report a novel regulatory role in ATC.
2. Materials and Methods
2.1. Cell Lines
ATC cell line T238 (RRID:CVCL_6299) and immortalized normal thyroid follicular epithelial cell line Nthy-ori-3-1 (RRID:CVCL_2659) were maintained in complete media composed of 10% FBS 1X RPMI 1640 + L-glutamine and phenol red pH indicator. Two genetically engineered cell lines, T238-ΔDUXAP10#1 and T238-ΔDUXAP10#2, were generated using lentiviral transduction and confirmed with qRT-PCR and RNAseq analysis. HEK293T cells (CRL-3216) used for lentiviral harvesting were maintained in complete media composed of 10% FBS 1X DMEM + 4.5 g/L glucose, L-glutamine, and sodium pyruvate, and phenol red pH indicator. Cells were grown in a cell culture water-jacketed incubator at 37 °C and 5% CO
2. Cell passage was completed using a 0.25% Trypsin, 2.21 mM EDTA, and 1X [-] sodium bicarbonate solution. Processes for cell maintenance and passage were completed as previously described [
14].
2.2. Bioinformatic Analysis
Publicly available NCBI GEO datasets were used for selection of a DEG using the provided GEO2R software. Limma package and GEO2R query combined R version 2.11.0 and Bioconductor 2.6 platforms for identification and ranking of the top 250 DEGs in ATC vs. normal thyroid tissue (GSE33630). RNAseq data provided by NCBI GEO was analyzed using the DESeq2 R package to generate normalized transcripts per million (TPM) expression counts (GSE85457).
2.3. qRT-PCR
Total RNA was extracted from 175 cm2 tissue culture flasks at 70% confluence using Zymo Research Quick RNA Miniprep Kit (cat no. 11237) according to manufacturer’s protocol. RNA concentrations and sample purity was determined using an IMPLEN Nanophotometer Pearl (serial no. 3464) nanodrop reader. RNA with a concentration of 50 ng/μL were used for qRT-PCR.
Primer sequences of approximately 18–30 bases in length were designed using NCBI Blast and ordered dry from GeneWiz. Primer set for DUXAP10 was F: 5′-GGTTCAACAGTATGGCTCCAAAG-3′; R: 5′-GACTGCCATCCACAGATGAAG-3′. GAPDH F: 5′-GTCTCCTCTGACTTCAACAGCG-3′; R: 5′-ACCACCCTGTTGCTGTAGCCAA-3′ was used as the endogenous control. Primers were reconstituted in nuclease-free H2O for a 100 μM concentration. Primers were diluted to 10 μM for qRT-PCR protocol.
The quantitative reaction was completed using qPCRBIO SyGreen 1-Step Go Lo-ROX (Genesee Scientific, El Cajon, CA, USA; cat no. 17-602B) according to manufacturer’s protocol. The RT-PCR was run using a QuantStudio 5 Real-Time PCR Instrument (96-Well) 0.2 mL Block (Applied Biosystems by ThermoFisher, Waltham, MA, USA; reference no. A28134) with a denaturation step for 10 min at 45 °C–55 °C, annealing step for 2 min at 95 °C, and a final extension step for 4 s at 95 °C and 20 s at 60 °C–65 °C, repeated 40 times. QuantStudio Design & Analysis software was used for determining the relative quantification (RQ) of the gene of interest.
2.4. CRISPR-Interference
CRISPRi methodology was used for selective transcriptional repression of DUXAP10 using the protocol described below.
2.5. Preparation of Selection Media and Selection Plates
Luria Broth with 100 μg/mL ampicillin was used as selection media and prepared with 10 g/L NaCl, 10 g/L tryptone, and 5 g/L yeast extract. Luria Broth selection plates were made using 12.5 g of Luria Broth mixture combined with 10 g of agar in 1 L of sterile H2O with 100 μg/mL ampicillin.
2.6. Single Guide RNA Selection
Single guide RNA (sgRNA) sequences were generated using CRISPick software provided by the Broad Institute [
13,
14] with a Human GRCh38 reference genome and SpyoCas9 as the enzyme (PAM policy, NGG). The top two ranked sgRNA sequences were selected and restriction enzyme overhangs were added for recombination (GeneWiz). Oligonucleotide sequences used:
DUXAP10_sg1 (F) 5′-CACCGGGCGTGGTCAGAGCGAGCTT-3′; (R) 5′-AAAACAAGCTCGCTCTGACCACGCC-3′; DUXAP10_sg2 (F) 5′-CACCGGAGCTTCGGAGAAGCAGTGG-3′; (R) 5′-AAACCCACTGCTTCTCCGAAGCTCCGGTG-3′;
Non-specific (NS)_sg (F) 5′-CACCAATCTCGCTTATATAACGAG-3′; (R) 5′-AAACCTCGTTATATAAGCGAGATT-3′.
A stock and working solution of 100 μM using 1X TE solution was prepared upon sgRNA arrival for both forward (F) and reverse (R) oligonucleotides.
2.7. Single Guide RNA Annealing
The sgRNAs were annealed in a reaction composed of 100 μM F and of 100 μM R sgRNAs, T4 Ligase Buffer, and Polynucleotide Kinase (PNK), quantum satis molecular grade water for a 40 μL reaction. The annealing mixture was incubated at 37 °C for 60 min followed by 95 °C for 5 min, decreasing by 1 °C each minute until a temperature of 12 °C was reached.
2.8. Restriction Enzyme Plasmid Digestion
The pLV hU6-sgRNA hUbC-dCas9-KRAB-T2a-Puro (Addgene #71236) used for recombination was gifted from the Charles Gersbach laboratory. The restriction enzyme digestion reaction was made per μg of p71236 plasmid DNA and contained 5 μL of 10X NEB Buffer 3.1, 1 μL of BsmbI Restriction Enzyme, quantum satis molecular grade water for a 50 μL reaction. The digestion mixture incubated at 55 °C for 30 min. Calf intestinal phosphatase (CIP) was added for the remaining 30 min at 37 °C. The reaction mixture was PCR purified prior use for ligation reaction. All 10 μL (500 ng) of PCR purified BsmbI-digested CIP-treated dCas9-KRAB-Puro was used for ligation.
2.9. Plasmid Ligation and Recombination
The entirety of the PCR purified digested plasmid was combined with previously annealed sgRNA oligonucleotide mixture with T4 Ligase Buffer and T4 Ligase and incubated at room temperature for 2 h.
2.10. Bacterial Transformation of E. coli
NEB-5 alpha
E. coli was used for transformation and subsequent replication of plasmid DNA.
E. coli cells were transformed with a 30 s heat shock at 42 °C followed immediately with ice. SOC media was added for recovery and newly transformed bacteria were incubated at 37 °C and 250 rpm. Bacteria were plated on prepared ampicillin selection plates and incubated overnight at 37 °C. Individual colonies were selected and grown in Luria Broth overnight at 37 °C at 250 rpm. Bacterial plasmids were isolated as previously described [
15,
16]. Recombinant plasmids were sequenced using a primer for the U6 promoter (5′-GACTATCATATGCTTACCGT-3′).
2.11. Lentivirus Preparation
HEK293T cells were used for lentiviral harvesting. Components for lentiviral assembly included a pCMV-VSV-G envelope glycoprotein vector (Addgene, Watertown, MA, USA; #12259), psPAX2 (Addgene, Watertown, MA, USA; #12260) packaging vector, and recombined pLV hU6-sgRNA hUbC-dCas9-KRAB-T2a-Puro with gene target. Lentiviral packaging was accomplished using assembly plasmids and a solution of OptiMEM (ThermoFisher, Waltham, MA, USA; cat no. 31985062) and TransIT LT1 Reagent (Millipore, Burlington, MA, USA; cat no. MIR2304).
2.12. Lentivirus Transduction
Filtered lentiviral complexes were transduced into target cells plated at 30% confluence and completed using Polybrene Transfection Reagent (10 mg/mL) (Millipore, Burlington, MA, USA; cat no. TR-1003-G) diluted 1:2.5 in 1X sterile PBS and then diluted again 1:1000 in complete media. Cells were incubated at 5% CO2 and 37 °C for 48 h.
2.13. Puromycin Selection
Concentration of puromycin needed for successful target cell selection following transduction was previously standardized. Cell media was replaced with 8 μg/mL puromycin diluted into complete media for T238. Cells were incubated for 48–72 h at 5% CO2 and 37 °C for full selection process. Selected cells were used for all further experimentation.
2.14. RNA-Seq
Total isolated RNA from T238-NS and T238-ΔDUXAP10#2 was prepared, in triplicate, as described using Zymo Research Quick RNA Miniprep Kit (cat no. 11237) according to manufacturer’s protocol. RNA was sent frozen for Next Generation Sequencing analysis provided by Azenta, Burlington, MA, USA. Top DEGs and corresponding gene ontology analysis was completed and used for further experimental evaluation.
2.15. Click-IT RNA Imaging Assay
The Click-iT RNA Imaging assay was completed according to protocol (ThermoFisher, Waltham, MA, USA; cat nos. C10329, C10330) and was used for nascent RNA labeling. T238 cells were seeded into 6-well plates at a density of 50,000 cells per well to achieve 40–50% confluence in 24 h. Cells were imaged using a Nikon Eclipse TS100 Inverted Microscope green fluorescence channel. Captured cells containing newly synthesized, labeled RNA were quantified using ImageJ. Complete Total Cell Fluorescence (CTCF) was calculated using cell fluorescence measures with subtracted background.
2.16. RNA-FISH
Cells were seeded in a 24-well tissue culture treated plate at densities required for 30–40% confluence. Fluorescent labeling of DUXAP10 was completed using QuantiGene ViewRNA ISH Cell Assay according to protocol (cat no. QVC001) and ThermoFisher compatible custom probe sets (cat no. VA4-310-2900). A working probe set solution was prepared at a 1:100 ratio and cells were incubated for 5 hr at in a dry incubator set to 40 °C following lysis with Triton X-100. Cells were imaged using the green fluorescent channel (Alexa FluorTM 488) using a Morrel Nikon Eclipse TS100 Inverted Microscope.
2.17. Colony Forming Unit Assay
Colony forming units (CFU) were used to evaluate clonogenic capacity. Cells were plated at sparse even densities (200 cells/well) in a 6-well tissue culture treated plate. Cells were incubated at 37 °C and 5% CO2 and monitored for colony formation. Endpoint was reached when wells containing control T238 had maximum colony formation. Cells were fixed using 100% methanol and stained using 1% toluidine blue in 1% borax solution.
2.18. XTT Assay
Non-radioactive cell viability was quantified over 72 h using a sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy6-nitro) benzene sulfonic acid hydrate (XTT) substrate. Colorimetric XTT assay was completed according to manufacturer’s instructions (Millipore Sigma, St. Louis, MO, USA; product no. 11465015001). 1 mg of XTT with 2.5 μL phenazine methosulfate (PMS) (Sigma, St. Louis, MO, USA; cat no. P58-12) was added to clear 1X RPMI. Incubation time was previously evaluated for each cell line and was determined to be 6 h at 37 °C and 5% CO2 for T238. Absorbances were measured with wavelengths set to 450 nm/640 nm.
2.19. Cell Death Detection ELISA
Apoptotic induction in response to starvation of cells was evaluated and measured using a Cell Death Detection ELISA (Sigma, St. Louis, MO, USA cat no. 11544675001) according to manufacturer’s instructions. Samples for analysis were prepared using adherent cells in a 96-well tissue culture treated plate at a density of 1000 cells in serum-free media. Cells were incubated for 4 h at 37 °C and 5% CO2.
2.20. Invasion Assay
Invasion Index was evaluated using Corning Chambers coated with a Matrigel Matrix according to manufacturer’s instructions (Corning, Corning, NY, USA; cat no. 354483). Approximately 10,000 cells were seeded into each coated insert containing serum-free media. Complete media with chemoattractant was placed in the wells. Cells were incubated for 18 h at 37 °C and 5% CO2. Invaded cells were stained with 1% toluidine blue in 1% borax solution for 2 min and placed in distilled H2O for 2 min for rinsing. Invaded cells were then imaged using Bright Field Microscopy at 40X. Cell counts were normalized to number of cells migrated to determine Invasion Index.
2.21. Migration Assay
Migration was evaluated using Corning Falcon Multiwell Insert System control inserts according to manufacturer’s instructions (cat no. 351185). Approximately 10,000 cells were seeded into each insert containing serum-free media. Complete media with chemoattractant was placed in the wells. Cells were incubated for 18 h at 37 °C and 5% CO2. Migrated cells were stained with 1% toluidine blue in 1% borax solution for 2 min and placed in distilled H2O for 2 min for rinsing. Migrated cells were then imaged using Bright Field Microscopy at 40X.
2.22. Scratch Wound Assay
Cells were seeded into 24-well tissue culture treated plates at a density of 150,000 cells per well. Cells were incubated for 24 h at 37 °C and 5% CO
2 and checked to ensure 100% confluence. A sterile 20 μL pipette tip was used to introduce a wound field directly through the center of the well. The wound field was measured using Bright Field Microscopy. Cells were incubated for 18–20 h at 37 °C and 5% CO
2. Wound field measurements were recorded, and calculations were made according to the following equation:
2.23. Tumorigenesis Mouse Model
Cells were grown and harvested using complete media. Approximately 500,000 cells were resuspended in sterile 1X PBS and kept on ice. Using a 24-gauge needle and 1 cc syringe, approximately 200 μL of cell suspension was administered into the hind leg flank of Female NU/J Mice Strain 002019 (Jackson Laboratory). Palpable tumors present after 3 weeks were measured using a vernier caliper and recorded for volume calculations. At the termination of the experiment (tumors > 2 cm), tumors were removed and weighed for evaluation of tumorigenesis. Gross lung images were taken and using ImageJ v2 software, were used to calculate the Metastatic Area using the following formula:
All in vivo experiments were conducted in the Department of Comparative Medicine at New York Medical College under the approved Institutional Animal Care and Use Committee (IACUC) protocol number 23755.
4. Discussion
Cancer genomics is a complex and evolving field of research. Studying uncharacterized molecular patterns has led to the discovery of novel biomarkers and therapeutic targets. Furthermore, researchers have begun to describe cancer types that exhibit unique biological puzzles. The inherent metastatic landscape of ATC presents significant diagnostic, prognostic, and therapeutic hurdles. Although mutational burden contributes to ATC pathology, it provides limited insight for diagnosis or treatment. Defining the genomic and transcriptomic landscape of ATC, as well as PTC and follicular thyroid cancer (FTC) tumors, can help improve early detection, treatment regimen, and ultimately, patient outcomes [
17,
18,
19]. In support of our research findings, it is reported that gene expression analysis can help curate molecular signatures that can determine tumor staging and therapeutic responsiveness—revolutionizing the standard of care in the field of oncology [
20,
21,
22,
23]. Moreover, the novelty and success of transcriptomic investigation, specifically the non-coding portion, cannot be overlooked [
24].
The limited success of using small-molecule inhibitors in ATC, as well as the inability to surgically resect or treat metastatic ATC, further highlights the need for additional avenues of intervention. This study aimed to identify a novel dysregulated gene that can serve as either a prognostic, diagnostic, or therapeutic target in ATC. Advances in sequencing and biomarker discovery serve as promising avenues for combating ATC [
25,
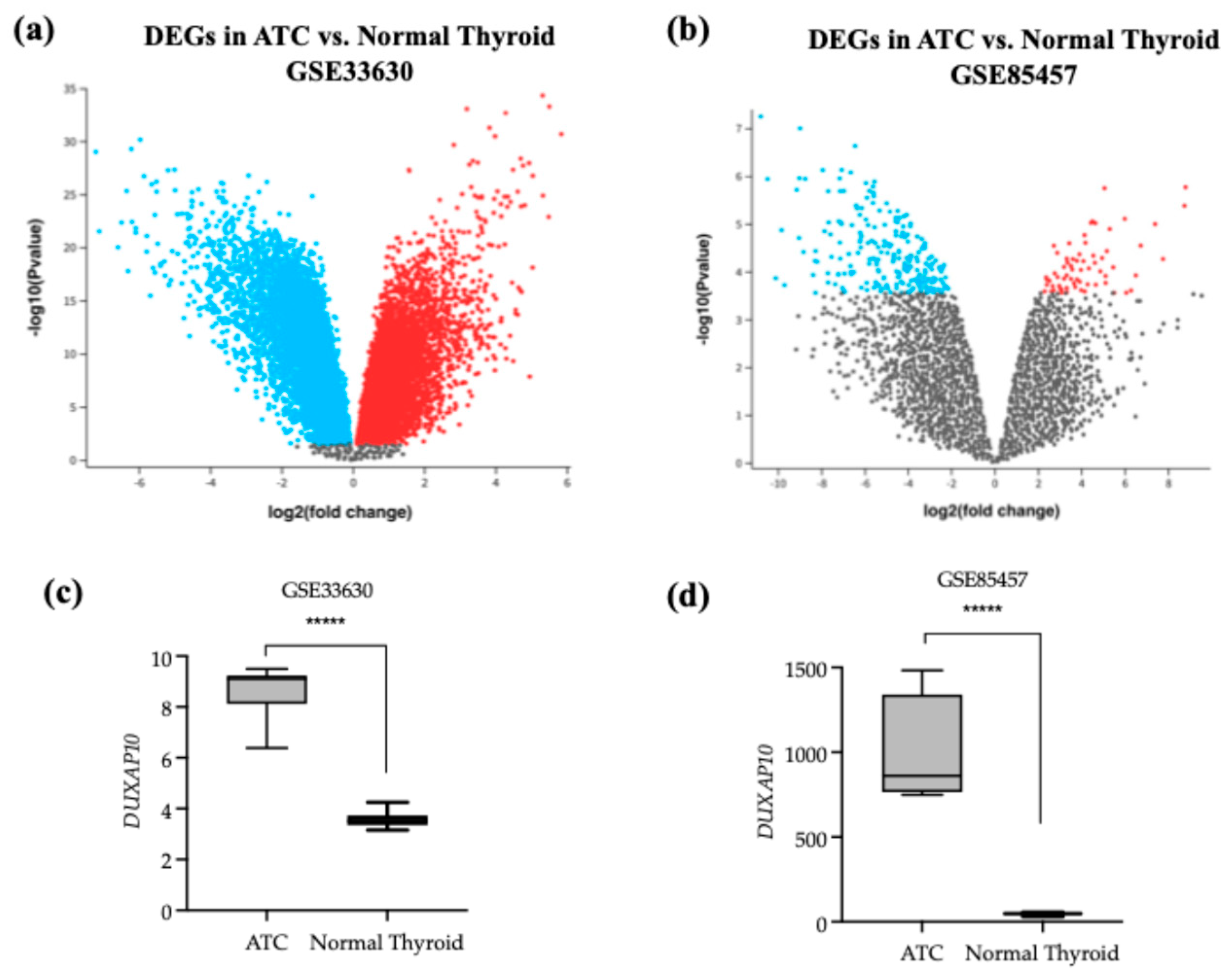
26]. Molecular signatures that orchestrate ATC’s unique pathology will significantly aid in disease management and curative efforts. Investigation of a more novel set of cellular regulators, such as lncRNAs, will help decipher the ways in which ATC is initiated and further driven— exceeding the bounds of mutational study. This pipeline of research has been successfully applied to many other cancer types. Publicly available datasets provided by NCBI were analyzed and indicated that DUXAP10 is a significantly overexpressed transcript and was the top statistically significant DEG in dataset GSE33630 (
Figure 1). The study provided by cBioPortal contained genomic datasets of well-differentiated thyroid cancer patients and found that the initial mutational drivers have striking similarity (i.e., mutations in MAPK and RAS); however, ATC acquires a large mutational burden as it progresses. This concept was also exhibited in ATC with concomitant well-differentiated thyroid cancer (i.e., PTC). It was reported that ATC and well-differentiated thyroid cancer share common ancestral cells and diverge as disease progresses [
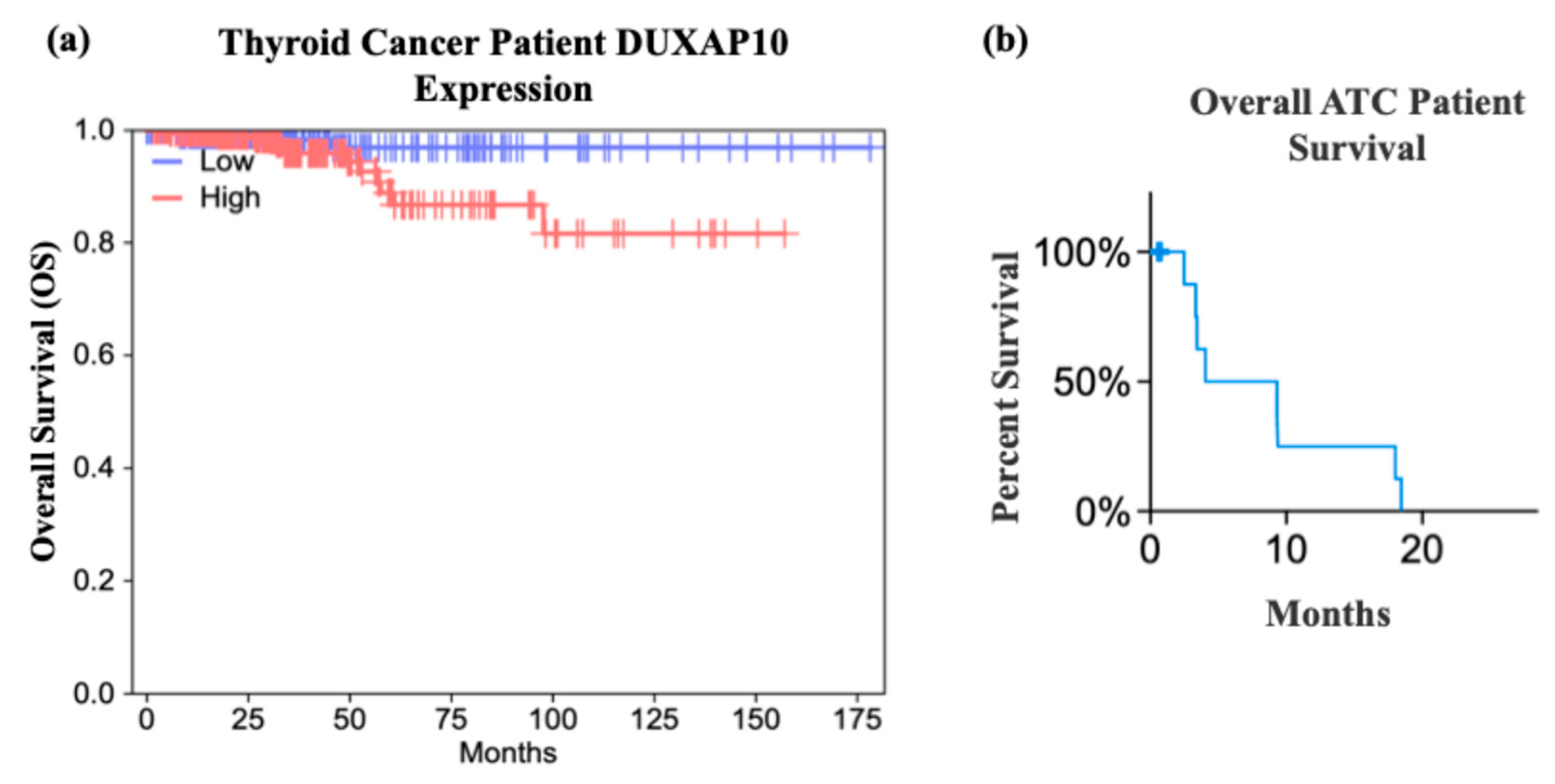
27]. These data support our hypothesis that DUXAP10 expression may serve as a prognostic marker in early ATC, and/or its upregulation heavily impacts the transition of patients into a stage IVC metastatic diagnosis, and GEPIA confirms a significant impact on patient survival when overexpressed (
Figure 2). In support of the initial selection of DUXAP10, this molecule is derived from homeobox genes, which are indispensable molecular elements during embryonic development. It has been discovered in recent years that homeobox genes, encoding for distinct homeobox proteins, serve as oncogenes or tumor suppressors in nearly all cancer types [
28]. Thus, a homeobox pseudogene-derived lncRNA, such as DUXAP10, is an extremely attractive molecule for research and intervention.
To understand the consequences of DUXAP10 overexpression in ATC, we employed CRISPRi technology to transcriptionally repress DUXAP10 at the genomic level—targeting two distinct regions of its promoter. The generation of stable CRISPRi cell lines facilitated comprehensive functional analyses of DUXAP10 and addressed the inherent limitations of transient RNA interference methodologies (
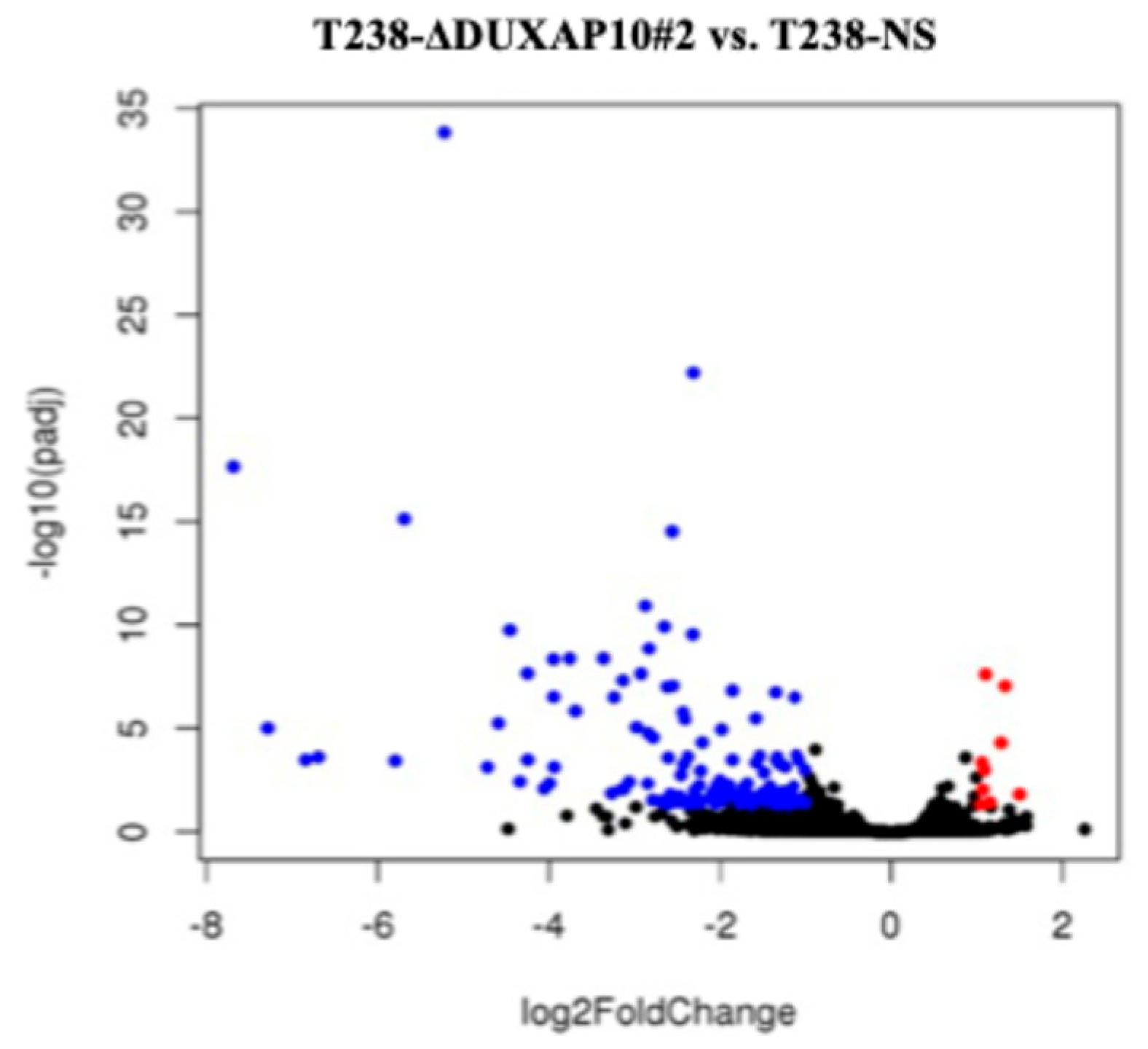
Figure 3). By result of DUXAP10 transcriptional repression in ATC cell line T238, a substantial impact of gene expression was observed using RNA-seq analysis (
Figure 4). A majority of these DEGs encode for proteins that are known to play pivotal, or minor roles across many cancer types. LncRNAs are functionally classified as miRNA sponges, protein scaffolds, and signal decoys. Therefore, studying DUXAP10, a large lncRNA molecule, is advantageous and likely has multi-faceted biological capabilities, which explains the drastic alteration of gene expression shown in
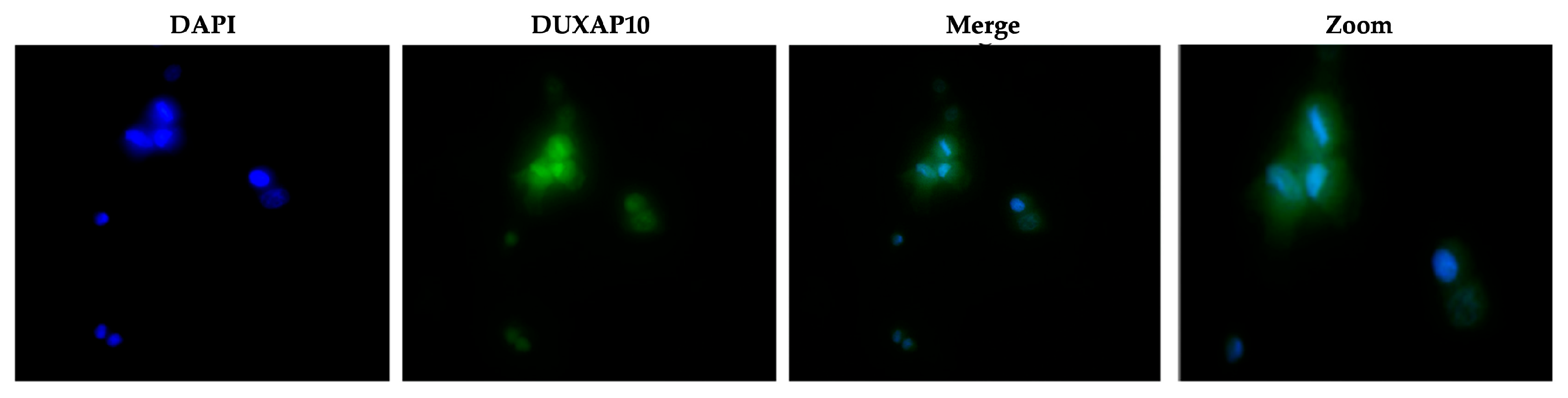
Figure 4. We observed that DUXAP10 is expressed in both the nucleus and cytoplasm of ATC cells, with a strong nuclear localization. This suggests that DUXAP10, functionally, can regulate DNA and RNA expression, as well as serve as a protein-binding molecule (
Figure 5). A critical, functional role for DUXAP10 was confirmed again by the marked reduction in RNA output, a key component of cell survival. These results provided evidence that DUXAP10 expression does significantly impact the S-phase of the cell cycle (
Figure 6). The rate at which RNA is synthesized is drastically increased in cancer due to the dysregulated expression of transcription factors and RNA polymerases [
29]. Previous studies have also shown the significant impact that targeting RNA polymerase II as a therapeutic option in some cancers— further supporting our finding that DUXAP10 expression alters this output [
30]. Data shown in
Figure 6 supported the RNA-seq data, which revealed the impact of DUXAP10 expression on the output of over 100 cancer-promoting genes. Published work on these genes has described them as bona fide regulators of proliferation, apoptosis, invasion, migration, etc. encompassing a broad spectrum of cancer-associated phenotypes. Therefore, we wanted to investigate if there is a phenotypic translation of this data.
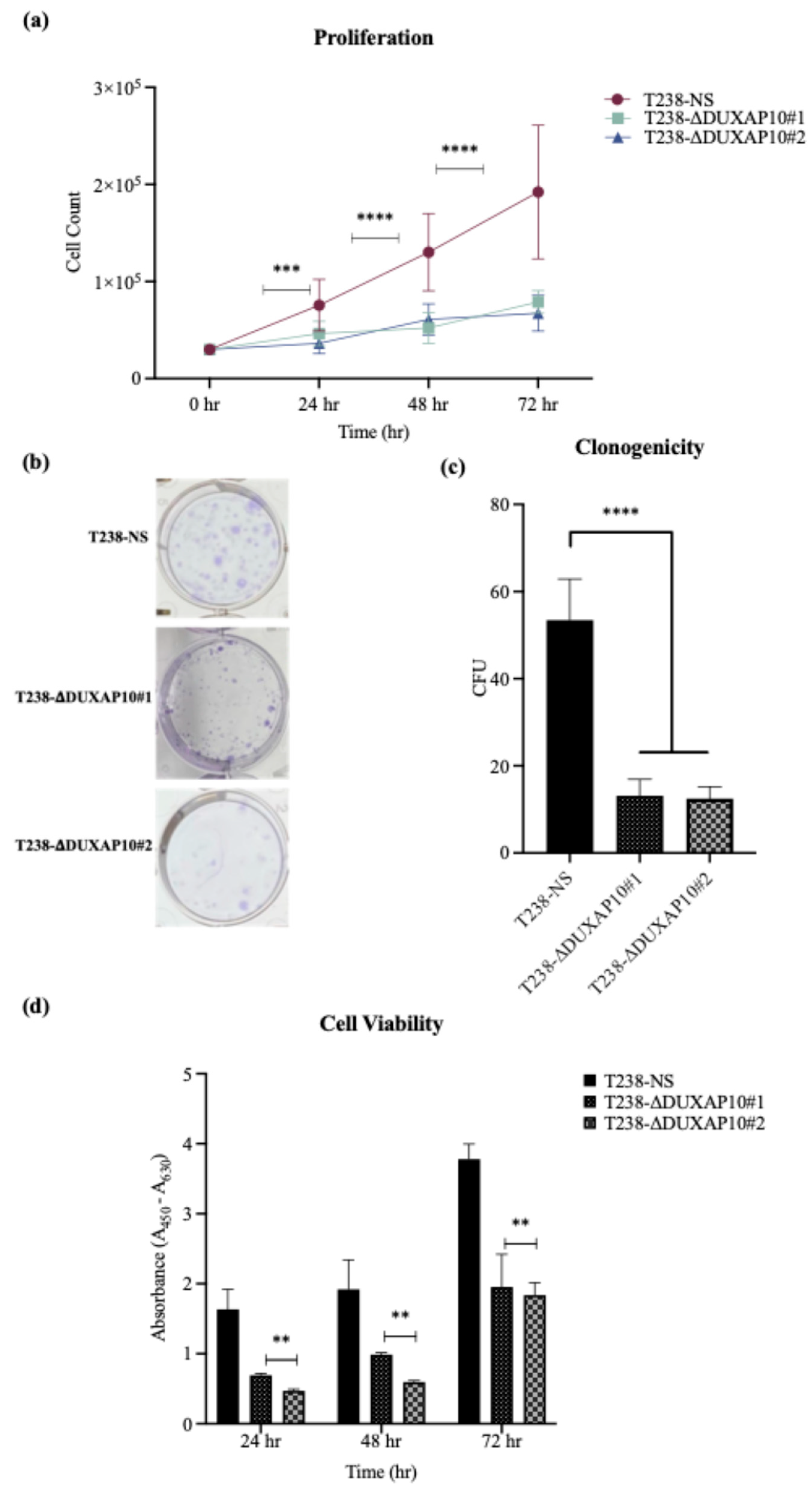
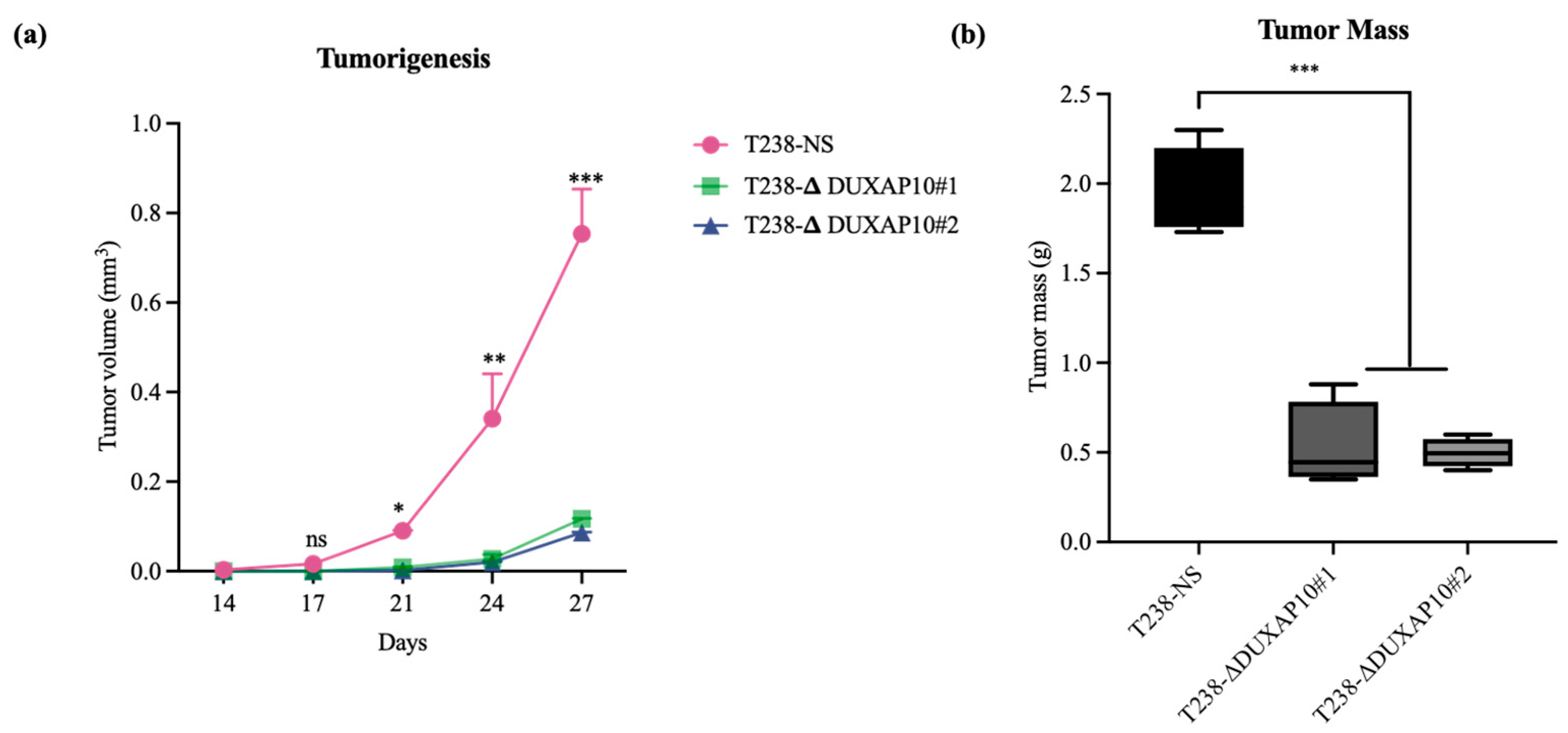
Ultimately, we described a significant role for DUXAP10 in phenotypes associated with ATC cell sustainability and tumorigenesis (
Figure 7 and
Figure 8). Hence, the reduction in proliferation, clonogenic capacity, and cell viability in both T238-ΔDUXAP10 knockdown cell lines supported the previously observed alteration in global transcription, as well as gene-specific regulation (
Figure 4 and
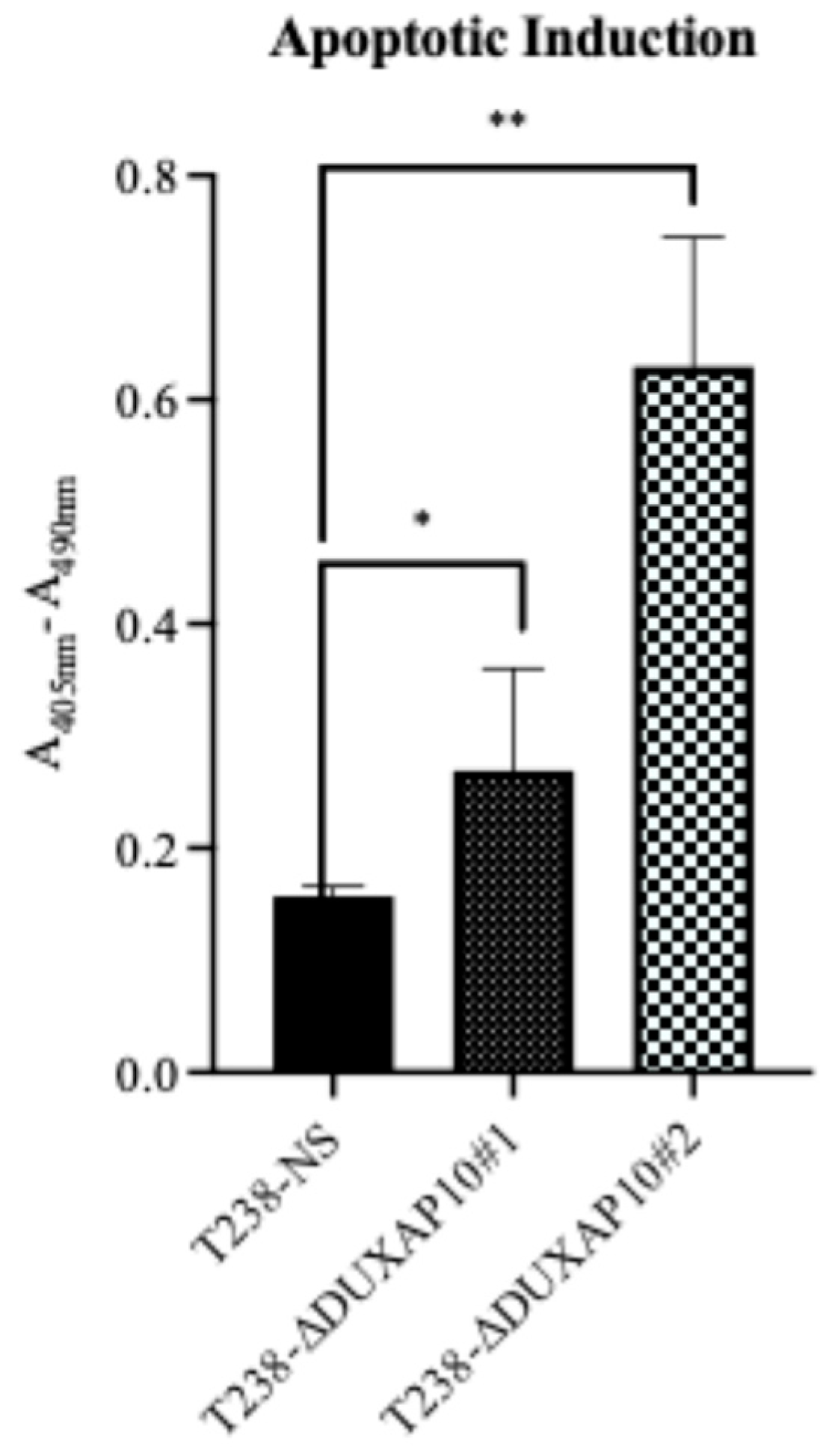
Figure 6). The balance of immortality of cancer cells lies in the upregulation of proliferative pathways, followed by the loss of negative regulation that is controlled by apoptotic induction. LncRNA molecules can drive proliferative signaling while promoting the loss of cell-death signals. Our data showed that in response to starvation, our T238-ΔDUXAP10 knockdown cell lines had significantly increased the sensitivity and activation of apoptosis, which was marked by the presence of mono-and oligo-nucleosome production— key markers of cell death (
Figure 9).
A key component of ATC is its inherent metastatic propensity. The activation of Epithelial-to-Mesenchymal Transition (EMT) processes aids in metastasis of cancer cells to distant secondary, tertiary, etc., sites [
31]. In ATC, metastasis occurs rapidly, typically in the weeks that follow disease propagation. The rapid proliferation at the ATC tumor sites contributes to the loss of nutrient supply and inherent need for evacuation and re-establishment at distant anatomical sites [
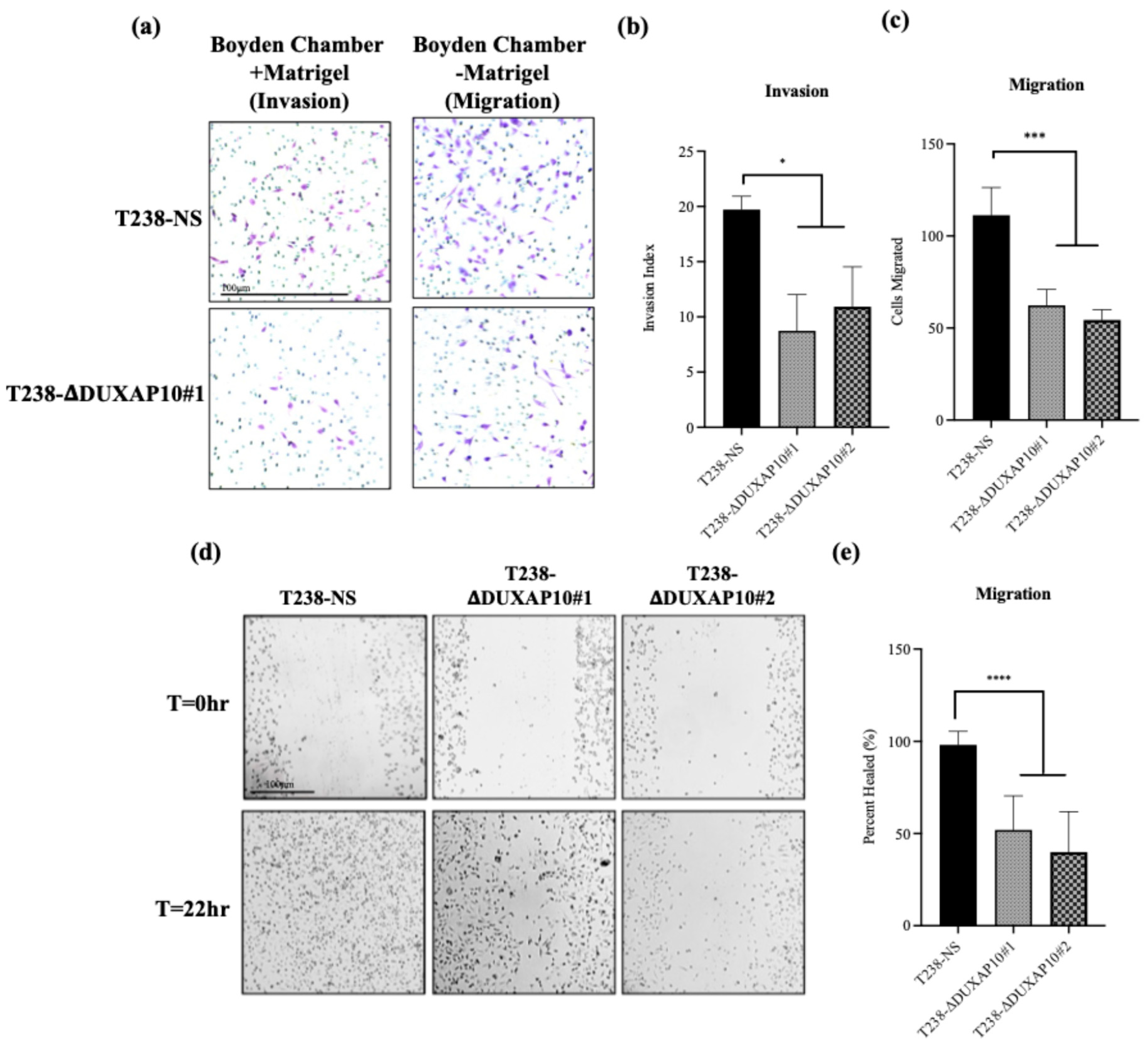
32]. We observed that both T238-ΔDUXAP10 knockdown cell lines significantly reduced the migratory and invasive capabilities of ATC in vitro and in vivo. EMT is supported by the loss of a stagnant, epithelial-like phenotype, followed by the upregulation of a motile, mesenchymal-like phenotype. We observed, using a pseudo-extracellular Matrigel matrix, that the T238-ΔDUXAP10 knockdowns had a reduced ability to degrade the components that make up a physiological extracellular matrix (
Figure 10a,b). This was supported by the significant reduction in migratory capacity, measured by both the reduction in movement towards a chemoattractant (
Figure 10a,c) and ability to seal a wound field (
Figure 10d,e). When implanted in our athymic mouse model, the significant reduction in tumor growth (
Figure 8) was supported by a reduction in metastatic lung lesions observed post-mortem (
Figure 11). Thus, our results heavily support a simultaneous role for DUXAP10 in the orchestration of ATC cell growth, cell death, and metastatic capabilities. We propose that DUXAP10 employs its protein- and nucleic acid-binding domains to modulate gene expression. Whether this occurs through direct interaction with chromatin, transcription factors, or other regulatory complexes remains an important avenue for future investigation. Further research regarding the molecular interactions of DUXAP10 that regulate these functions is warranted.