Temperature-Dependent Effects of Induced Hyperthermia, Including Whole-Body Hyperthermia, on the Hallmarks of Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
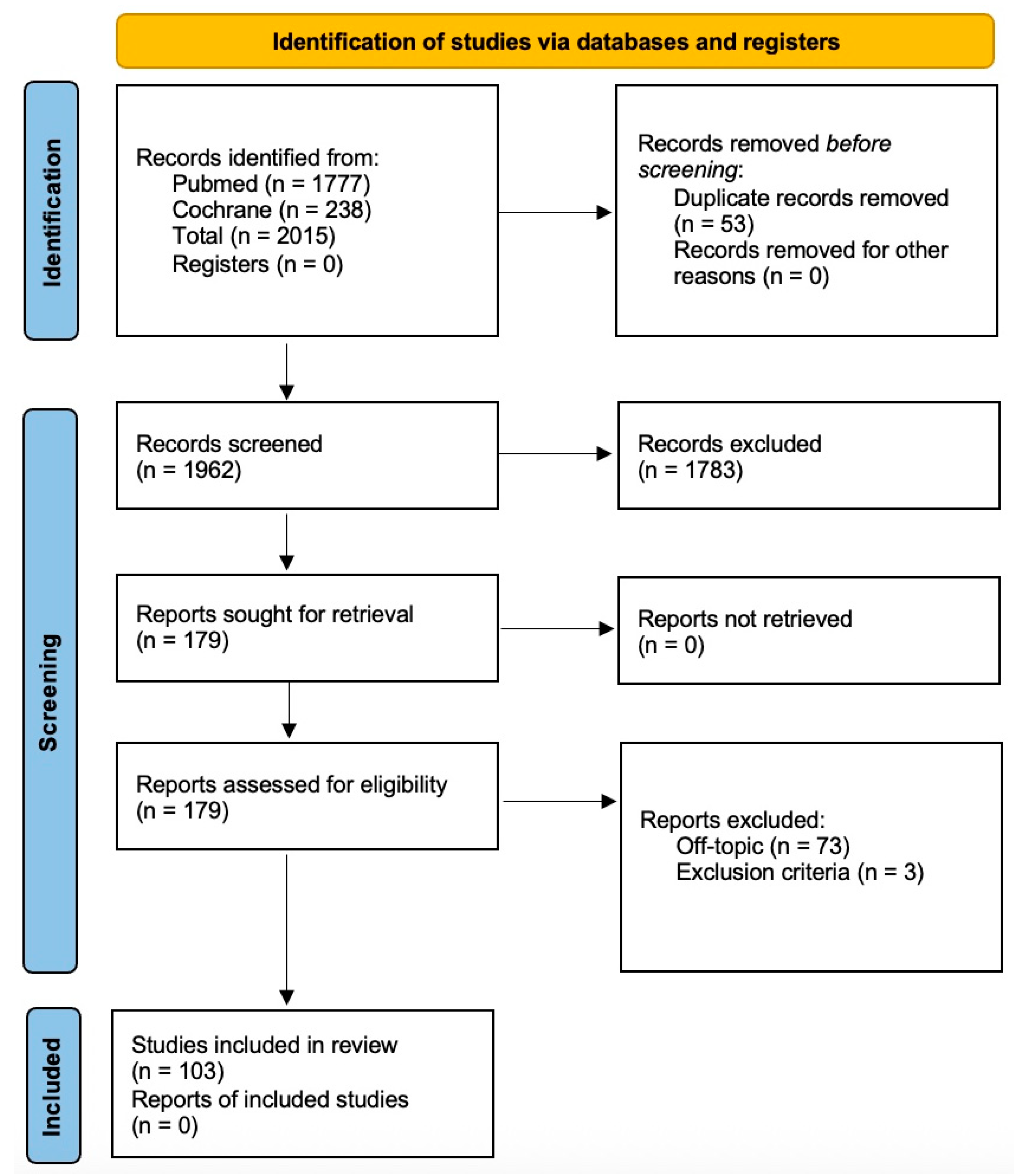
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Databases
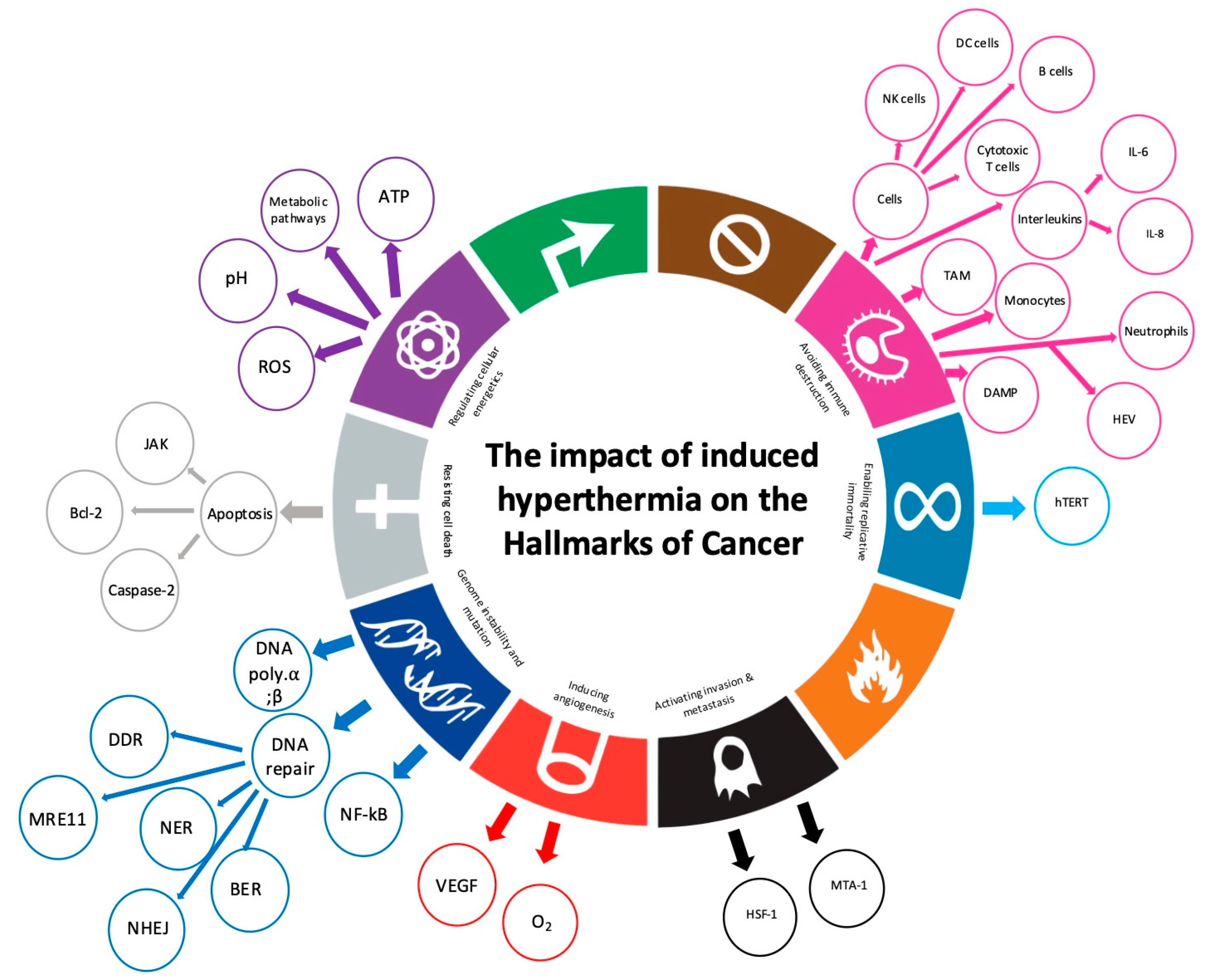
3. Results
3.1. Avoiding Immune Destruction
3.1.1. Induced Hyperthermia Triggers Immune-Mediated Cancer Destruction
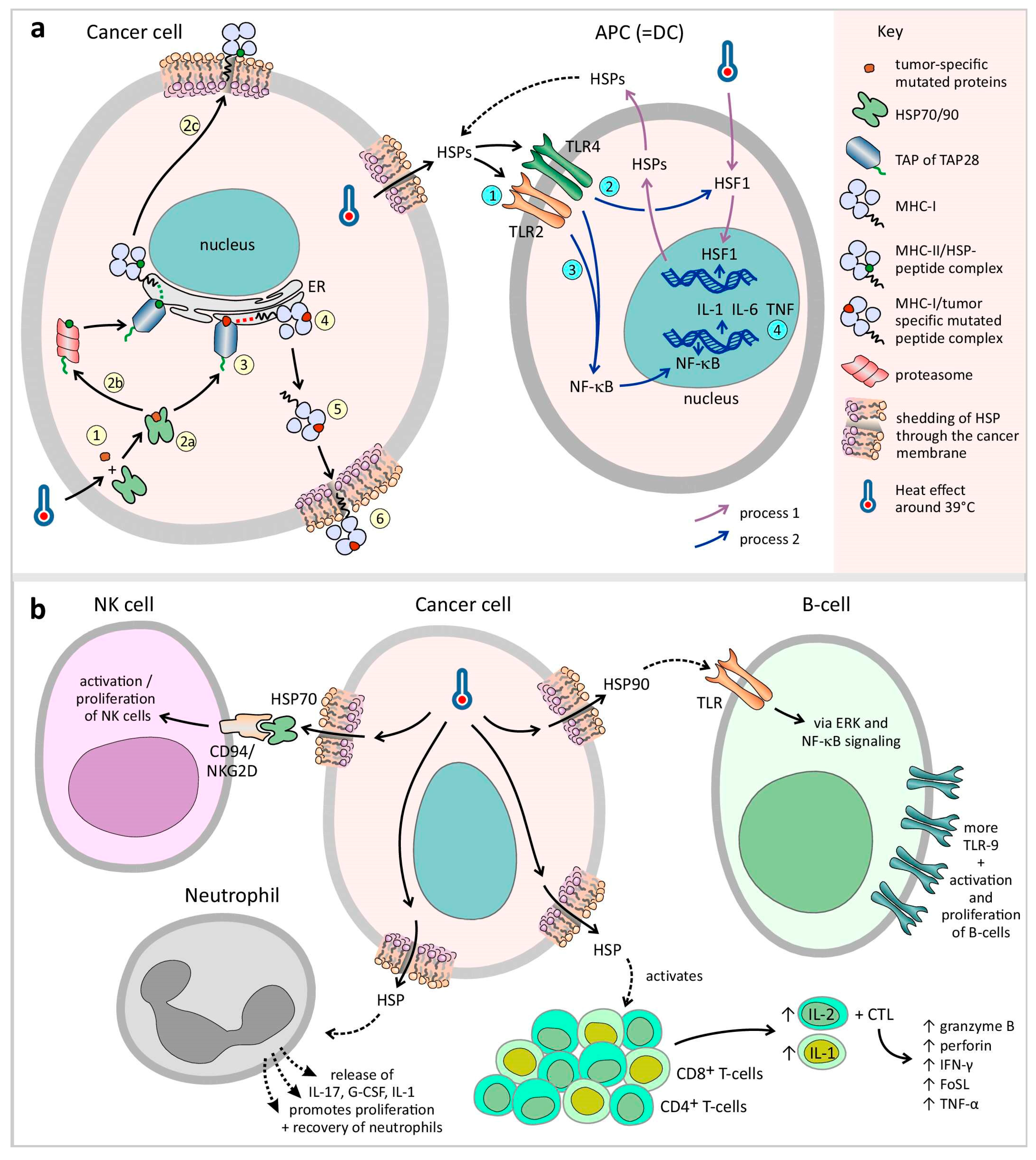
3.1.2. Central Role of Heat Shock Proteins (HSPs)
3.1.3. Antigen-Presenting Cells
Dendritic Cells (DCs)
Macrophages
3.1.4. Effector Cells
NK Cells
T Cells
B Cells
Neutrophils
3.1.5. Cytokines
IL-6
3.1.6. Immune Cell Trafficking
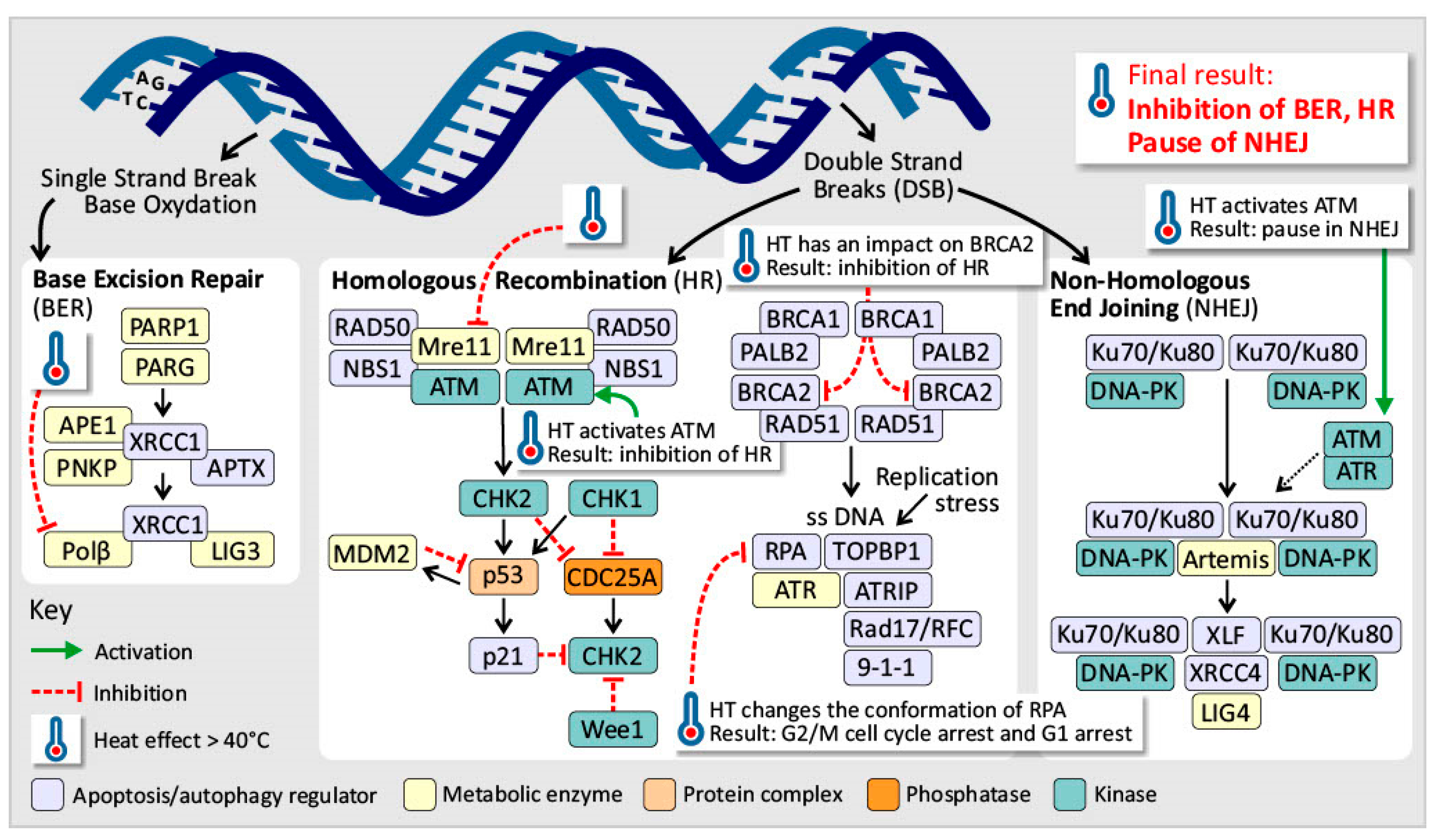
3.2. Genome Instability and Mutation
Induced Hyperthermia Disrupts the DNA Repair Mechanisms
3.3. Resisting Cell Death
Induced Hyperthermia Modulates Cell Death Pathways
3.4. Deregulating Cellular Energetics
Induced Hyperthermia Disrupts Cancer Cell Metabolism
3.5. Inducing Angiogenesis
Induced Hyperthermia Exerts Dual Effects on Tumor Angiogenesis
3.6. Activating Invasion and Metastasis
Induced Hyperthermia Has a Dual Role in the Process of Cancer Metastasis, Addressing the Protumoral Effect
3.7. Enabling Replicative Immortality
Induced Hyperthermia Supports Replicative Immortality of Cancer Cells
4. Discussion
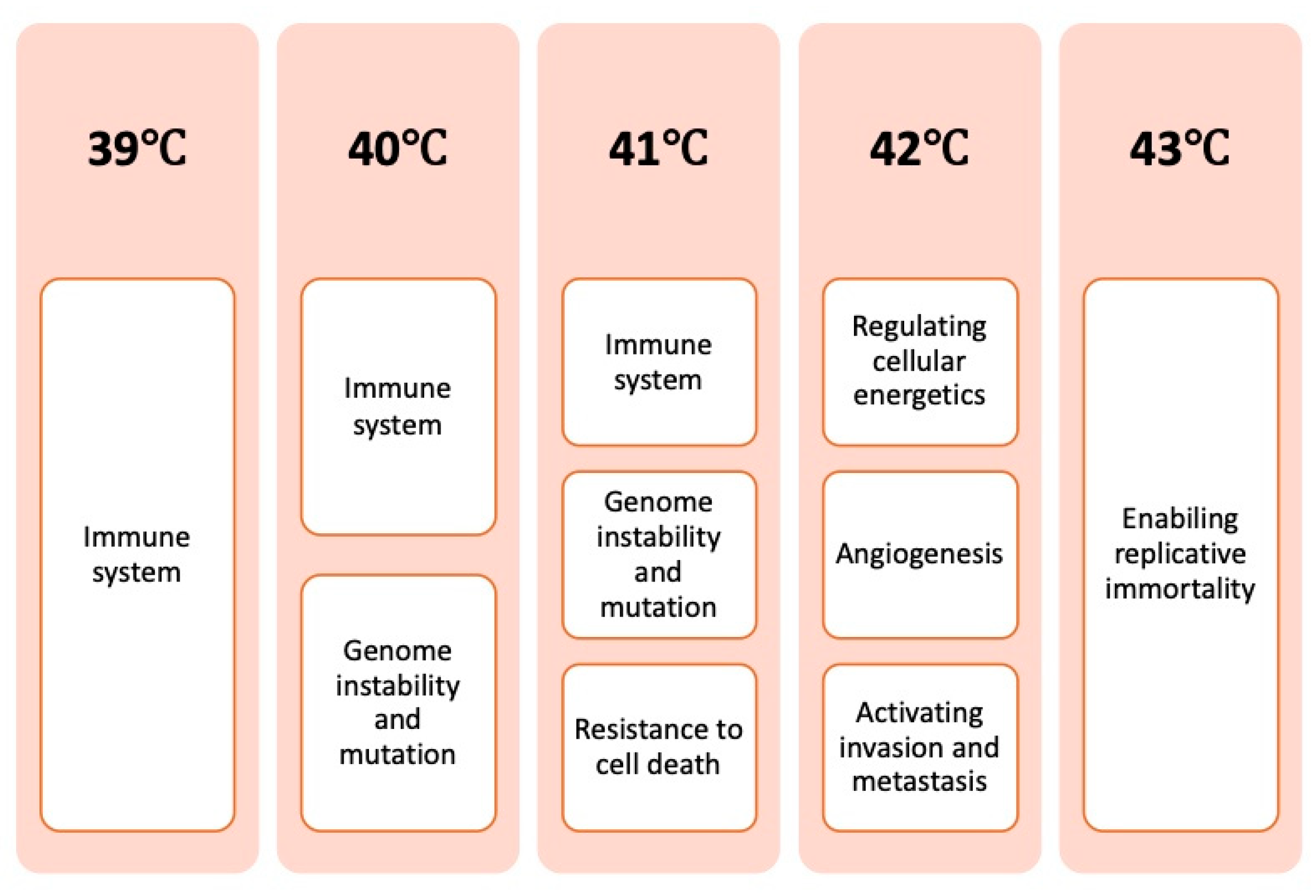
4.1. Temperature-Dependent Effects
4.2. Clinical Translation and Future Directions
4.3. Critical Discussion of the Reviewed Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 53BP1 | p53-binding protein 1 |
| AIF | apoptosis-inducing factor |
| Akt, p38, ERK | antiapoptotic pathways |
| AMPK | AMP-activated protein kinase |
| Apaf-1 | apoptotic protease-activating factor 1 |
| APE1 | apurinic/Apyrimidinic Endonuclease 1 |
| APCs | antigen-presenting cells |
| ATM | ataxia–telangiectasia Mutated |
| ATR | ataxia–telangiectasia and Rad3-related protein kinase |
| ATP | adenosine triphosphate |
| BAG3 | Bcl-2-associated athanogene 3 |
| Bax, Bim, Puma | proapoptotic factors |
| Bcl-2, Bcl-xL | antiapoptotic proteins |
| BER | Base Excision Repair |
| Bid, Bim, Bmf | BH3 proteins |
| BRCA | human tumor suppressor gene |
| CCL | chemokine (C-C motif) ligand |
| CCR | chemokine receptor |
| CD | cluster of differentiation |
| Chk1 | checkpoint kinase 1 |
| CTL | cytotoxic T lymphocytes |
| CXCL | chemokine (C-X-C motif) ligand 1 |
| DAMP | damage-associated molecular pattern |
| DCs | dendritic cells |
| DDR | DNA damage response |
| DNA | ceoxyribonucleic acid |
| DSBs | double-strand breaks |
| EMT | epithelial–mesenchymal Transition |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| FancD2 | Fanconi anemia group D2 protein |
| Fas | death receptor |
| FasL | fas ligand |
| FoxP3 | forkhead box P3 |
| G-CSF | granulocyte colony-stimulating factor |
| G1 cell cycle | gap 1 phase |
| G2 cell cycle | gap 2 phase |
| Gb3 | globotriaosylceramide |
| gp96 | glycoprotein 96 |
| HIF | hypoxia-inducible factor |
| HIFU | high-intensity focused ultrasound |
| HLA | human leukocyte antigens |
| HMGB1 | high mobility group box 1 |
| HR | homologous recombination |
| HSE | heat shock response element |
| HSF-1 | heat shock factor 1 |
| HSPB11 | heat shock protein family B (small), member 11 |
| HSPs | heat shock proteins |
| hTERT | human telomerase reverse transcriptase |
| HT | hyperthermia |
| ICAM | intercellular adhesion molecule |
| IFN | interferon |
| IGFBP | insulin-like growth factor binding protein |
| Igfl/2 | survival signals |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LAK | lymphokine-activated killer cells |
| Lox-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| LPS | lipopolysaccharide endotoxins |
| M cell cycle | mitotic phase |
| MDSCs | myeloid-derived suppressor cells |
| MDC1 | mediator of DNA damage checkpoint protein 1 |
| MEKK1 | mitogen-activated protein kinase kinase kinase |
| MHC | major histocompatibility complex |
| MnSOD | manganese superoxide dismutase |
| MOMP | mitochondrial outer membrane permeabilization |
| MRE11 | meiotic recombination 11 homolog A |
| MRN | protein complex consisting of Mre11, Rad50 and Nbs1 |
| MTA-1 | metastasis-associated protein 1 |
| mGFP-GPI | glycosylphosphatidylinositol-anchored monomeric GFP |
| mp53 | mutated p53 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-kB | nuclear factor-kappa B |
| NHEJ | nonhomologous end joining |
| NK | natural killer cells |
| NKG2D | natural killer group 2D |
| NO | nitric oxide |
| PAI-1 | plasminogen activator inhibitor-1 |
| PDT | photodynamic therapy |
| p-H2AX | phosphorylation of H2AX |
| PKC | protein kinase C |
| PPP | pentose phosphate pathway |
| PS | phosphatidylserine |
| Rad | DNA repair and recombination protein |
| RAIDD | RIP-associated ICH-1/CED-3 homologous protein with a death domain |
| RBC | red blood cells |
| ROS | reactive oxygen species |
| RPA | replication protein A |
| RT | radiotherapy |
| sCLU | clusterin |
| SICD | stress-induced apoptosis-like cell death |
| SIa1CD | stress-induced apoptosis-like cell death |
| S cell cycle | synthesis phase |
| SOD-1 | superoxide dismutase 1 |
| SR-A | scavenger receptor A |
| SSBS | single-strand breaks |
| STAT | signal transducers and activators of transcription |
| TAP | transporter associated with antigen processing |
| TGF-β | transforming growth factor beta |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| TP53 | tumor protein p53 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| VCAM | vascular cell adhesion protein 1 |
| WAF1 | cyclin-dependent kinase inhibitor 1 |
| WBHT | whole-body hyperthermia |
| wtp53 | Wild-type protein 53 |
| XRCC | X-ray repair cross-complementing protein 1 |
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hegyi, G.; Szasz, O.; Szasz, A. Oncothermia: A New Paradigm and Promising Method in Cancer Therapies. Acupunct. Electro-Ther. Res. 2013, 38, 161–197. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Roussakow, S. The History of Hyperthermia Rise and Decline. Conf. Pap. Med. 2013, 2013, 1–40. [Google Scholar] [CrossRef]
- Vertrees, R.A.; Leeth, A.; Girouard, M.; Roach, J.D.; Zwischenberger, J.B. Whole-Body Hyperthermia: A Review of Theory, Design and Application. Perfusion 2002, 17, 279–290. [Google Scholar] [CrossRef]
- Wylleman, B.; Brancato, L.; Gorbaslieva, I.; van Zwol, E.; Mori da Cunha, M.G.M.C.; Benoit, J.; Tierny, D.; Vueghs, P.; Van den Bossche, J.; Rudenko, O.; et al. Tolerability of Long-Term Temperature Controlled Whole-Body Thermal Treatment in Advanced Cancer-Bearing Dogs. Int. J. Hyperth. 2022, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Jung, H. A Generalized Concept for Cell Killing by Heat. Radiat. Res. 1986, 106, 56–72. [Google Scholar] [CrossRef]
- Jordan, B.F.; Sonveaux, P. Targeting Tumor Perfusion and Oxygenation to Improve the Outcome of Anticancer Therapy. Front. Pharmacol. 2012, 3, 22593. [Google Scholar] [CrossRef]
- Habash, R.W.Y.; Krewski, D.; Bansal, R.; Alhafid, H.T. Principles, Applications, Risks and Benefits of Therapeutic Hyperthermia. Front. Biosci.-Elite 2011, E3, 1169–1181. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Swann, J.B.; Koebel, C.M.; Schreiber, R.D.; Smyth, M.J. Immune-Mediated Dormancy: An Equilibrium with Cancer. J. Leukoc. Biol. 2008, 84, 988–993. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer Immunoediting from Immune Surveillance to Immune Escape. Immunology 2007, 121, 1–14. [Google Scholar] [CrossRef]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.H. Immune Infiltration in Human Tumors: A Prognostic Factor That Should Not Be Ignored. Oncogene 2010, 29, 1093–1102. [Google Scholar] [CrossRef]
- Nelson, B.H. The Impact of T-Cell Immunity on Ovarian Cancer Outcomes. Immunol. Rev. 2008, 222, 101–116. [Google Scholar] [CrossRef]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of Lymphoidlike Stroma and Immune Escape by Tumors That Express the Chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Weiss, E.-M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and New Facts about Hyperthermia-Induced Modulations of the Immune System. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Dieing, A.; Ahlers, O.; Hildebrandt, B.; Kerner, T.; Tamm, I.; Possinger, K.; Wust, P. The Effect of Induced Hyperthermia on the Immune System. Prog. Brain Res. 2007, 162, 137–152. Available online: http://sfxit.ugent.be/ugent?sid=EMBASE&issn=00796123&id=doi:10.1016%2FS0079-6123%2806%2962008-6&atitle=The+effect+of+induced+hyperthermia+on+the+immune+system&stitle=Prog.+Brain+Res.&title=Progress+in+Brain+Research&volume=162&issue=&spage=137&epage=152&aulast=Dieing&aufirst=Annette&auinit=A.&aufull=Dieing+A.&coden=PBRRA&isbn=volume9780444519269&pages=137-152&date=2007&auinit1=A&auinitm= (accessed on 22 August 2022). [CrossRef]
- Zhang, H.-G.; Mehta, K.; Cohen, P.; Guha, C. Hyperthermia on Immune Regulation: A Temperature’s Story. Cancer Lett. 2008, 271, 191–204. [Google Scholar] [CrossRef]
- Baronzio, G.; Gramaglia, A.; Fiorentini, G. Hyperthermia and Immunity. A Brief Overview. In Vivo 2006, 20, 689–695. [Google Scholar]
- Bastianpillai, C.; Petrides, N.; Shah, T.; Guillaumier, S.; Ahmed, H.U.; Arya, M. Harnessing the Immunomodulatory Effect of Thermal and Non-Thermal Ablative Therapies for Cancer Treatment. Tumour Biol. 2015, 36, 9137–9146. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Fisher, D.T.; Skitzki, J.J.; Chen, Q. Targeted Regulation of a Lymphocyte-Endothelial-Interleukin-6 Axis by Thermal Stress. Int. J. Hyperth. 2008, 24, 67–78. [Google Scholar] [CrossRef]
- Van Dieren, L.; Quisenaerts, T.; Licata, M.; Beddok, A.; Lellouch, A.G.; Ysebaert, D.; Saldien, V.; Peeters, M.; Gorbaslieva, I. Combined Radiotherapy and Hyperthermia: A Systematic Review of Immunological Synergies for Amplifying Radiation-Induced Abscopal Effects. Cancers 2024, 16, 3656. [Google Scholar] [CrossRef]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune Biological Rationales for the Design of Combined Radio- and Immunotherapies. Cancer Immunol. Immunother. 2020, 69, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Son, B.; Park, G.; Kim, H.; Kang, H.; Jeon, J.; Youn, H.; Youn, B. Immunogenic Effect of Hyperthermia on Enhancing Radiotherapeutic Efficacy. Int. J. Mol. Sci. 2018, 19, 2795. [Google Scholar] [CrossRef]
- Bai, J.F.; Liu, P.; Xu, L.X. Recent Advances in Thermal Treatment Techniques and Thermally Induced Immune Responses against Cancer. IEEE Trans. Biomed. Eng. 2014, 61, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Ruckert, M.; Deloch, L.; Ruhle, P.F.; Derer, A.; Fietkau, R.; Gaipl, U.S. Immunomodulation by Ionizing Radiation-Impact for Design of Radio-Immunotherapies and for Treatment of Inflammatory Diseases. Immunol. Rev. 2017, 280, 231–248. [Google Scholar] [CrossRef]
- Weiss, E.-M.; Frey, B.; Rodel, F.; Herrmann, M.; Schlucker, E.; Voll, R.E.; Fietkau, R.; Gaipl, U.S. Ex Vivo- and in Vivo-Induced Dead Tumor Cells as Modulators of Antitumor Responses. Ann. N. Y. Acad. Sci. 2010, 1209, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Schildkopf, P.; Ott, O.J.; Frey, B.; Wadepohl, M.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Biological Rationales and Clinical Applications of Temperature Controlled Hyperthermia–Implications for Multimodal Cancer Treatments. Curr. Med. Chem. 2010, 17, 3045–3057. [Google Scholar] [CrossRef]
- Ito, A.; Honda, H.; Kobayashi, T. Cancer Immunotherapy Based on Intracellular Hyperthermia Using Magnetite Nanoparticles: A Novel Concept of “Heat-Controlled Necrosis” with Heat Shock Protein Expression. Cancer Immunol. Immunother. 2006, 55, 320–328. [Google Scholar] [CrossRef]
- Manjili, M.H.; Wang, X.Y.; Park, J.; Macdonald, I.J.; Li, Y.; Van Schie, R.C.A.A.; Subjeck, J.R. Cancer Immunotherapy: Stress Proteins and Hyperthermia. Int. J. Hyperth. 2002, 18, 506–520. [Google Scholar] [CrossRef]
- Skitzki, J.J.; Repasky, E.A.; Evans, S.S. Hyperthermia as an Immunotherapy Strategy for Cancer. Curr. Opin. Investig. Drugs 2009, 10, 550–558. [Google Scholar]
- Multhoff, G.; Habl, G.; Combs, S.E. Rationale of Hyperthermia for Radio(Chemo)Therapy and Immune Responses in Patients with Bladder Cancer: Biological Concepts, Clinical Data, Interdisciplinary Treatment Decisions and Biological Tumour Imaging. Int. J. Hyperth. 2016, 32, 455–463. [Google Scholar] [CrossRef]
- Coss, R.A. Inhibiting Induction of Heat Shock Proteins as a Strategy to Enhance Cancer Therapy. Int. J. Hyperth. 2005, 21, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Morimoto, R.I. Role of the Heat Shock Response and Molecular Chaperones in Oncogenesis and Cell Death. J. Natl. Cancer Inst. 2000, 92, 1564–1572. [Google Scholar] [CrossRef]
- Lauber, K.; Brix, N.; Ernst, A.; Hennel, R.; Krombach, J.; Anders, H.; Belka, C. Targeting the Heat Shock Response in Combination with Radiotherapy: Sensitizing Cancer Cells to Irradiation-Induced Cell Death and Heating up Their Immunogenicity. Cancer Lett. 2015, 368, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The Cellular and Molecular Basis of Hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Keisari, Y. Tumor Abolition and Antitumor Immunostimulation by Physico-Chemical Tumor Ablation. Front. Biosci. (Landmark Ed.) 2017, 22, 310–347. [Google Scholar] [CrossRef]
- Milani, V.; Noessner, E. Effects of Thermal Stress on Tumor Antigenicity and Recognition by Immune Effector Cells. Cancer Immunol. Immunother. 2006, 55, 312–319. [Google Scholar] [CrossRef]
- Appenheimer, M.M.; Evans, S.S. Temperature and Adaptive Immunity. Handb. Clin. Neurol. 2018, 156, 397–415. [Google Scholar] [CrossRef]
- Milani, V.; Noessner, E.; Ghose, S.; Kuppner, M.; Ahrens, B.; Scharner, A.; Gastpar, R.; Issels, R.D. Heat Shock Protein 70: Role in Antigen Presentation and Immune Stimulation. Int. J. Hyperth. 2002, 18, 563–575. [Google Scholar] [CrossRef]
- Noessner, E. Thermal Stress-Related Modulation of Tumor Cell Physiology and Immune Responses. Cancer Immunol. Immunother. 2006, 55, 289–291. [Google Scholar] [CrossRef]
- Payne, M.; Bossmann, S.H.; Basel, M.T. Direct Treatment versus Indirect: Thermo-Ablative and Mild Hyperthermia Effects. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2020, 12, e1638. [Google Scholar] [CrossRef]
- Muthana, M.; Multhoff, G.; Pockley, A.G. Tumour Infiltrating Host Cells and Their Significance for Hyperthermia. Int. J. Hyperth. 2010, 26, 247–255. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J. Heating the Patient: A Promising Approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Overgaard, J.; Nielsen, O.S. The Importance of Thermotolerance for the Clinical Treatment with Hyperthermia. Radiother. Oncol. 1983, 1, 167–178. [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Fiering, S. Local Tumour Hyperthermia as Immunotherapy for Metastatic Cancer. Int. J. Hyperth. 2014, 30, 531–539. [Google Scholar] [CrossRef]
- Dayanc, B.E.; Beachy, S.H.; Ostberg, J.R.; Repasky, E.A. Dissecting the Role of Hyperthermia in Natural Killer Cell Mediated Anti-Tumor Responses. Int. J. Hyperth. 2008, 24, 41–56. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Fanelli, M.A.; Cuello-Carrion, F.D.; Castro, G.N. Heat Shock Proteins in Prostate Cancer: From Tumorigenesis to the Clinic. Int. J. Hyperth. 2010, 26, 737–747. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Ciocca, D.R. Heat Shock Proteins: Stress Proteins with Janus-like Properties in Cancer. Int. J. Hyperth. 2008, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, G.M.; Kaur, P.; Asea, A. Role of Human and Mouse HspB1 in Metastasis. Curr. Mol. Med. 2012, 12, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J.; Stevenson, M.A.; Murshid, A. Cellular and Molecular Chaperone Fusion Vaccines: Targeting Resistant Cancer Cell Populations. Int. J. Hyperth. 2013, 29, 376–379. [Google Scholar] [CrossRef]
- Mahmood, J.; Shukla, H.D.; Soman, S.; Samanta, S.; Singh, P.; Kamlapurkar, S.; Saeed, A.; Amin, N.P.; Vujaskovic, Z. Immunotherapy, Radiotherapy, and Hyperthermia: A Combined Therapeutic Approach in Pancreatic Cancer Treatment. Cancers 2018, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, M.; Ren, X.; Tang, Y.; Liang, X. Local Hyperthermia in Head and Neck Cancer: Mechanism, Application and Advance. Oncotarget 2016, 7, 57367–57378. [Google Scholar] [CrossRef] [PubMed]
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature Matters! And Why It Should Matter to Tumor Immunologists. Cancer Immunol. Res. 2013, 1, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Deng, Z.S.; Liu, J. A Review of Hyperthermia Combined with Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in Combined Treatment of Cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Pennacchioli, E.; Fiore, M.; Gronchi, A. Hyperthermia as an Adjunctive Treatment for Soft-Tissue Sarcoma. Expert. Rev. Anticancer Ther. 2009, 9, 199–210. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lee, C.-T.; Ashcraft, K.A. The Future of Biology in Driving the Field of Hyperthermia. Int. J. Hyperth. 2016, 32, 4–13. [Google Scholar] [CrossRef]
- Lee, C.-T.; Mace, T.; Repasky, E.A. Hypoxia-Driven Immunosuppression: A New Reason to Use Thermal Therapy in the Treatment of Cancer? Int. J. Hyperth. 2010, 26, 232–246. [Google Scholar] [CrossRef]
- Liso, A.; Capitanio, N.; Gerli, R.; Conese, M. From Fever to Immunity: A New Role for IGFBP-6? J. Cell Mol. Med. 2018, 22, 4588–4596. [Google Scholar] [CrossRef]
- Ostberg, J.R.; Repasky, E.A. Emerging Evidence Indicates That Physiologically Relevant Thermal Stress Regulates Dendritic Cell Function. Cancer Immunol. Immunother. 2006, 55, 292–298. [Google Scholar] [CrossRef]
- Tzeng, A.; Tzeng, T.H.; Vasdev, S.; Korth, K.; Healey, T.; Parvizi, J.; Saleh, K.J. Treating Periprosthetic Joint Infections as Biofilms: Key Diagnosis and Management Strategies. Diagn. Microbiol. Infect. Dis. 2015, 81, 192–200. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress Biomarkers and Proteomics Alteration to Thermal Stress in Ruminants: A Review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef]
- Rylander, M.N.; Feng, Y.; Bass, J.; Diller, K.R. Thermally Induced Injury and Heat-Shock Protein Expression in Cells and Tissues. Ann. N. Y. Acad. Sci. 2005, 1066, 222–242. [Google Scholar] [CrossRef] [PubMed]
- Mikucki, M.E.; Fisher, D.T.; Ku, A.W.; Appenheimer, M.M.; Muhitch, J.B.; Evans, S.S. Preconditioning Thermal Therapy: Flipping the Switch on IL-6 for Anti-Tumour Immunity. Int. J. Hyperth. 2013, 29, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. ScienceDirect-Molecular Cell: The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef]
- Kastan, M.B. DNA Damage Responses: Mechanisms and Roles in Human Disease. Mol. Cancer Res. 2008, 6, 517–524. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lora-Michiels, M.; Viglianti, B.L.; Dewey, W.C.; Repacholi, M. Carcinogenic Effects of Hyperthermia. Int. J. Hyperth. 2003, 19, 236–251. [Google Scholar] [CrossRef]
- Eppink, B.; Krawczyk, P.M.; Stap, J.; Kanaar, R. Hyperthermia-Induced DNA Repair Deficiency Suggests Novel Therapeutic Anti-Cancer Strategies. Int. J. Hyperth. 2012, 28, 509–517. [Google Scholar] [CrossRef]
- Turner, T.; Caspari, T. When Heat Casts a Spell on the DNA Damage Checkpoints. Open Biol. 2014, 4, 140008. [Google Scholar] [CrossRef] [PubMed]
- Youssef, I.; Zulfiqar, H.; Amin, N.P. Hyperthermia for Chest Wall Recurrence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Roti Roti, J.L. Cellular Responses to Hyperthermia (40–46 Degrees C): Cell Killing and Molecular Events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Ohnishi, T. The Role of the P53 Molecule in Cancer Therapies with Radiation and/or Hyperthermia. J. Cancer Res. Ther. 2005, 1, 147–150. [Google Scholar] [CrossRef]
- Ahmed, K.; Tabuchi, Y.; Kondo, T. Hyperthermia: An Effective Strategy to Induce Apoptosis in Cancer Cells. Apoptosis 2015, 20, 1411–1419. [Google Scholar] [CrossRef]
- Walther, W.; Stein, U. Heat-Responsive Gene Expression for Gene Therapy. Adv. Drug Deliv. Rev. 2009, 61, 641–649. [Google Scholar] [CrossRef]
- Mantso, T.; Goussetis, G.; Franco, R.; Botaitis, S.; Pappa, A.; Panayiotidis, M. Effects of Hyperthermia as a Mitigation Strategy in DNA Damage-Based Cancer Therapies. Semin. Cancer Biol. 2016, 37–38, 96–105. [Google Scholar] [CrossRef]
- van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving Efficacy of Hyperthermia in Oncology by Exploiting Biological Mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef]
- Pandita, T.K.; Pandita, S.; Bhaumik, S.R. Molecular Parameters of Hyperthermia for Radiosensitization. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 235–251. [Google Scholar] [CrossRef]
- Pandita, T.K. Role of HSPs and Telomerase in Radiotherapy. Int. J. Hyperth. 2005, 21, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R. Realistic Biological Approaches for Improving Thermoradiotherapy. Int. J. Hyperth. 2016, 32, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, G.; Wu, W.; Wang, M. DNA Double Strand Break Repair Inhibition as a Cause of Heat Radiosensitization: Re-Evaluation Considering Backup Pathways of NHEJ. Int. J. Hyperth. 2008, 24, 17–29. [Google Scholar] [CrossRef]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L.H. Hallmarks of Hyperthermia in Driving the Future of Clinical Hyperthermia as Targeted Therapy: Translation into Clinical Application. Int. J. Hyperth. 2016, 32, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Zaidi, S.F.; Rehman, M.U.; Rehman, R.; Kondo, T. Hyperthermia and Protein Homeostasis: Cytoprotection and Cell Death. J. Therm. Biol. 2020, 91, 102615. [Google Scholar] [CrossRef]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 Apoptotic Switch in Cancer Development and Therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.; Littlewood, T. A Matter of Life and Cell Death. Science 1998, 281, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Cepero, E.; Evan, G. Intrinsic Tumour Suppression. Nature 2004, 432, 307–315. [Google Scholar] [CrossRef]
- Willis, S.N.; Adams, J.M. Life in the Balance: How BH3-Only Proteins Induce Apoptosis. Curr. Opin. Cell Biol. 2005, 17, 617–625. [Google Scholar] [CrossRef]
- Signaling, C. Autophagy Signaling Pathway Map. Cell Signal. 2010. [Google Scholar]
- Mizushima, N. Autophagy: Process and Function. Genes. Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- White, E.; DiPaola, R.S. The Double-Edged Sword of Autophagy Modulation in Cancer. Clin. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Thompson, C.B. The Roles of Therapy-Induced Autophagy and Necrosis in Cancer Treatment. Clin. Cancer Res. 2007, 13, 7271–7279. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Ahmed, K.; Zaidi, S.F. Treating Cancer with Heat: Hyperthermia as Promising Strategy to Enhance Apoptosis. J. Pak. Med. Assoc. 2013, 63, 504–508. [Google Scholar] [PubMed]
- Peer, A.J.; Grimm, M.J.; Zynda, E.R.; Repasky, E.A. Diverse Immune Mechanisms May Contribute to the Survival Benefit Seen in Cancer Patients Receiving Hyperthermia. Immunol. Res. 2010, 46, 137–154. [Google Scholar] [CrossRef]
- Kejík, Z.; Jakubek, M.; Kaplánek, R.; Králová, J.; Mikula, I.; Martásek, P.; Král, V. Epigenetic Agents in Combined Anticancer Therapy. Future Med. Chem. 2018, 10, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Ohnishi, T. Heat-Induced P53-Dependent Signal Transduction and Its Role in Hyperthermic Cancer Therapy. Int. J. Hyperth. 2001, 17, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Roufayel, R.; Kadry, S. Expression of MiR-23a by Apoptotic Regulators in Human Cancer: A Review. Cancer Biol. Ther. 2017, 18, 269–276. [Google Scholar] [CrossRef]
- Burattini, S.; Battistelli, M.; Falcieri, E. Morpho-Functional Features of in-Vitro Cell Death Induced by Physical Agents. Curr. Pharm. Des. 2010, 16, 1376–1386. [Google Scholar] [CrossRef]
- Komarova, E.A.; Gudkov, A. V Chemoprotection from P53-Dependent Apoptosis: Potential Clinical Applications of the P53 Inhibitors. Biochem. Pharmacol. 2001, 62, 657–667. [Google Scholar] [CrossRef]
- Milleron, R.S.; Bratton, S.B. “Heated” Debates in Apoptosis. Cell Mol. Life Sci. 2007, 64, 2329–2333. [Google Scholar] [CrossRef]
- Ghaffari, H.; Beik, J.; Talebi, A.; Mahdavi, S.R.; Abdollahi, H. New Physical Approaches to Treat Cancer Stem Cells: A Review. Clin. Transl. Oncol. 2018, 20, 1502–1521. [Google Scholar] [CrossRef]
- Zhang, Y.; Calderwood, S.K. Autophagy, Protein Aggregation and Hyperthermia: A Mini-Review. Int. J. Hyperth. 2011, 27, 409–414. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Zhou, X.-D.; Yang, J.; Chen, L.-X.; Ran, D.-H. Inhibition of Autophagy Enhances Heat-Induced Apoptosis in Human Non-Small Cell Lung Cancer Cells through ER Stress Pathways. Arch. Biochem. Biophys. 2016, 607, 55–66. [Google Scholar] [CrossRef]
- Baust, J.M.; Rabin, Y.; Polascik, T.J.; Santucci, K.L.; Snyder, K.K.; Van Buskirk, R.G.; Baust, J.G. Defeating Cancers’ Adaptive Defensive Strategies Using Thermal Therapies: Examining Cancer’s Therapeutic Resistance, Ablative, and Computational Modeling Strategies as a Means for Improving Therapeutic Outcome. Technol. Cancer Res. Treat. 2018, 17, 1533033818762207. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, X.; Jin, L.; Zhou, R.; Liu, S.; Xu, N.; Liao, D.J. Necrosis, and Then Stress Induced Necrosis-like Cell Death, but Not Apoptosis, Should Be the Preferred Cell Death Mode for Chemotherapy: Clearance of a Few Misconceptions. Oncoscience 2014, 1, 407–422. [Google Scholar] [CrossRef]
- Jones, R.G.; Thompson, C.B. Tumor Suppressors and Cell Metabolism: A Recipe for Cancer Growth. Genes. Dev. 2009, 23, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Pouyssegur, J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Dewhirst, M.W. Tumor Metabolism of Lactate: The Influence and Therapeutic Potential for MCT and CD147 Regulation. Futur. Oncol. 2009, 6, 127–148. [Google Scholar] [CrossRef]
- Hardee, M.; Dewhirst, M.; Agarwal, N.; Sorg, B. Novel Imaging Provides New Insights into Mechanisms of Oxygen Transport in Tumors. Curr. Mol. Med. 2009, 9, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Piazena, H.; Notter, M.; Thomsen, A.R.; Grosu, A.L.; Scholkmann, F.; Pockley, A.G.; Multhoff, G. From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy. Cancers 2023, 15, 1394. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.W.; Kelleher, D.K. Blood Flow and Associated Pathophysiology of Uterine Cervix Cancers: Characterisation and Relevance for Localised Hyperthermia. Int. J. Hyperth. 2012, 28, 518–527. [Google Scholar] [CrossRef]
- Vaupel, P.W.; Kelleher, D.K. Pathophysiological and Vascular Characteristics of Tumours and Their Importance for Hyperthermia: Heterogeneity Is the Key Issue. Int. J. Hyperth. 2010, 26, 211–223. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Frank, J. Antioxidants in Cancer Therapy: Is There a Rationale to Recommend Antioxidants during Cancer Therapy? Biofactors 2003, 17, 229–240. [Google Scholar] [CrossRef]
- Amendola, R.; Cervelli, M.; Tempera, G.; Fratini, E.; Varesio, L.; Mariottini, P.; Agostinelli, E. Spermine Metabolism and Radiation-Derived Reactive Oxygen Species for Future Therapeutic Implications in Cancer: An Additive or Adaptive Response. Amino Acids 2014, 46, 487–498. [Google Scholar] [CrossRef]
- Gius, D.; Mattson, D.; Bradbury, C.M.; Smart, D.K.; Spitz, D.R. Thermal Stress and the Disruption of Redox-Sensitive Signalling and Transcription Factor Activation: Possible Role in Radiosensitization. Int. J. Hyperth. 2004, 20, 213–223. [Google Scholar] [CrossRef]
- Horsman, M.R. Angiogenesis and Vascular Targeting: Relevance for Hyperthermia. Int. J. Hyperth. 2008, 24, 57–65. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.M.; Jamshidi-Parsian, A.; Song, C.W. Mild Temperature Hyperthermia and Radiation Therapy: Role of Tumour Vascular Thermotolerance and Relevant Physiological Factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Csoboz, B.; Balogh, G.E.; Kusz, E.; Gombos, I.; Peter, M.; Crul, T.; Gungor, B.; Haracska, L.; Bogdanovics, G.; Torok, Z.; et al. Membrane Fluidity Matters: Hyperthermia from the Aspects of Lipids and Membranes. Int. J. Hyperth. 2013, 29, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hokland, S.L.; Nielsen, T.; Busk, M.; Horsman, M.R. Imaging Tumour Physiology and Vasculature to Predict and Assess Response to Heat. Int. J. Hyperth. 2010, 26, 264–272. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The Angiogenic Switch in Carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Pathways Mediating VEGF-Independent Tumor Angiogenesis. Cytokine Growth Factor. Rev. 2010, 21, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Crezee, H.; van Leeuwen, C.M.; Oei, A.L.; Stalpers, L.J.A.; Bel, A.; Franken, N.A.; Kok, H.P. Thermoradiotherapy Planning: Integration in Routine Clinical Practice. Int. J. Hyperth. 2016, 32, 41–49. [Google Scholar] [CrossRef]
- Horsman, M.R. Tissue Physiology and the Response to Heat. Int. J. Hyperth. 2006, 22, 197–203. [Google Scholar] [CrossRef]
- Knisely, J.P.S.; Rockwell, S. Importance of Hypoxia in the Biology and Treatment of Brain Tumors. Neuroimaging Clin. N. Am. 2002, 12, 525–536. [Google Scholar] [CrossRef]
- Curtis, L.T.; Frieboes, H.B. The Tumor Microenvironment as a Barrier to Cancer Nanotherapy. Adv. Exp. Med. Biol. 2016, 936, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Dahl, O.; Borkamo, E.D.; Fluge, O. Current Status of Antivascular Therapy and Targeted Treatment in the Clinic. Int. J. Hyperth. 2008, 24, 97–110. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.; Griffin, R.J. Improvement of Tumor Oxygenation by Mild Hyperthermia. Radiat. Res. 2001, 155, 515–528. [Google Scholar] [CrossRef]
- Curtis, K.K.; Wong, W.W.; Ross, H.J. Past Approaches and Future Directions for Targeting Tumor Hypoxia in Squamous Cell Carcinomas of the Head and Neck. Crit. Rev. Oncol. Hematol. 2016, 103, 86–98. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of Increased Tumor Blood Flow and Oxygenation Caused by Mild Temperature Hyperthermia in Tumor Treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef]
- Calderwood, S.K. Heat Shock Proteins in Breast Cancer Progression–a Suitable Case for Treatment? Int. J. Hyperth. 2010, 26, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Hayflick, His Limit, and Cellular Ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomeres and Human Disease: Ageing, Cancer and Beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef]
- Ince, T.A.; Richardson, A.L.; Bell, G.W.; Saitoh, M.; Godar, S.; Karnoub, A.E.; Iglehart, J.D.; Weinberg, R.A. Transformation of Different Human Breast Epithelial Cell Types Leads to Distinct Tumor Phenotypes. Cancer Cell 2007, 12, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Herbert, B.S.; Pan, K.H.; Shay, J.W.; Cohen, S.N. Disparate Effects of Telomere Attrition on Gene Expression during Replicative Senescence of Human Mammary Epithelial Cells Cultured under Different Conditions. Oncogene 2004, 23, 6193–6198. [Google Scholar] [CrossRef]
- Artandi, S.E.; DePinho, R.A. Telomeres and Telomerase in Cancer. Carcinogenesis 2009, 31, 9–18. [Google Scholar] [CrossRef]
- Huaqi, Y.; Bingqi, D.; Yanhui, Z.; Yongkang, M.; Shiming, Z.; Zhenghui, S.; Zheng, D.; Jiangshan, P.; Tiejun, Y. Hyperthermia Inhibits Cellular Function and Induces Immunogenic Cell Death in Renal Cell Carcinoma. BMC Cancer 2023, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.M.; Chocron, A.F.; Smadja, D.M. From Cold to Hot: Mechanisms of Hyperthermia in Modulating Tumor Immunology for Enhanced Immunotherapy. Front. Immunol. 2025, 16, 1487296. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Polónia, B.; Santos, L.L.; Vasconcelos, M.H.; Xavier, C.P.R. Drug Repurposing Opportunities in Pancreatic Ductal Adenocarcinoma. Pharmaceuticals 2021, 14, 280. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbaslieva, I.; Quisenaerts, T.; Bogers, J.J.P.M.; Peeters, M.; Saldien, V.; Ysebaert, D. Temperature-Dependent Effects of Induced Hyperthermia, Including Whole-Body Hyperthermia, on the Hallmarks of Cancer: A Systematic Review. Cancers 2025, 17, 3824. https://doi.org/10.3390/cancers17233824
Gorbaslieva I, Quisenaerts T, Bogers JJPM, Peeters M, Saldien V, Ysebaert D. Temperature-Dependent Effects of Induced Hyperthermia, Including Whole-Body Hyperthermia, on the Hallmarks of Cancer: A Systematic Review. Cancers. 2025; 17(23):3824. https://doi.org/10.3390/cancers17233824
Chicago/Turabian StyleGorbaslieva, Ivana, Tom Quisenaerts, Johannes J. P. M. Bogers, Marc Peeters, Vera Saldien, and Dirk Ysebaert. 2025. "Temperature-Dependent Effects of Induced Hyperthermia, Including Whole-Body Hyperthermia, on the Hallmarks of Cancer: A Systematic Review" Cancers 17, no. 23: 3824. https://doi.org/10.3390/cancers17233824
APA StyleGorbaslieva, I., Quisenaerts, T., Bogers, J. J. P. M., Peeters, M., Saldien, V., & Ysebaert, D. (2025). Temperature-Dependent Effects of Induced Hyperthermia, Including Whole-Body Hyperthermia, on the Hallmarks of Cancer: A Systematic Review. Cancers, 17(23), 3824. https://doi.org/10.3390/cancers17233824





