Bartholin Gland Carcinoma: A State-of-the-Art Review of Epidemiology, Histopathology, Molecular Testing, and Clinical Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Data Synthesis
2.2. Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection and Data Extraction
2.5. Quality Considerations
2.6. Aims
3. Anatomy of the Bartholin Glands
3.1. Morphology
3.2. Histology
3.3. Function
4. Bartholin Gland Carcinoma
4.1. Definition
4.2. Epidemiology
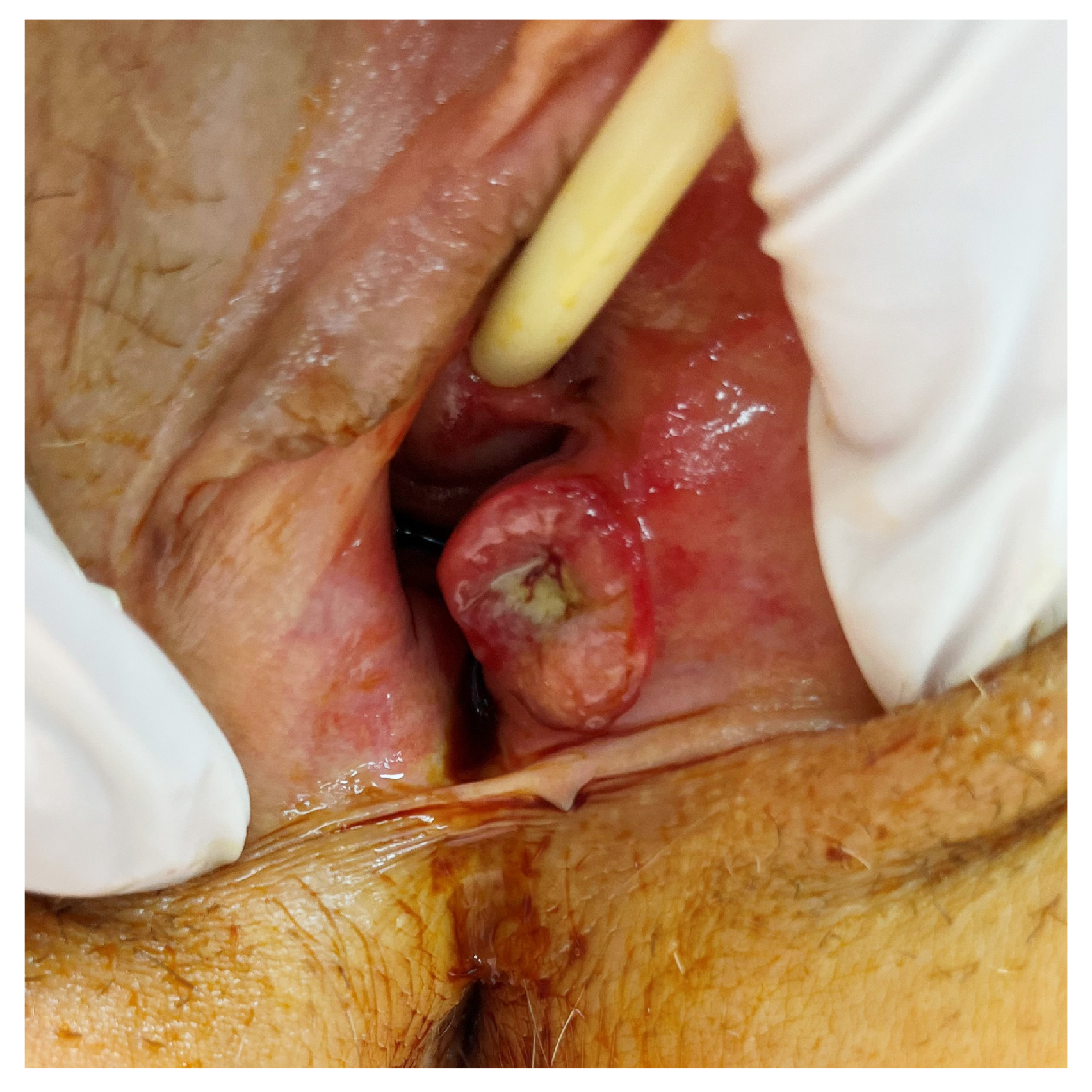
4.3. Clinical Manifestations
4.4. Diagnosis
4.5. Staging
4.6. Differential Diagnosis
5. Histological BGC Subtypes
5.1. Histological Distribution
- SCC: 80 cases (30.7%);
- AdCC: 77 cases (29.6%);
- Adenocarcinoma: 65 cases (25%);
- Transitional cell carcinoma: 7 cases (2.6%);
- Sarcoma: 7 cases (2.6%);
- Other rare subtypes: 38 cases (14.6%)
5.2. Squamous Cell Carcinoma
5.3. Adenocarcinoma
5.4. Intestinal-Type (Cloacogenic) Adenocarcinoma
5.5. Adenoid Cystic Carcinoma
5.6. Transitional Cell Carcinoma
5.7. Epithelial-Myoepithelial Carcinoma
5.8. Neuroendocrine Carcinoma (Small-Cell Type)
5.9. Mixed Tumors
6. Molecular Profiles of BGC
6.1. Squamous Cell BGC
6.2. Adenocarcinoma
6.3. Adenoid Cystic Carcinoma
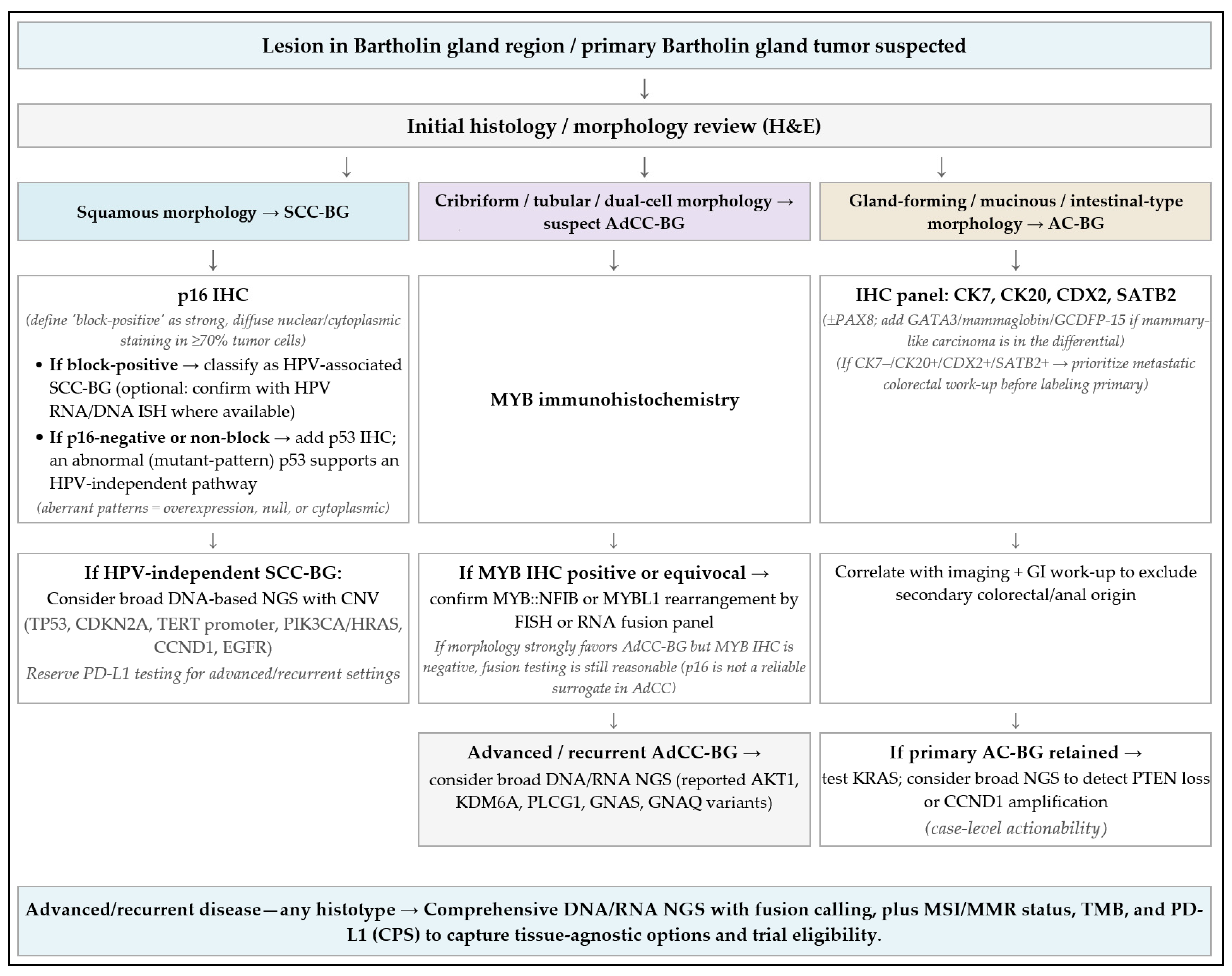
6.4. Testing Algorithm
7. Treatment
7.1. Surgical Treatment of the Primary Tumor
Practical Surgical Considerations
7.2. Surgical Treatment of the Groin
Sentinel Lymph-Node Biopsy (SLNB)
7.3. Radiotherapy Alone
7.3.1. Adjuvant EBRT to the Vulvar Region
7.3.2. Adjuvant EBRT to the Groin
- Micrometastases after SLNB (<2 mm) or isolated tumor cells, as an alternative to groin dissection;
- Positive sentinel-node metastasis >2 mm and/or extracapsular spread, after completion dissection;
- As an alternative to groin dissection in the presence of bulky metastatic nodes;
- When contralateral inguinofemoral nodes are not dissected.
7.3.3. Adjuvant EBRT to the Pelvis
- When groin nodes are metastatic (field typically to the distal iliac chain up to the iliac bifurcation).
- When pelvic nodal metastases are suspected on imaging or pathologically proven (field one level above the highest involved node).
7.3.4. Brachytherapy
7.3.5. Practical Considerations
7.4. Chemotherapy Alone
7.5. Combined Therapy—Chemoradiation
7.6. Targeted Therapies
7.7. Treatment of Metastatic/Recurrent Disease
7.8. Follow-Up
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Preoperative Diagnostic Modality | VSCC | BGC |
|---|---|---|
| Biopsy of the primary tumor | Small biopsy of all suspicious areas—L-5, [116] | Formal en bloc excision—L-5, direct, [4] |
| Evaluation of cervix, vagina and anus, including cytology and HPV test from cervix/vagina | L-4, [116] | L-4, direct [3,5,28] |
| Imaging for pT1a tumors not required | L-3, [116] | L-5, indirect [116] |
| Imaging for all other stages [expert vulvar sonography, CT or PET/CT] | L-3, [116] | L-5, indirect [116] |
| Lymph node status–ultrasound-guided fine-needle aspiration or core needle biopsy | L-3 [6,37,116] | L-5 indirect [36,37,102] |
| MRI in locally advanced tumor with involvement of surrounding tissue | L-4 [116] | L-4, direct [2,3,5] |
| Biopsy of distant metastases | L-5 [116] | L-5, indirect [116] |
References
- Ireland, A.; Francis, K.; Naran, A.; Stewart, C.; Mesbah Ardakani, N. Bartholin gland adenocarcinoma with micropapillary features: A case report with molecular evaluation. Pathology 2023, 55, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Ouldamer, L.; Chraibi, Z.; Arbion, F.; Barillot, I.; Body, G. Bartholin’s gland carcinoma: Epidemiology and therapeutic management. Surg. Oncol. 2013, 22, 117–122. [Google Scholar] [CrossRef]
- Di Donato, V.; Casorelli, A.; Bardhi, E.; Vena, F.; Marchetti, C.; Muzii, L.; Benedetti Panici, P. Bartholin gland cancer. Crit. Rev. Oncol. Hematol. 2017, 117, 1–11. [Google Scholar] [CrossRef]
- Addley, S.; Sadeghi, N.; Smyth, S.L.; Johnson, C.; Damato, S.; Soleymani Majd, H. Bartholin’s gland carcinoma: The diagnostic and management challenges of a rare malignancy—A case report and review of current literature. Transl. Cancer Res. 2023, 12, 201–208. [Google Scholar] [CrossRef]
- Bhalwal, A.B.; Nick, A.M.; Dos Reis, R.; Chen, C.L.; Munsell, M.F.; Ramalingam, P.; Salcedo, M.P.; Ramirez, P.T.; Sood, A.K.; Schmeler, K.M. Carcinoma of the Bartholin Gland: A Review of 33 Cases. Int. J. Gynecol. Cancer 2016, 26, 785–789. [Google Scholar] [CrossRef]
- Cardosi, R.J.; Speights, A.; Fiorica, J.V.; Grendys, E.C., Jr.; Hakam, A.; Hoffman, M.S. Bartholin’s gland carcinoma: A 15-year experience. Gynecol. Oncol. 2001, 82, 247–251. [Google Scholar] [CrossRef]
- Turetta, C.; Mazzeo, R.; Capalbo, G.; Miano, S.; Fruscio, R.; Di Donato, V.; Falcone, F.; Mangili, G.; Pignata, S.; Palaia, I. Management of primary and recurrent Bartholin’s gland carcinoma: A systematic review on behalf of MITO Rare Cancer Group. Tumori 2024, 110, 96–108. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document); Oxford Centre for Evidence-Based Medicine: Oxford, UK, 2011; Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 23 June 2025).
- Perelmuter, S.; Giovannetti, O.; Tomalty, D. Review of the vestibular glands: Functional anatomy, clinical significance, and role in sexual function. Sex. Med. Rev. 2025, 13, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Dalpiaz, A.; Schwamb, R.; Miao, Y.; Waltzer, W.; Khan, A. Clinical Pathology of Bartholin’s Glands: A Review of the Literature. Curr. Urol. 2015, 8, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Puppo, V. Embryology and anatomy of the vulva: The female orgasm and women’s sexual health. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 154, 3–8. [Google Scholar] [CrossRef]
- Lintoiu, B.; Bacalbasa, N. Bartholin’s gland carcinoma. Rev. Med. Rom 2017, 64, 16–20. [Google Scholar] [CrossRef]
- Quaresma, C.; Sparzak, P.B. Anatomy, Abdomen and Pelvis: Bartholin Gland. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Serre, E.; Destrieux, C.; Body, G.; Ouldamer, L. Lymphatic Drainage of Bartholin’s Gland in Fresh Adult Cadavers: Implications in Terms of Groin Surg. ery in Case of Bartholin’s Gland Carcinoma. J. Surg. Oncol. 2022, 1, 1. [Google Scholar] [CrossRef]
- Kozakiewicz, B.; Dmoch-Gajzlerska, E.; Roszkowska-Purska, K. Carcinomas and sarcomas of the Bartholin gland. A report of nine cases and review of the literature. Eur. J. Gynaecol. Oncol. 2014, 35, 243–249. [Google Scholar]
- Yoshida, H.; Uno, M.; Kato, T. Bartholin’s glands commonly express NKX3.1: Reconsideration of “prostatic differentiation” in gynaecological pathology. Histopathology 2022, 80, 1013–1015. [Google Scholar] [CrossRef]
- Wesselmann, U.; Burnett, A.L.; Heinberg, L.J. The urogenital and rectal pain syndromes. Pain 1997, 73, 269–294. [Google Scholar] [CrossRef] [PubMed]
- Tympanidis, P.; Terenghi, G.; Dowd, P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br. J. Dermatol. 2003, 148, 1021–1027. [Google Scholar] [CrossRef]
- Bornstein, J.; Goldschmid, N.; Sabo, E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol. Obstet. Investig. 2004, 58, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Balat, Ö.; Edwards, C.L.; Delclos, L. Advanced primary carcinoma of the Bartholin gland: Report of 18 patients. Eur. J. Gynaecol Oncol. 2001, 22, 46–49. [Google Scholar]
- Chamlian, D.L.; Taylor, H.B. Primary carcinoma of the Bartholin’s gland. A report of 24 patients. Obstet. Gynecol. 1972, 39, 489–494. [Google Scholar]
- Wilkinson, E.; Rush, D. Bartholin Gland Carcinoma. In The Histopathology of Vulvar Neoplasia; Global Library of Women’s Medicine (GLOWM): Preston, UK, 2017. [Google Scholar] [CrossRef]
- Jones, M.A.; Mann, E.W.; Caldwell, C.L.; Tarraza, H.M.; Dickersin, G.R.; Young, R.H. Small Cell Neuroendocrine Carcinoma of Bartholin’s Gland. Am. J. Clin. Pathol. 1990, 94, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, C.; Jackson, A.; Loreen, A. 29-year-old with dyspareunia and vulvar mass: An unusual diagnosis of Bartholin’s gland carcinoma. Gynecol. Oncol. Rep. 2018, 27, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.W.; Leow, J.B.Y.; Naing, K.; Jaaback, K.; Thachil, T. Adenoid Cystic Carcinoma of the Bartholin’s Gland: A Diagnostic Dilemma. Case Rep. Obstet. Gynecol. 2019, 2019, 1784949. [Google Scholar] [CrossRef]
- Akhtar, M.; Chishti, U.; Idress, R. Bartholin gland carcinoma in a young female: A rare disease in an unusual age group. BMJ Case Rep. 2021, 14, e236821. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.C.; Cote, R.J.; Kramer, E.E.; Saigo, P.; Goldman, G.H. Carcinomas of Bartholin’s gland. Histogenesis and the etiological role of human papillomavirus. Am. J. Pathol. 1993, 142, 925–933. [Google Scholar]
- Palicelli, A.; Torricelli, F.; Tonni, G.; Bisagni, A.; Zanetti, E.; Zanelli, M.; Medina-Illueca, V.D.; Melli, B.; Zizzo, M.; Morini, A.; et al. Primary Carcinomas of the Episiotomy Scar Site: A Systematic Literature Review. Curr. Oncol. 2025, 32, 65. [Google Scholar] [CrossRef]
- Schweppe, K.W.; Schlake, W. Adenokarzinom der Bartholinschen Drüse. Geburtshilfe Frauenheilkd 1980, 40, 437–443. [Google Scholar] [CrossRef]
- Eppel, W.; Frigo, P.; Worda, C.; Bettelheim, D. Ultrasound Imaging of Bartholin’s Cysts. Gynecol. Obstet. Invest. 2000, 49, 179–182. [Google Scholar] [CrossRef]
- Robotti, G.; Canepari, E.; Torresi, M. Premenstrual Inguinal Swelling and Pain Caused by Endometriosis in the Bartholin Gland: A Case Report. J. Ultrasound 2015, 18, 71–72. [Google Scholar] [CrossRef]
- Visco, A.G.; Del Priore, G. Postmenopausal Bartholin Gland Enlargement: A Hospital-Based Cancer Risk Assessment. Obstet. Gynecol. 1996, 87, 286–290. [Google Scholar] [CrossRef]
- Omole, F.; Kelsey, R.C.; Phillips, K.; Cunningham, K. Bartholin Duct Cyst and Gland Abscess: Office Management. Am. Fam. Physician 2019, 99, 760–766. [Google Scholar]
- Morrison, J.; Baldwin, P.; Hanna, L.; Andreou, A.; Buckley, L.; Durrant, L.; Edey, K.; Faruqi, A.; Fotopoulou, C.; Ganesan, R.; et al. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: An update on recommendations for practice 2023. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 210–238. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Santoro, A.; Inzani, F.; Spadola, S.; Fiorentino, V.; Cianfrini, F.; Carbone, C.; Garganese, G.; Rossi, E.D.; Scambia, G.; et al. Ultrasound-guided FNA cytology of groin lymph nodes improves the management of squamous cell carcinoma of the vulva: Results from a comparative cytohistological study. Cancer CytoPathol. 2019, 127, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Fischerová, D.; Planchamp, F.; Alcázar, J.L.; Dundr, P.; Epstein, E.; Felix, A.; Frühauf, F.; Garganese, G.; Salvesen Haldorsen, I.; Jurkovic, D.; et al. ISUOG/ESGO Consensus Statement on ultrasound-guided biopsy in gynecological oncology. Int. J. Gynecol. Cancer 2025, 35, 101732. [Google Scholar] [CrossRef]
- Patsner, B. Bartholin’s gland metastases from breast cancer: A case report. Eur. J. Gynaecol Oncol. 1996, 17, 96–98. [Google Scholar] [PubMed]
- Menzin, A.W.; De Risi, D.; Smilari, T.F.; Kalish, P.E.; Vinciguerra, V. Lobular breast carcinoma metastatic to the vulva: A case report and literature review. Gynecol. Oncol. 1998, 69, 84–88. [Google Scholar] [CrossRef]
- Ray, K.; Rocconi, R.P.; Novak, L.; Straughn, J.M., Jr. Recurrence of endometrial adenocarcinoma in a prior Bartholin’s cyst marsupialization incision. Gynecol. Oncol. 2006, 103, 749–751. [Google Scholar] [CrossRef]
- Olmes, G.L.; Breitbach, G.P.; Tepikin, A.; Nistor, A.; Solomayer, E.F.; Hamoud, B.H. A Metastasis of Ovarian Cancer in the Bartholin Gland: A Case Report with Systematic Literature Review. Reprod. Sci. 2024, 31, 550–554. [Google Scholar] [CrossRef]
- El-Tawab, S.S.; Kehoe, S. Synchronous ovarian and Bartholin gland carcinoma: Case report and review of literature. Int. J. Gynecol. Obstet. 2023, 163, 744–746. [Google Scholar] [CrossRef]
- McAlinden, J.; Caruso, V.; Hammond, I. Metastatic lung cancer involving the vulva: Two new cases and literature review. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 175–176. [Google Scholar] [CrossRef]
- Ramanah, R.; Allam-Ndoul, E.; Baeza, C.; Riethmuller, D. Brain and Lung Metastasis of Bartholin’s Gland Adenoid Cystic Carcinoma: A Case Report. J. Med. Case Rep. 2013, 7, 208. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Cuello, M.A.; Rogers, L.J. Cancer of the vulva: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 7–18. [Google Scholar] [CrossRef]
- Koenig, C.; Tavassoli, F.A. Nodular hyperplasia, adenoma, and adenomyoma of Bartholin’s gland. Int. J. Gynecol. Pathol. 1998, 17, 289–294. [Google Scholar] [CrossRef]
- Heller, D.S.; Bean, S. Lesions of the Bartholin gland: A review. J. Low. Genit. Tract Dis. 2014, 18, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.A.; Wilcox, F.L.; Sissons, M. Bartholin’s gland leiomyoma: A diagnostic and management dilemma. J. Obstet. Gynaecol Res. 2012, 38, 941–943. [Google Scholar] [CrossRef]
- González-Bugatto, F.; Añón-Requena, M.J.; López-Guerrero, M.A.; Báez-Perea, J.M.; Bartha, J.L.; Hervías-Vivancos, B. Vulvar leiomyosarcoma in Bartholin’s gland area: A case report and literature review. Arch. Gynecol. Obstet. 2009, 279, 171–174. [Google Scholar] [CrossRef]
- Gocmen, A.; Inaloz, H.S.; Sari, I.; Inaloz, S.S. Endometriosis in the Bartholin gland. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 114, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Heijink, T.; Bogers, H.; Steensma, A. Endometriosis of the Bartholin gland: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Dhall, K.; Das, S.S.; Dey, P. Tuberculosis of Bartholin’s gland. Int. J. Gynaecol Obstet. 1995, 48, 223–224. [Google Scholar] [CrossRef]
- Tresserra, F.; Grases, P.J.; Cararach, M.; Fabregas, R. Nodular hyperplasia of the bartholin gland increasing in size during sexual intercourse. J. Low. Genit. Tract Dis. 2000, 4, 18–20. [Google Scholar] [CrossRef]
- Plouffe, L.; Tulandi, T.; Rosenberg, A.; Ferenczy, A. Non-Hodgkin’s Lymphoma in Bartholin’s Gland: Case Report and Review of Literature. Am. J. Obstet. Gynecol. 1984, 148, 608–609. [Google Scholar] [CrossRef]
- Lavanderos, S.; Puebla, V.; Barboza, O. Endometriosis of the Bartholin Gland in a Patient with Deep Endometriosis. Am. J. Obstet. Gynecol. 2025, 232, 232–234. [Google Scholar] [CrossRef]
- Topolovec, Z.; Blažičević, V.; Šijanović, S.; Vidosavljević, D. Squamous cell carcinoma of Bartholin gland coexistent with human papillomavirus. Eur. J. Gynaecol Oncol. 2015, 36, 482–484. [Google Scholar]
- Nazeran, T.; Cheng, A.S.; Karnezis, A.N.; Tinker, A.V.; Gilks, C.B. Bartholin Gland Carcinoma: Clinicopathologic Features, Including p16 Expression and Clinical Outcome. Int. J. Gynecol. Pathol. 2019, 38, 189–195. [Google Scholar] [CrossRef]
- Niller, H.H.; Wolf, H.; Minarovits, J. Viral hit and run-oncogenesis: Genetic and epigenetic scenarios. Cancer Lett. 2011, 305, 200–217. [Google Scholar] [CrossRef]
- Kostov, S.; Dzhenkov, D.; Metodiev, D.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Yordanov, A. A case of human papillomavirus infection and vulvar cancer in a young patient—“Hit and run” theory. Gynecol. Oncol. Rep. 2021, 36, 100760. [Google Scholar] [CrossRef]
- Cao, H.; Wang, S.; Zhang, Z.; Lou, J. Prognostic Value of Overexpressed p16INK4a in Vulvar Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0152459. [Google Scholar] [CrossRef]
- Downs, L.S.; Ghosh, K.; Dusenbery, K.E.; Cosin, J.A. Stage IV carcinoma of the Bartholin gland managed with primary chemoradiation. Gynecol. Oncol. 2002, 87, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Kacerovska, D.; Nemcova, J.; Petrik, R.; Michal, M.; Kazakov, D.V. Lymphoepithelioma-like carcinoma of the Bartholin gland. Am. J. Dermatopathol. 2008, 30, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Nakano, T.; Abe, A.; Sano, T.; Niibe, Y.; Oka, K. Mucinous adenocarcinoma of Bartholin gland treated with radiation therapy: A case report. Jpn. J. Clin. Oncol. 2001, 31, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Lubis, L.D.; Dina, S.; Khaidirman, D.K. Primary Bartholin Adenocarcinoma: A Rare Case and Radiotherapy as Definitive Treatment. Folia Med. 2021, 63, 985–989. [Google Scholar] [CrossRef]
- Robinson, H.; Karpe, M.; Edidi, I.; Fisher, A.; Drew, Y.; Ralte, A.; O’Donnell, R.L. Enteric Type Bartholin Gland Adenocarcinoma: An Unusual Variant of a Rare Neoplasm. Int. J. Gynecol. Pathol. 2021, 40, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vallejo, J.; Molina-Bellido, P.; Laforga, J.B.; Clemente-Pérez, P.A. Intestinal-type adenocarcinoma of the Bartholin gland: A case report and literature review. Gynecol. Oncol. Rep. 2021, 37, 100836. [Google Scholar] [CrossRef]
- Chatzistamatiou, K.; Tanimanidis, P.; Xirou, P.; Patakiouta, F.; Kaplanis, K. Primary adenocarcinoma of the Bartholin gland: An extremely rare case report. J. Obstet. Gynaecol. 2015, 35, 536–537. [Google Scholar] [CrossRef]
- Dellino, M.; Cicogna, S.; Falcone, F.; Mitidieri, M.; Mazzeo, R.; Pignata, S.; Mangili, G.; Cormio, G. “Intestinal-Type” Vulvar Adenocarcinoma: A Review of the MITO Rare Tumors Group. Cancers 2022, 14, 5171. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Isaac, M.A.; Hidalgo, E.; Márquez, B.; Nogales, F.F. Villoglandular adenocarcinoma of the vulva. Gynecol. Oncol. 2001, 83, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Laforga, J.B.; Martin, J. Intestinal-type mucinous adenocarcinoma of the Bartholin gland in a perimenopausal woman. A case report and review of the literature. Rev. Esp. Patol. 2021, 54, 197–200. [Google Scholar] [CrossRef]
- Verta, S.; Christmann, C.; Brambs, C.E. Adenoid Cystic Carcinoma of Bartholin’s Gland: A Case Report with Emphasis on Surg. ical Management. Am. J. Case Rep. 2022, 23, e935707. [Google Scholar] [CrossRef]
- Segarra Vidal, B.; Cañete Mota, S.; Andrade Cadena, P.; Llueca Abella, A. Adenoid cystic carcinoma of the Bartholin’s gland. Int. J. Gynecol. Cancer 2021, 31, 292–298. [Google Scholar] [CrossRef]
- Barcellini, A.; Gadducci, A.; Laliscia, C.; Imparato, S.; Vitolo, V.; Preda, L.; Valvo, F. Adenoid Cystic Carcinoma of Bartholin’s Gland: What Is the Best Approach? Oncology 2020, 98, 513–519. [Google Scholar] [CrossRef]
- Pellizzon, A.C.A. The adenoid cystic carcinoma of the Bartholin’s gland: A literature review. Appl. Cancer Res. 2018, 38, 6. [Google Scholar] [CrossRef]
- Santiago, A.E.; Teunissen, N.; Ricardo, B.F.P.; Cândido, E.B.; Furtado, R.S.; Silva Filho, A.L.D. High-Grade Transformation in Adenoid Cystic Carcinoma of the Bartholin Gland: Case Report. Rev. Bras. Ginecol Obstet. 2021, 43, 980–984. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, H.S.; Lee, Y.Y.; Kim, T.J.; Choi, C.H.; Song, S.Y.; Kim, B.G.; Bae, D.S.; Lee, J.W. Analysis of clinical outcomes of patients with adenoid cystic carcinoma of Bartholin glands. Int. J. Clin. Exp Pathol. 2015, 8, 5688–5694. [Google Scholar]
- Şahin Aker, S.; Cansız Ersöz, C.; Ortaç, F. Adenoid cystic carcinoma of Bartholin’s gland diagnosed after lung lobectomy: Review of the literature and a case presentation. Turk. J. Obstet. Gynecol. 2020, 17, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuyzen-de Boer, G.M.; Dasgupta, S.; Ewing-Graham, P.C.; Van Bockstal, M.R. Adenoid cystic carcinoma of the Bartholin gland is not HPV-related: A case report and review of literature. Pathol. Res. Pract. 2020, 216, 152968. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.J.; Sneige, N.; Gershenson, D.M.; Saul, P.B.; Stringer, C.A.; Seski, J.C. Adenoid cystic carcinoma of Bartholin gland. Obstet. Gynecol. 1986, 67, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, T.; Vesterinen, E.; Saksela, E. Primary carcinoma of Bartholin’s glands: A morphological and clinical study of six cases including a transitional cell carcinoma. Gynecol. Oncol. 1978, 6, 354–362. [Google Scholar] [CrossRef]
- Fujiwaki, R.; Takahashi, K.; Nishiki, Y.; Ryuko, K.; Kitao, M. Rare case of transitional cell carcinoma originating in Bartholin’s gland duct. Gynecol. Obstet. Investig. 1995, 40, 278–280. [Google Scholar] [CrossRef]
- Scinicariello, F.; Rady, P.; Hannigan, E.; Dinh, T.V.; Tyring, S.K. Human papillomavirus type 16 found in primary transitional cell carcinoma of the Bartholin’s gland and in a lymph node metastasis. Gynecol. Oncol. 1992, 47, 263–266. [Google Scholar] [CrossRef]
- Kyriazi, M.A.; Carvounis, E.E.; Kitsou, M.; Arkadopoulos, N.; Nicolaidou, E.; Fotiou, S.; Smyrniotis, V. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: Case report and review of literature. Int. J. Gynecol. Pathol. 2010, 29, 501–504. [Google Scholar] [CrossRef]
- Noronha, V.; Cooper, D.L.; Higgins, S.A.; Murren, J.R.; Kluger, H.M. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006, 7, 270–271. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Aydin, N.E.; Wong, N.A.; Cooper, K. Low-grade epithelial–myoepithelial carcinoma of Bartholin gland: Report of 2 cases of a distinctive neoplasm arising in the vulvovaginal region. Int. J. Gynecol. Pathol. 2009, 28, 286–291. [Google Scholar] [CrossRef]
- Obermair, A.; Koller, S.; Crandon, A.J.; Perrin, L.; Nicklin, J.L. Primary Bartholin gland carcinoma: A report of seven cases. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 78–81. [Google Scholar] [CrossRef]
- Wu, J.C.; Xi, M.L.; Wang, Y.Q.; Tang, W.B.; Zhang, Y.Q. Primary small cell neuroendocrine carcinoma of the Bartholin’s gland: A case report. Oncol. Lett. 2018, 16, 4434–4438. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zheng, A.; Chen, Y. A rare case of primary small cell neuroendocrine carcinoma of the Bartholin gland. Int. J. Gynaecol. Obstet. 2021, 155, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Fetissof, F.; Arbeille, B.; Bellet, D.; Barre, I.; Lansac, J. Endocrine cells in human Bartholin’s glands. An immunohistochemical and ultrastructural analysis. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 57, 117–121. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Elliott, K.S.; Lee, Y.C.; Chen, P.C.; Abulafia, O. Merkel cell carcinoma of the Bartholin’s gland. Gynecol. Oncol. 2005, 97, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.B.; Lott, M.; O’Sullivan, J.C.; Azzopardi, J.G. Combined adenoid cystic and squamous carcinoma of Bartholin’s gland. Case report. Br. J. Obstet. Gynaecol. 1984, 91, 291–295. [Google Scholar] [CrossRef]
- Ordóñez, N.G.; Manning, J.T.; Luna, M.A. Mixed tumor of the vulva: A report of two cases probably arising in Bartholin’s gland. Cancer 1981, 48, 181–186. [Google Scholar] [CrossRef]
- Sieiński, W.; Bobkiewicz, P. Bartholin’s gland mixed carcinoma predominantly of the transitional cell type following radiotherapy for a cervical carcinoma. Zentralbl. Allg. Pathol. 1990, 136, 265–268. [Google Scholar]
- Carreras-Dieguez, N.; Saco, A.; Del Pino, M.; Marimon, L.; López Del Campo, R.; Manzotti, C.; Fusté, P.; Pumarola, C.; Torné, A.; Garcia, A.; et al. Human papillomavirus and p53 status define three types of vulvar squamous cell carcinomas with distinct clinical, pathological, and prognostic features. Histopathology 2023, 83, 17–30. [Google Scholar] [CrossRef]
- Carreras-Dieguez, N.; Saco, A.; Del Pino, M.; Pumarola, C.; Del Campo, R.L.; Manzotti, C.; Garcia, A.; Marimon, L.; Diaz-Mercedes, S.; Fuste, P.; et al. Vulvar squamous cell carcinoma arising on human papillomavirus-independent precursors mimicking high-grade squamous intra-epithelial lesion: A distinct and highly recurrent subtype of vulvar cancer. Histopathology 2023, 82, 731–744. [Google Scholar] [CrossRef]
- Horn, L.-C.; Brambs, C.E.; Gilks, B.; Hoang, L.; Singh, N.; Hiller, G.G.R.; Hering, K.; McAlpine, J.N.; Jamieson, A.; Alfaraidi, M.; et al. Molecular Subtypes of Vulvar Squamous Cell Carcinoma: The Significance of HPV-Independent/p53 Wild Type. Cancers 2024, 16, 4216. [Google Scholar] [CrossRef]
- Ordi, O.; Saco, A.; Peñuelas, N.; Blanco-Irazuegui, O.; Pino, M.D.; Carreras-Dieguez, N.; Marimon, L.; Rodrigo-Calvo, M.T.; Morató, A.; Sisuashvili, L.; et al. Whole-Exome Sequencing of Vulvar Squamous Cell Carcinomas Reveals an Impaired Prognosis in Patients with TP53 Mutations and Concurrent CCND1. Gains. Mod. Pathol. 2024, 37, 100574. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Dieguez, N.; Ordi, O.; Peñuelas, N.; Del Pino, M.; Diez-Ahijado, L.; Sisuashvili, L.; Darecka, K.; Marimon, L.; Vega, N.; Torné, A.; et al. CyClin. D1 Overexpression Predicts Poor Disease-Specific Survival in Human Papillomavirus-Independent Vulvar Squamous Cell Carcinoma. Mod. Pathol. 2025, 38, 100833. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Dieguez, N.; Guerrero, J.; Rodrigo-Calvo, M.T.; Ribera-Cortada, I.; Trias, I.; Jares, P.; López Del Campo, R.; Saco, A.; Munmany, M.; Marimon, L.; et al. Molecular Landscape of Vulvar Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 7069. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Wallbillich, J.J.; Wu, S.; Farrell, A.; Hodges, K.; Xiu, J.; Nabhan, C.; Guastella, A.; Kheil, M.; Gogoi, R.; et al. The Genomic Landscape of Vulvar Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2023, 42, 515–522. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zeng, X.; Yu, W.; Zhang, Z.; Wei, B. Adenoid cystic carcinoma of the breast: Molecular profiling updates and multidimensional detection of MYB. Pathol. Res Pr. 2025, 272, 156091. [Google Scholar] [CrossRef]
- Evin, C.; Just, P.A.; Borghese, B.; Fabiano, E.; Bennani, S.; Canny, E.; Marisa, L.; Derive, N.; Laurent-Puig, P.; Alexandre, J.; et al. Adenoid cystic carcinoma of Bartholin’s gland, a case report with genomic data and literature review. Cancer Radiother. 2023, 27, 328–336. [Google Scholar] [CrossRef]
- Bayrak, R.; Haltas, H.; Yenidunya, S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: Cytokeratin 7-/20+ phenotype is more specific than CDX2 antibody. Diagn. Pathol. 2012, 7, 9. [Google Scholar] [CrossRef]
- Dabir, P.D.; Svanholm, H.; Christiansen, J.J. SATB2 is a supplementary immunohistochemical marker to CDX2 in the diagnosis of colorectal carcinoma metastasis in an unknown primary. APMIS 2018, 126, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Trecourt, A.; Treilleux, I.; Pissaloux, D.; Donzel, M.; Thamphya, B.; Thirode, F.; Houlier, A.; Paindavoine, S.; Franceschi, T.; Baltrès, A.; et al. Primary Vulvar and Vaginal Adenocarcinomas of Intestinal Type Are Closer to Colorectal Adenocarcinomas Than to Carcinomas of Müllerian Origin. Mod. Pathol. 2025, 38, 100649. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Donisi, C.; Sanna, E.; Chiappe, G.; Nemolato, S.; Melis, L.; Oppi, S.; Mola, B.; Madeddu, C. Next-Generation Sequencing Whole-Genome Analysis for Targeted Treatment Approach of Metastatic Bartholin Gland Adenocarcinoma: An Emblematic Case Report and Review of the Literature. Diagnostics 2021, 11, 2085. [Google Scholar] [CrossRef]
- Rakislova, N.; Clavero, O.; Alemany, L.; Saco, A.; Quirós, B.; Lloveras, B.; Alejo, M.; Pawlita, M.; Quint, W.; Del Pino, M.; et al. Histological characteristics of HPV-associated and -independent squamous cell carcinomas of the vulva: A study of 1,594 cases. Int. J. Cancer. 2017, 141, 2517–2527. [Google Scholar] [CrossRef]
- Growdon, W.B.; Boisvert, S.L.; Akhavanfard, S.; Oliva, E.; Dias-Santagata, D.C.; Kojiro, S.; Horowitz, N.S.; Iafrate, A.J.; Borger, D.R.; Rueda, B.R. Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol. Oncol. 2008, 111, 289–297. [Google Scholar] [CrossRef]
- Williams, E.A.; Werth, A.J.; Sharaf, R.; Montesion, M.; Sokol, E.S.; Pavlick, D.C.; McLaughlin-Drubin, M.; Erlich, R.; Toma, H.; Williams, K.J.; et al. Vulvar Squamous Cell Carcinoma: Comprehensive Genomic Profiling of HPV+ Versus HPV− Forms Reveals Distinct Sets of Potentially Actionable Molecular Targets. JCO Precis. Oncol. 2020, 4, PO.19.00406. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Pors, J.; Thompson, E.; Ho, J.; Prentice, L.; McConechy, M.; Aguirre-Hernandez, R.; Miller, R.; Leung, S.; Proctor, L.; et al. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome. Mod. Pathol. 2021, 34, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Alemany, L.; Clavero, O.; Del Pino, M.; Saco, A.; Marimon, L.; Quirós, B.; Lloveras, B.; Ribera-Cortada, I.; Alejo, M.; et al. HPV-independent Precursors Mimicking High-grade Squamous Intraepithelial Lesions (HSIL) of the Vulva. Am. J. Surg. Pathol. 2020, 44, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Aimono, E.; Tanishima, S.; Nomura, H.; Imai, M.; Hayashi, H.; Nishihara, H. Genetic profiling of patients with adenoid cystic carcinoma of the Bartholin’s glands reveals potential new routes for targeted therapies: A case report. Diagn. Pathol. 2020, 15, 64. [Google Scholar] [CrossRef]
- Feinberg, J.; Da Cruz Paula, A.; da Silva, E.M.; Pareja, F.; Patel, J.; Zhu, Y.; Selenica, P.; Leitao, M.M., Jr.; Abu-Rustum, N.R.; Reis-Filho, J.S.; et al. Adenoid cystic carcinoma of the Bartholin’s gland is underpinned by MYB- and MYBL1- rearrangements. Gynecol. Oncol. 2024, 185, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Bishop, J.A.; Hansen, T.V.; Westra, W.H.; Bilde, A.; von Buchwald, C.; Kiss, K. Human papillomavirus-related carcinoma with adenoid cystic-like features of the sinonasal tract: Clinical and morphological characterization of six new cases. Histopathology 2017, 70, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Frommer, R.; Mileshkin, L.; Manzyuk, L.; Penel, N.; Burge, M.; Piha-Paul, S.A.; Girda, E.; Lopez Martin, J.A.; van Dongen, M.G.J.; Italiano, A.; et al. Efficacy and safety of pembrolizumab for patients with previously treated advanced vulvar squamous cell carcinoma: Results from the phase 2 KEYNOTE-158 study. Gynecol. Oncol. 2022, 166, 211–218. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef]
- Lelle, R.J.; Davis, K.P.; Roberts, J.A. Adenoid cystic carcinoma of the Bartholin’s gland: The University of Michigan experience. Int. J. Gynecol. Cancer 1994, 4, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rathore, R.; Singhal, A.; Maddirala, H.; Mathur, S.; Bhatla, N. Adeno-squamous Carcinoma of Bartholin Gland: Challenges in Diagnosis and Management of a Less Known Vulvar Cancer—A Case Report. Curr. Probl. Cancer Case Rep. 2024, 16, 100330. [Google Scholar] [CrossRef]
- Thibault, I.; Lavallée, M.C.; Aubin, S.; Jain, S.; Laflamme, N.; Vigneault, É. Management of Bartholin’s gland carcinoma using high-dose-rate interstitial brachytherapy boost. Brachytherapy 2013, 12, 500–507. [Google Scholar] [CrossRef]
- Gien, L.T.; Slomovitz, B.; Van der Zee, A.; Oonk, M. Phase II activity trial of high-dose radiation and chemosensitization in patients with macrometastatic lymph node spread after sentinel node biopsy in vulvar cancer: GROINSS-V III/NRG-GY024. Int. J. Gynecol. Cancer 2023, 33, 619–622. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Li, C. Neoadjuvant chemotherapy followed by radical vulvectomy for adenoid cystic carcinoma of Bartholin’s gland: A case report. Transl. Cancer Res. 2022, 11, 3883–3889. [Google Scholar] [CrossRef]
- López-Varela, E.; Oliva, E.; McIntyre, J.F.; Fuller, A.F., Jr. Primary treatment of Bartholin’s gland carcinoma with radiation and chemoradiation: A report on ten consecutive cases. Int. J. Gynecol. Cancer 2007, 17, 661–667. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. Vulvar Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2024, 22, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Watrowski, R.; Friebe, Z. Synchronous squamous vulvar and cervical cancer--a case report with literature review and analysis of therapeutic strategies. Przegl. Lek. 2008, 65, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H. Surg. ical Treatment for Locally Advanced Adenoid Cystic Carcinoma of the Bartholin’s Gland: A Case Report. Tokai J. Exp. Clin. Med. 2019, 44, 68–72. [Google Scholar]
- de Mooij, Y.; Burger, M.P.; Schilthuis, M.S.; Buist, M.; van der Velden, J. Partial urethral resection in the Surg. ical treatment of vulvar cancer does not have a significant impact on urinary continence. A confirmation of an authority-based opinion. Int. J. Gynecol. Cancer 2007, 17, 294–297. [Google Scholar] [CrossRef]
- Kunos, C.; Simpkins, F.; Gibbons, H.; Tian, C.; Homesley, H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: A randomized controlled trial. Obstet. Gynecol. 2009, 114, 537–546. [Google Scholar] [CrossRef]
- Woelber, L.; Prieske, K.; Eulenburg, C.Z.; Corradini, S.; Petersen, C.; Bommert, M.; Blankenstein, T.; Hilpert, F.; de Gregorio, N.; Iborra, S.; et al. Adjuvant radiotherapy and local recurrence in vulvar cancer—A subset analysis of the AGO-CaRE-1 study. Gynecol. Oncol. 2022, 164, 68–75. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brännström, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients with Micrometastases in the Sentinel Node: Results of GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.; Simonsen, E.; Risberg, B. Adenoid cystic carcinoma of Bartholin’s gland: A report of five new cases treated with Surg. ery and radiotherapy. Gynecol. Oncol. 1989, 34, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Irfan, H.I.; Ben-Tarifite, Y.; Mahmood, R. Recurrent adenoid cystic carcinoma of the vulva: A case report of salvage stereotactic ablative radiotherapy to the pudendal nerve. Radiol. Case Rep. 2025, 20, 5148–5155. [Google Scholar] [CrossRef]
- Forner, D.M.; Mallmann, P. Neoadjuvant and definitive chemotherapy or chemoradiation for stage III and IV vulvar cancer: A pooled reanalysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 115–118. [Google Scholar] [CrossRef]
- Rydzewski, N.R.; Kanis, M.J.; Donnelly, E.D.; Lurain, J.R.; Strauss, J.B. Role of adjuvant external beAm. radiotherapy and chemotherapy in one versus two or more node-positive vulvar cancer: A National Cancer Database study. Radiother. Oncol. 2018, 129, 534–539. [Google Scholar] [CrossRef]
- Mousa, A.; Rahimi, K.; Warkus, T. Neoadjuvant chemoradiotherapy followed by radical vulvectomy for adenoid cystic carcinoma of Bartholin’s gland: A case report and review of the literature. Eur. J. Gynaecol Oncol. 2016, 37, 113–116. [Google Scholar] [CrossRef]
- Cunningham, M.J.; Goyer, R.P.; Gibbons, S.K.; Kredentser, D.C.; Malfetano, J.H.; Keys, H. Primary radiation, cisplatin, and 5-fluorouracil for advanced squamous carcinoma of the vulva. Gynecol. Oncol. 1997, 66, 258–261. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lee, J.W.; Kim, W.S.; Jung, K.L.; Lee, S.J.; Lee, J.H.; Bae, D.S.; Kim, B.G. Adenoid cystic carcinoma of the Bartholin’s gland: Report of two cases and review of the literature. Gynecol. Oncol. 2006, 100, 422–425. [Google Scholar] [CrossRef]
- Hsu, S.T.; Wang, R.C.; Lu, C.H.; Ke, Y.M.; Chen, Y.T.; Chou, M.M.; Ho, E.S. Report of two cases of adenoid cystic carcinoma of Bartholin’s gland and review of literature. Taiwan J. Obstet. Gynecol. 2013, 52, 113–116. [Google Scholar] [CrossRef]
- Takatori, E.; Shoji, T.; Miura, J.; Takeuchi, S.; Sugiyama, T. Chemoradiotherapy with irinotecan (CPT-11) for adenoid cystic carcinoma of Bartholin’s gland: A case report and review of the literature. Gynecol. Oncol. Case Rep. 2012, 4, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Preti, M.; Bertero, L.; Collemi, G.; Castellano, I.; Cassoni, P.; Cosma, S.; Carosso, A.R.; Bevilacqua, F.; Gallio, N.; et al. Is There a Place for Immune CheckpoInt. Inhibitors in Vulvar Neoplasms? A State of the Art Review. Int. J. Mol Sci. 2020, 22, 190. [Google Scholar] [CrossRef] [PubMed]
- Praiss, A.; Navitski, A.; Cohen, S.; Tessier-Cloutier, B.; Broach, V.; O’Cearbhaill, R.E. Immunotherapy for recurrent or metastatic vulvar carcinoma: A case report and review of current guidelines. Gynecol. Oncol. Rep. 2022, 41, 100982. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.P.; Delord, J.P.; Evans, T.R.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef]
- Dillon, P.M.; Petroni, G.R.; Horton, B.J.; Moskaluk, C.A.; Fracasso, P.M.; Douvas, M.G.; Varhegyi, N.; Zaja-Milatovic, S.; Thomas, C.Y. A Phase II Study of Dovitinib in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma. Clin. Cancer Res. 2017, 23, 4138–4145. [Google Scholar] [CrossRef]

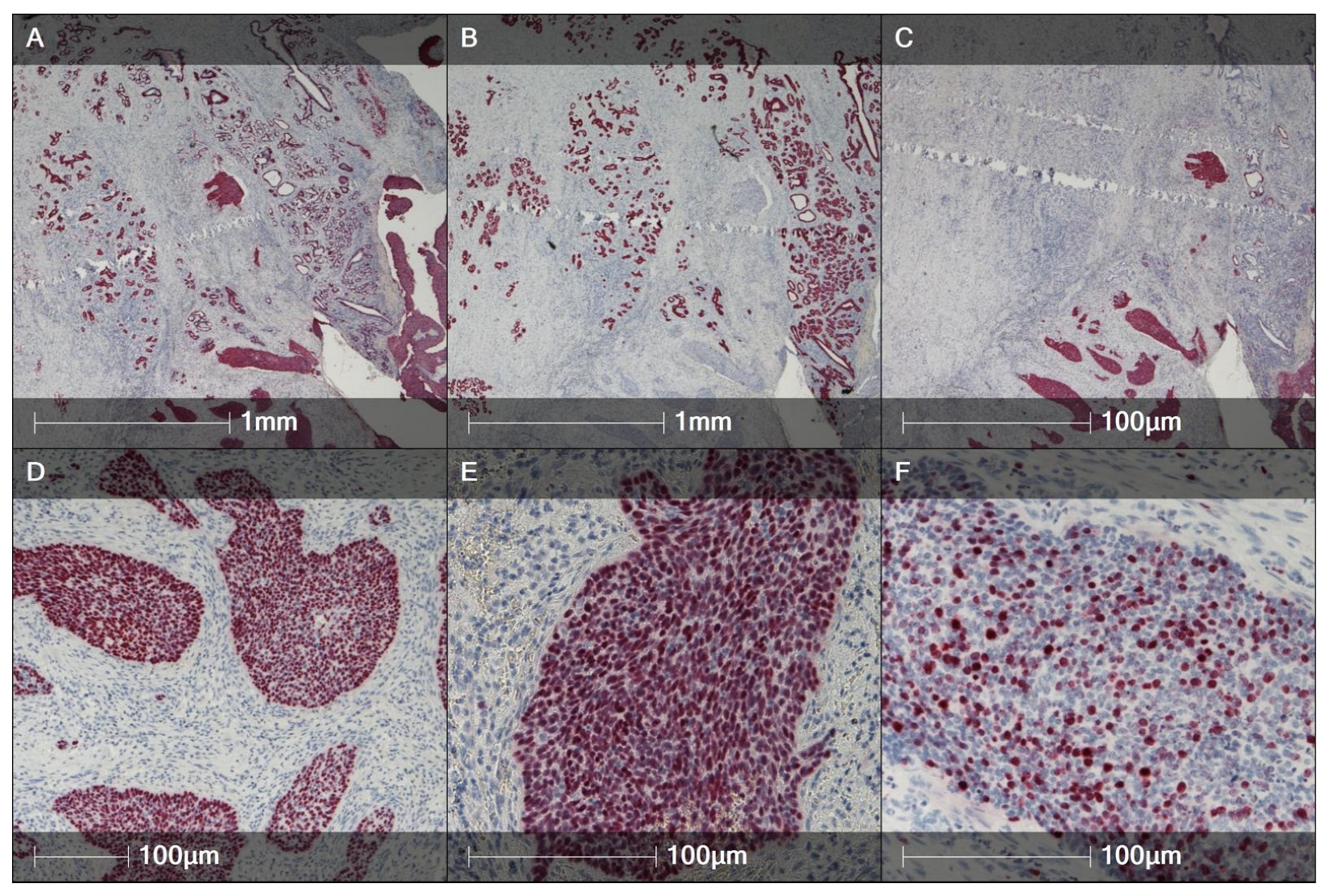

| Histotype | High-Value Molecular/ Biomarker Features | Practical Ancillary Tests | Diagnostic Relevance | Key References |
|---|---|---|---|---|
| SCC HPV-associated | HPV16/18 association; p16 block-positive | p16 IHC; HPV RNA/DNA ISH | Confirms HPV-driven pathway; segregates from HPV-independent SCC | [28,57] |
| SCC HPV-independent | Aberrant p53; TP53/CDKN2A; PIK3CA/HRAS; TERT-promoter; frequent CCND1 gains (± cyclin D1 overexpression); occasional EGFR amp; MMR-proficient (extrapolated) | p53 IHC; cyclin D1 IHC (CCND1 surrogate); broad NGS incl. CNV; EGFR FISH if relevant; PD-L1 IHC; eventually MMR/MSI/TMB (usually proficient/low) | Defines HPV-independent biology; CCND1/cyclin D1 refines prognosis (extrapolated) | [94,95,96,97,98,99,100] |
| AdCC-BG | MYB::NFIB or MYBL1 rearrangements; otherwise “quiet” genomes; rare AKT1/KDM6A/GNAS/GNAQ (case-level) | MYB IHC; MYB/MYBL1 FISH or RNA-seq | Fusion detection is diagnostic; not HPV-related. | [78,101,102] |
| Adenocarcinoma, intestinal-type | CK20+/CDX2+, variable CK7; SATB2 frequent; HPV-negative; KRAS/TP53 in a subset (extrapolated) | CK7/CK20/CDX2/SATB2 ± PAX8; GI work-up | Overlap with colorectal/anal primaries; metastases should be excluded. | [103,104,105] |
| Adenocarcinoma, non-intestinal | Case-level: PTEN loss (exons 2–5), CCND1 amplification by WGS | Broad NGS; to be discussed at molecular tumor board | Supports compassionate mTORi → CDK4/6i sequencing in selected cases | [106] |
| Biomarker/Pathway | Diagnostic/Biological Rationale | Preferred Method/ Platform | Potential Therapy/Management Impact | Evidence in BGC (vs. Extrapolated) | Key Refs |
|---|---|---|---|---|---|
| HPV status (SCC-BG) | Confirms HPV-driven pathway, aligns SCC-BG with HPV-related VSCC, explains p16 overexpression, younger age, better stage profile. | p16 IHC ± HPV RNA/DNA ISH | No de-escalation data in BGC, but HPV-positive tumors are the ones most analogous to ‘good-prognosis’ VSCC and to immunotherapy series in vulvar SCC. | Direct BG data (small series) | [28,57,99] |
| p53 IHC pattern | Separates HPV-independent squamous tumors from HPV-related ones; in VSCC, aberrant p53 is associated with worse outcome. The current three-tier classification system used in VSCC is applicable by analogy to SCC-BG. | p53 IHC, pattern-based interpretation | Frames prognosis in HPV-negative SCC-BG and tells you which tumors deserve broader sequencing. | Extrapolated from VSCC molecular subclassification | [94,96,100,110,111] |
| Integrated HPV/p16 + p53 status | Three prognostic VSCC groups (HPV+/p16+, HPV–/p53abn, HPV–/p53wt) have been defined in molecular subclassification; this framework can be applied analogously to SCC-BG. | p16 IHC + p53 IHC (both mandatory) | Helps reporting and follow-up stratification; still not a BGC-specific de-/escalation tool. | Extrapolated from VSCC | [95,96,97,98,105,106] |
| CCND1 gain/Cyclin D1 overexpression | CCND1/Cyclin D1 is a bad-risk signal in HPV-independent disease. Same biology is expected in HPV-independent SCC-BG. | Cyclin D1 IHC (screen) ± copy-number from targeted DNA NGS | Risk contextualization; in advanced/recurrent setting, could support considering CDK4/6 inhibitor concepts. | Extrapolated from VSCC | [97,98,100] |
| MYB/MYBL1 rearrangements (AdCC-BG) | Near-pathognomonic for BG adenoid cystic carcinoma; absence should trigger re-evaluation. | MYB IHC → FISH or RNA-based fusion panel | Diagnostic confirmation; occasionally eligibility for ACC-type trials. | Direct BG AdCC case series/reports | [78,102,113] |
| PI3K–mTOR/cell-cycle lesions in AC-BG (e.g., PTEN loss, CCND1 amp) | Whole-genome BG adenocarcinoma with PTEN loss + CCND1 amp that responded to everolimus then palbociclib. | Broad DNA NGS with CNV calling; RNA optional | Supports off-label/compassionate use (mTORi → CDK4/6i sequence). | Direct but single-patient | [99,106] |
| EGFR amplification/9p24 gains (SCC-BG) | Seen in VSCC WES; marks a more aggressive HPV-negative subset. | DNA NGS with CNV or FISH | Currently only for trial or n-of-1 decisions; no BG-specific response data. | Extrapolated from VSCC | [98,100] |
| MMR/MSI, TMB-H, PD-L1 | Tissue-agnostic biomarkers; PD-L1 positivity is more common in HPV-negative VSCC, so likely also in HPV-negative SCC-BG. | IHC for MMR and PD-L1; NGS for MSI/TMB | Supports use of pembrolizumab or other checkpoint inhibitors as in KEYNOTE-158 vulvar SCC cohort. | Extrapolated from vulvar SCC trials | [115] |
| KRAS (intestinal-type AC-BG) | Confirms intestinal-type differentiation and supports exclusion of colorectal origin. KRAS p.G12D has been directly demonstrated in a true BG adenocarcinoma case. | Targeted DNA NGS (KRAS exon 2–4) | Argues against EGFR-targeted therapy; supports GI-style work-up when metastasis is suspected. | Direct single-case + extrapolated vulvar intestinal-type series | [68,70,105] |
| ‘Broad panel’/global actionability | VSCC WES shows virtually every tumor has ≥1 potentially targetable alteration or immune biomarker; reasonable to expect the same or higher in advanced BGC. | Comprehensive hybrid-capture DNA ± RNA NGS | Opens trial eligibility; informs compassionate treatment in recurrence. | Extrapolated from VSCC WES | [97,99,100,109,110] |
| Treatment Modality | VSCC | BGC |
|---|---|---|
| 1. Surgical treatment of the primary tumor | L 3–4, [116] | L 4–5, direct [1,2,3] |
| 2. Groin treatment | L 3–4, [116] | L 4–5, direct [1,2,3,35,64,117,118] |
| 3. Sentinel lymph node procedure | L 1–2, [35,116] | V, indirect, [35,116] |
| 4. Adjuvant radiotherapy to the vulva | L-4, [35,116] | L-4, direct, [5,6,119] |
| 5. Adjuvant radiotherapy to the groin | L 2–3, [35,116] | L-4, direct, [5,6,119] |
| 6. Adjuvant chemoradiotherapy | L-3, [116,120] | L-4, direct, [5,6,7] |
| 7. Neoadjuvant chemotherapy | L-4, [116] | L-5, direct, [121] |
| 8. Neoadjuvant chemoradiotherapy | L-3, [35,116] | L-4, direct, [122] |
| 9. Targeted therapies | L 3–4, [35,116] | L-5, direct, [102,106] |
| 10. Recurrent/metastatic disease [systemic therapy] | L 3–4, [35,116] | L-5, indirect, [35,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Kornovski, Y.; Ivanova, V.; Metodiev, D.; Yordanov, A.; Slavchev, S.; Ivanova, Y.; Seidel, A.; Juhasz-Böss, I.; Hasan, I.; et al. Bartholin Gland Carcinoma: A State-of-the-Art Review of Epidemiology, Histopathology, Molecular Testing, and Clinical Management. Cancers 2025, 17, 3819. https://doi.org/10.3390/cancers17233819
Kostov S, Kornovski Y, Ivanova V, Metodiev D, Yordanov A, Slavchev S, Ivanova Y, Seidel A, Juhasz-Böss I, Hasan I, et al. Bartholin Gland Carcinoma: A State-of-the-Art Review of Epidemiology, Histopathology, Molecular Testing, and Clinical Management. Cancers. 2025; 17(23):3819. https://doi.org/10.3390/cancers17233819
Chicago/Turabian StyleKostov, Stoyan, Yavor Kornovski, Vesela Ivanova, Dimitar Metodiev, Angel Yordanov, Stanislav Slavchev, Yonka Ivanova, Anke Seidel, Ingolf Juhasz-Böss, Ihsan Hasan, and et al. 2025. "Bartholin Gland Carcinoma: A State-of-the-Art Review of Epidemiology, Histopathology, Molecular Testing, and Clinical Management" Cancers 17, no. 23: 3819. https://doi.org/10.3390/cancers17233819
APA StyleKostov, S., Kornovski, Y., Ivanova, V., Metodiev, D., Yordanov, A., Slavchev, S., Ivanova, Y., Seidel, A., Juhasz-Böss, I., Hasan, I., Alkatout, I., & Watrowski, R. (2025). Bartholin Gland Carcinoma: A State-of-the-Art Review of Epidemiology, Histopathology, Molecular Testing, and Clinical Management. Cancers, 17(23), 3819. https://doi.org/10.3390/cancers17233819









