Multiparametric MRI Markers Associated with Breast Cancer Risk in Women with Dense Breasts
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. Image Acquisition and Interpretation
2.3. Quantitative Imaging Markers
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Follow-Up
3.2. Correlation Between Imaging Markers and Age
3.3. Association Between Imaging Markers and Tyrer–Cuzick Risk Classifications
3.4. Concordance Between Imaging Markers and Tyrer–Cuzick Risk Classifications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, H.D.; Tyne, K.; Naik, A.; Bougatsos, C.; Chan, B.K.; Humphrey, L. Screening for Breast Cancer: An Update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 151, 727–737. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017, 67, 439–448. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Cintolo-Gonzalez, J.A.; Braun, D.; Blackford, A.L.; Mazzola, E.; Acar, A.; Plichta, J.K.; Griffin, M.; Hughes, K.S. Breast cancer risk models: A comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res. Treat. 2017, 164, 263–284. [Google Scholar] [CrossRef]
- Boyd, N.F.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Chiarelli, A. Mammographic Density and the Risk and Detection of Breast Cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef]
- Dontchos, B.N.; Rahbar, H.; Partridge, S.C.; Korde, L.A.; Lam, D.L.; Scheel, J.R.; Peacock, S.; Lehman, C.D. Are Qualitative Assessments of Background Parenchymal Enhancement, Amount of Fibroglandular Tissue on MR Images, and Mammographic Density Associated with Breast Cancer Risk? Radiology 2015, 276, 371–380. [Google Scholar] [CrossRef]
- Arasu, V.A.; Miglioretti, D.L.; Sprague, B.L.; Alsheik, N.H.; Buist, D.S.M.; Henderson, L.M.; Herschorn, S.D.; Lee, J.M.; Onega, T.; Rauscher, G.H.; et al. Population-Based Assessment of the Association Between Magnetic Resonance Imaging Background Parenchymal Enhancement and Future Primary Breast Cancer Risk. J. Clin. Oncol. 2019, 37, 954–963. [Google Scholar] [CrossRef]
- Telegrafo, M.; Rella, L.; Stabile Ianora, A.A.; Angelelli, G.; Moschetta, M. Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn. Reson. Imaging 2016, 34, 173–176. [Google Scholar] [CrossRef] [PubMed]
- King, V.; Brooks, J.D.; Bernstein, J.L.; Reiner, A.S.; Pike, M.C.; Morris, E.A. Background Parenchymal Enhancement at Breast MR Imaging and Breast Cancer Risk. Radiology 2011, 260, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, L.; You, C.; Gu, Y. Fibroglandular Tissue and Background Parenchymal Enhancement on Breast MR Imaging Correlates With Breast Cancer. Front. Oncol. 2021, 11, 616716. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.J.; Bancroft, L.C.H.; Strigel, R.M.; Chitalia, R.D.; Kontos, D.; Moy, L.; Partridge, S.C.; Rahbar, H. Background parenchymal enhancement on breast MRI: A comprehensive review. J. Magn. Reson. Imaging 2020, 51, 43–61. [Google Scholar] [CrossRef]
- Karimi, Z.; Phillips, J.; Slanetz, P.; Lotfi, P.; Dialani, V.; Karimova, J.; Mehta, T. Factors Associated With Background Parenchymal Enhancement on Contrast-Enhanced Mammography. Am. J. Roentgenol. 2021, 216, 340–348. [Google Scholar] [CrossRef]
- Pujara, A.C.; Mikheev, A.; Rusinek, H.; Gao, Y.; Chhor, C.; Pysarenko, K.; Rallapalli, H.; Walczyk, J.; Moccaldi, M.; Babb, J.S.; et al. Comparison between qualitative and quantitative assessment of background parenchymal enhancement on breast MRI. J. Magn. Reson. Imaging 2018, 47, 1685–1691. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Obuchowski, N.A.; Kessler, L.G.; Raunig, D.L.; Gatsonis, C.; Huang, E.P.; Kondratovich, M.; McShane, L.M.; Reeves, A.P.; Barboriak, D.P.; et al. Metrology Standards for Quantitative Imaging Biomarkers. Radiology 2015, 277, 813–825. [Google Scholar] [CrossRef]
- Niell, B.L.; Abdalah, M.; Stringfield, O.; Raghunand, N.; Ataya, D.; Gillies, R.; Balagurunathan, Y. Quantitative Measures of Background Parenchymal Enhancement Predict Breast Cancer Risk. Am. J. Roentgenol. 2021, 217, 64–75. [Google Scholar] [CrossRef]
- Lam, D.L.; Hippe, D.S.; Kitsch, A.E.; Partridge, S.C.; Rahbar, H. Assessment of Quantitative Magnetic Resonance Imaging Background Parenchymal Enhancement Parameters to Improve Determination of Individual Breast Cancer Risk. J. Comput. Assist. Tomogr. 2019, 43, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Van Der Velden, B.H.M.; Verburg, E.; Bakker, M.F.; Pijnappel, R.M.; Veldhuis, W.B.; van Gils, C.H.; Gilhuijs, K.G.A. Assessing Quantitative Parenchymal Features at Baseline Dynamic Contrast-enhanced MRI and Cancer Occurrence in Women with Extremely Dense Breasts. Radiology 2023, 308, e222841. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. 2013 ACR BI-RADS Atlas: Breast Imaging Reporting and Data System [Internet], 5th ed.; American College of Radiology; Available online: https://books.google.com/books?vid=ISBN155903016X (accessed on 8 September 2025).
- Hahn, S.Y.; Ko, E.S.; Han, B.K.; Lim, Y.; Gu, S.; Ko, E.Y. Analysis of factors influencing the degree of detectability on diffusion-weighted MRI and diffusion background signals in patients with invasive breast cancer. Medicine 2016, 95, e4086. [Google Scholar] [CrossRef]
- Lew, C.O.; Harouni, M.; Kirksey, E.R.; Kang, E.J.; Dong, H.; Gu, H.; Grimm, L.J.; Walsh, R.; Lowell, D.A.; Mazurowski, M.A. A publicly available deep learning model and dataset for segmentation of breast, fibroglandular tissue, and vessels in breast MRI. Sci. Rep. 2024, 14, 5383. [Google Scholar] [CrossRef]
- Kuo, Y.T.; Kazerouni, A.S.; Park, V.Y.; Surento, W.; Hippe, D.S.; Rahbar, H.; Partridge, S.C. Validation of Fully-Automated Deep Learning-Based Fibroglandular Tissue Segmentation for Efficient and Reliable Quantitation of Background Parenchymal Enhancement in Breast MRI. arXiv 2025, arXiv:2511.07088. [Google Scholar]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Müller-Schimpfle, M.; Ohmenhaüser, K.; Stoll, P.; Dietz, K.; Claussen, C.D. Menstrual cycle and age: Influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997, 203, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Kim, Y.J.; Kang, B.J.; An, Y.Y.; Lee, A.W.; Song, B.J.; Park, Y.S.; Lee, H.B. Enhancement parameters on dynamic contrast enhanced breast MRI: Do they correlate with prognostic factors and subtypes of breast cancers? Magn. Reson. Imaging 2015, 33, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, L.; Li, Q.; Gu, Y. Quantitative assessment of background parenchymal enhancement in breast magnetic resonance images predicts the risk of breast cancer. Oncotarget 2017, 8, 10620–10627. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, R. Vascular Response of the Breast to Estrogen. J. Clin. Endocrinol. Metab. 1969, 29, 695–700. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Bieling, H.B.; Gieseke, J.; Kreft, B.P.; Sommer, T.; Lutterbey, G.; Schild, H.H. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: Normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997, 203, 137–144. [Google Scholar] [CrossRef]
- Bhatelia, K.; Singh, K.; Singh, R. TLRs: Linking inflammation and breast cancer. Cell Signal 2014, 26, 2350–2357. [Google Scholar] [CrossRef]
- Castañeda-Gill, J.; Vishwanatha, J. Antiangiogenic mechanisms and factors in breast cancer treatment. J. Carcinog. 2016, 15, 1. [Google Scholar]
- Vartanian, R.K.; Weidner, N. Correlation of Intratumoral Endothelial Cell Proliferation with Microvessel Density (Tumor Angiogenesis) and Tumor Cell Proliferation in Breast Carcinoma. Am. J. Pathol. 1994, 144, 1188. [Google Scholar]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef]
- Kristensen, T.; Knutsson, M.; Wehland, M.; Laursen, B.; Grimm, D.; Warnke, E.; Magnusson, N.E. Anti-Vascular Endothelial Growth Factor Therapy in Breast Cancer. Int. J. Mol. Sci. 2014, 15, 23024–23041. [Google Scholar] [CrossRef] [PubMed]
- Fakhrejahani, E.; Toi, M. Antiangiogenesis Therapy for Breast Cancer: An Update and Perspectives from Clinical Trials. Jpn. J. Clin. Oncol. 2014, 44, 197–207. [Google Scholar] [CrossRef]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [CrossRef]
- Choi, E.J.; Choi, H.; Choi, S.A.; Youk, J.H. Dynamic contrast-enhanced breast magnetic resonance imaging for the prediction of early and late recurrences in breast cancer. Medicine 2016, 95, e5330. [Google Scholar] [CrossRef]
- Sung, J.S.; Corben, A.D.; Brooks, J.D.; Edelweiss, M.; Keating, D.M.; Lin, C.; Morris, E.A.; Patel, P.; Robson, M.; Woods, M.; et al. Histopathologic characteristics of background parenchymal enhancement (BPE) on breast MRI. Breast Cancer Res. Treat. 2018, 172, 487–496. [Google Scholar] [CrossRef]
- Fuller, A.M.; Olsson, L.T.; Midkiff, B.R.; Kirk, E.L.; McNaughton, K.K.; Calhoun, B.C.; Troester, M.A. Vascular density of histologically benign breast tissue from women with breast cancer: Associations with tissue composition and tumor characteristics. Hum. Pathol. 2019, 91, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jesinger, R.A.; Lattin, G.E.; Ballard, E.A.; Zelasko, S.M.; Glassman, L.M. Vascular Abnormalities of the Breast: Arterial and Venous Disorders, Vascular Masses, and Mimic Lesions with Radiologic-Pathologic Correlation. RadioGraphics 2011, 31, E117–E136. [Google Scholar] [CrossRef] [PubMed]




| Protocol | DCE | DWI |
|---|---|---|
| Sequence Type | 3D Fast Field Echo | 2D single-shot—Echo Planar Imaging |
| Acquisition plane | axial | axial |
| Phase encode | R/L | A/P |
| Field-of-view (mm) | 240 × 360 | 360 × 360 |
| Slice thickness (mm) | 1.5 | 4 |
| Acquisition matrix | 480 × 720 | 200 × 200 |
| In-plane resolution (mm2) | 0.5 × 0.5 | 1.8 × 1.8 |
| TR (msec) | 5.7 | 3500 |
| TE (msec) | 3 | 75 |
| Flip angle | 10 | 90 |
| Fat suppression | SPAIR | SPAIR |
| b values, s/mm2 | 0, 100, 800, 1200 | |
| Gadolinium-based contrast agent | gadoteridol (0.1 mmol/kg body weight) | - |
| Acquisition time | 2 min per phase, (1 pre-contrast, 3 post-contrast phases with k0 at approx. 2, 4, and 6 min after contrast injection) <9 min total | 3 min |
| Derivation Formula | |
|---|---|
| Volume measures | |
| Breast volume (cm3) | number of voxels in Breast mask × volume per voxel |
| FGT volume (cm3) | number of voxels in FGT mask × volume per voxel |
| Vessel volume (mm3) | number of voxels in Vessel mask × volume per voxel |
| FGT:breast ratio (%) | (FGT volume/Breast volume) × 100% |
| Vessel:breast ratio (%) | (Vessel volume/Breast volume) × 100% |
| Vessel:FGT ratio (%) | (Vessel volume/FGT volume) × 100% |
| BPE measures | |
| Median PE (%) | median PE of voxels > 10% within FGT mask |
| BPE volume (cm3) | number of voxels with PE > 10% within FGT mask × volume per voxel |
| BPE:breast ratio (%) | (BPE volume/Breast volume) × 100% |
| BPE:FGT ratio (%) | (BPE volume/FGT volume) × 100% |
| BPE:vessel ratio | (BPE volume/Vessel volume) |
| Integrated intensity (cm3) | BPE volume × mean PE |
| Variable | Overall Cohort (n = 77) |
|---|---|
| Age at MRI, years | 45 (40, 52) |
| Menopausal status | |
| Pre-menopausal | 52 (68%) |
| Peri-menopausal | 4 (5%) |
| Post-menopausal | 21 (27%) |
| Race | |
| American Indian/Alaska Native | 3 (4%) |
| Asian | 5 (76%) |
| Black or African American | 1 (1%) |
| White | 63 (82%) |
| More Than Once Race | 1 (1%) |
| Unknown/Not Reported | 4 (5%) |
| Breast Density * | |
| Heterogeneous | 47 (61%) |
| Extreme | 30 (39%) |
| Tyrer–Cuzick risk | |
| Low (≤20% lifetime risk) | 20 (26%) |
| High (>20% lifetime risk) | 57 (74%) |
| Variable | Median (IQR) | Spearman’s rho vs. Age | p-Value |
|---|---|---|---|
| Qualitative metrics | |||
| Qualitative BPE | 3 (1, 4) | −0.31 | 0.006 |
| Qualitative BPDS | 2 (1, 3) | −0.39 | <0.001 |
| Quantitative metrics | |||
| Volume measures | |||
| Breast volume (cm3) | 1597 (1108, 2501) | 0.25 | 0.029 |
| FGT volume (cm3) | 204 (157, 326) | −0.30 | 0.007 |
| Vessel volume (mm3) | 13 (12, 14) | 0.31 | <0.001 |
| FGT:breast ratio (%) | 14.2 (7.6, 19.4) | −0.38 | <0.001 |
| Vessel:breast ratio (%) | 0.5 (0.4, 0.7) | 0.29 | 0.011 |
| Vessel:FGT ratio (%) | 4.0 (1.8, 7.2) | 0.38 | <0.001 |
| BPE measures | |||
| Median PE (%) | 28.1 (23.7, 36.1) | −0.40 | <0.001 |
| BPE volume (cm3) | 147 (85, 262) | −0.36 | <0.001 |
| BPE:breast ratio (%) | 7.9 (4.8, 14.0) | −0.47 | < 0.001 |
| BPE:FGT ratio (%) | 62.0 (54.7, 72.9) | −0.29 | 0.011 |
| BPE:vessel ratio | 13.7 (8.3, 28.5) | −0.47 | <0.001 |
| Integrated intensity (cm3) | 49 (26, 105) | −0.43 | <0.001 |
| Diffusion-weighted measures | |||
| Median ADC (×10−3 mm2/s) | 1.61 (1.43, 1.77) | −0.14 | 0.23 |
| ADC interquartile range (×10−3 mm2/s) | 0.49 (0.44, 0.57) | 0.24 | 0.040 |
| ADC skewness (mm2/s) | 0.03 (−0.27, 0.32) | −0.26 | 0.022 |
| Tyrer–Cuzick Risk Group * | |||||
|---|---|---|---|---|---|
| Variable | Low Risk (n = 20) | High Risk (n = 57) | AUC (95% CI) | Adjusted OR † (95% CI) | Adjusted p-Value ‡ |
| Qualitative markers | |||||
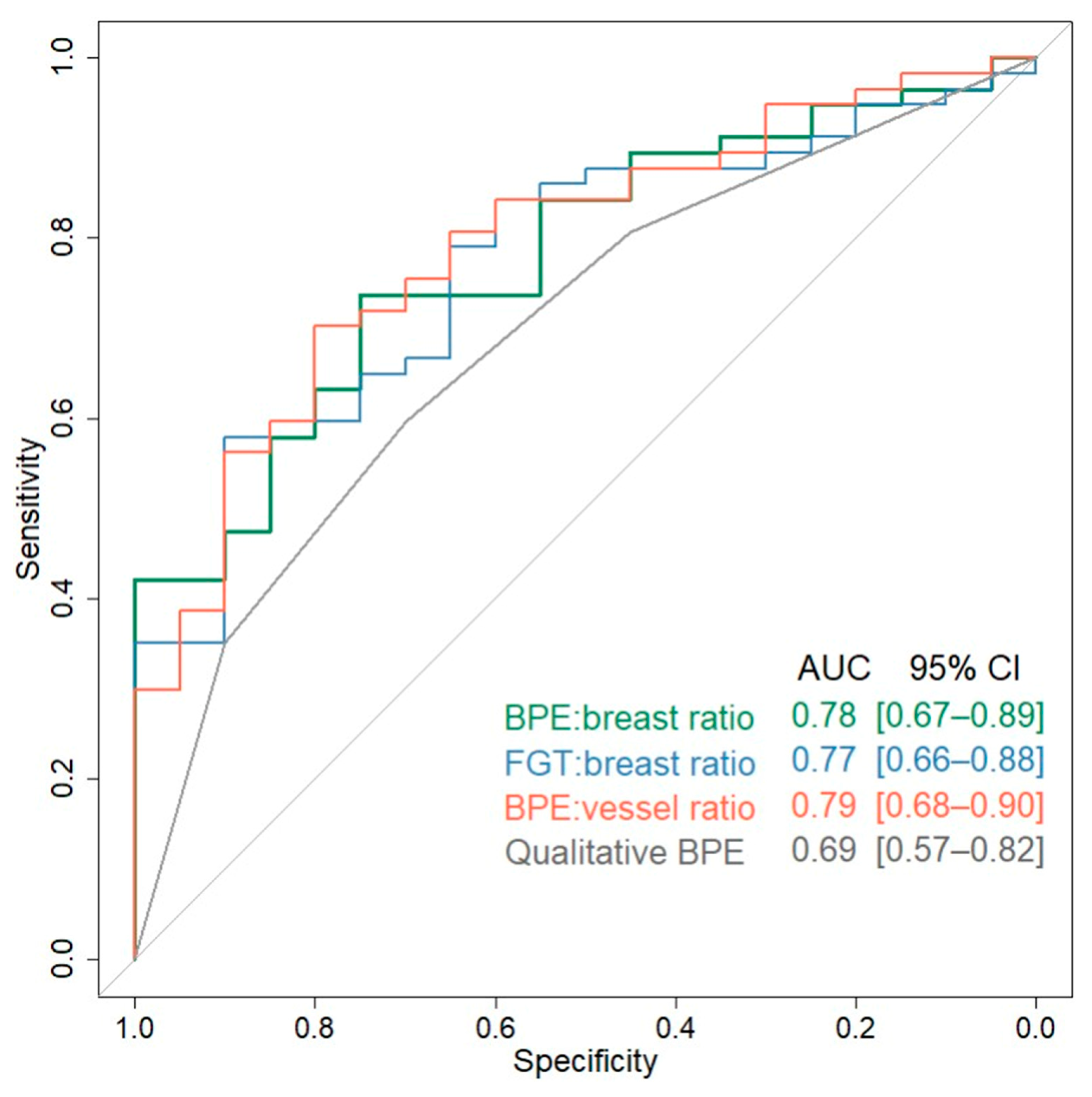
| Qualitative BPE | 2 (1, 3) | 3 (2, 4) | 0.69 (0.57–0.82) | 1.79 (1.00–3.39) | 0.11 |
| Qualitative BPDS | 2 (1, 2) | 2 (1, 3) | 0.63 (0.50–0.76) | 1.31 (0.72–2.50) | 0.38 |
| Quantitative markers | |||||
| Volume measures | |||||
| Breast volume (cm3) | 1918 (1355, 2833) | 1516 (1052, 2370) | 0.62 (0.49–0.76) | 0.74 (0.42–1.27) | 0.28 |
| FGT volume (cm3) | 178 (133, 204) | 254 (164, 376) | 0.70 (0.58–0.83) | 1.83 (1.01–3.52) | 0.22 |
| Vessel volume (mm3) | 11 (8, 15) | 7 (4, 14) | 0.66 (0.53–0.79) | 0.53 (0.24–1.02) | 0.24 |
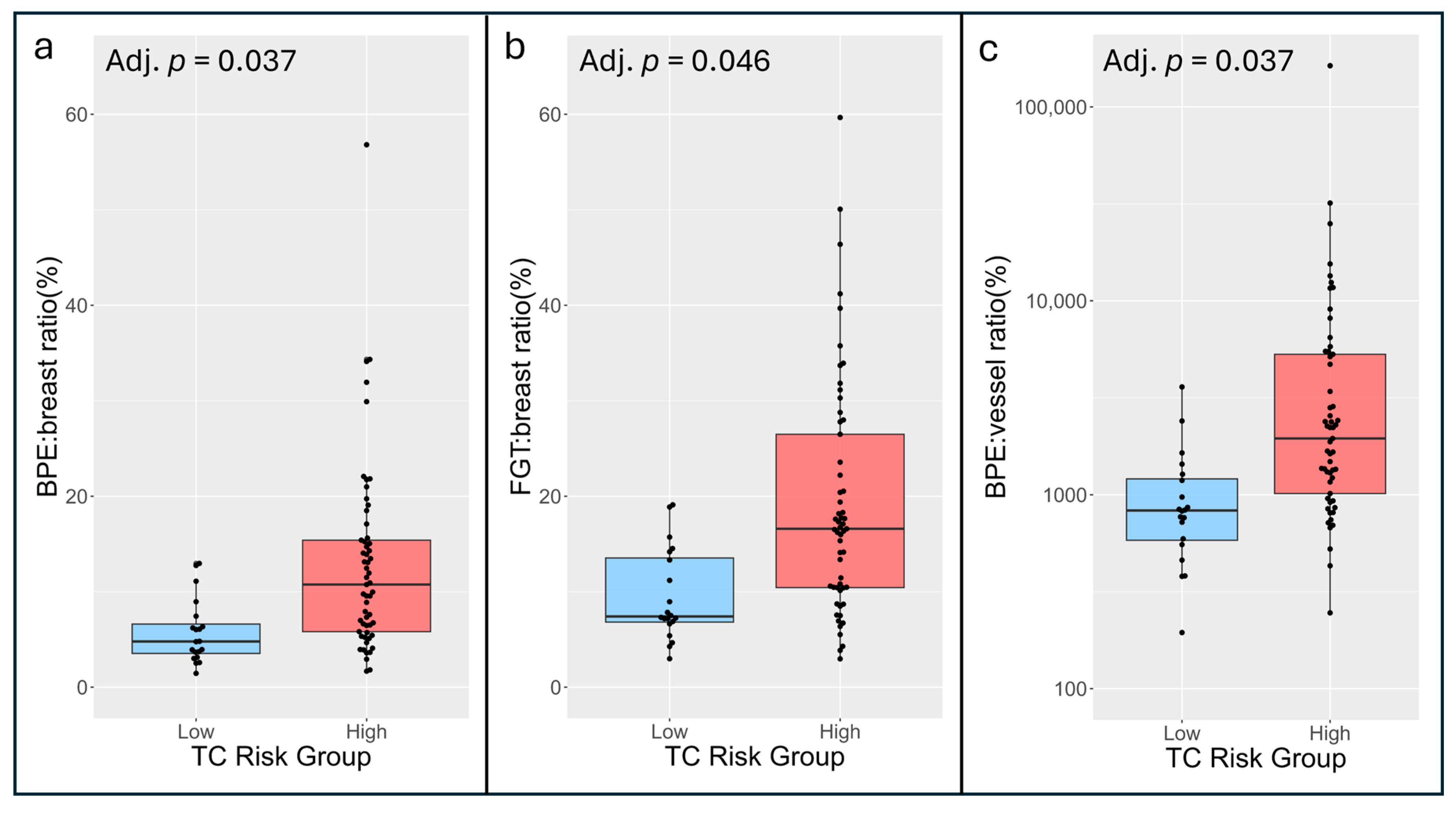
| FGT:breast ratio (%) | 7.4 (6.8, 13.5) | 16.6 (10.4, 26.5) | 0.77 (0.66–0.88) | 2.59 (1.34–5.56) | 0.046 |
| Vessel:breast ratio (%) | 0.6 (0.5, 0.7) | 0.5 (0.3, 0.6) | 0.68 (0.55–0.80) | 0.44 (0.15–0.97) | 0.24 |
| Vessel:FGT ratio (%) | 7.1 (4.6, 9.3) | 3.6 (1.3, 6.1) | 0.76 (0.65–0.87) | 0.28 (0.09–0.67) | 0.055 |
| BPE measures | |||||
| Median PE (%) | 25.9 (23.7, 38.0) | 29.2 (23.7, 34.7) | 0.55 (0.39–0.71) | 0.91 (0.52–1.64) | >0.9 |
| BPE volume (cm3) | 97 (64, 133) | 161 (94, 275) | 0.72 (0.60–0.85) | 1.81 (1.00–3.46) | 0.22 |
| BPE:breast ratio (%) | 4.8 (3.5, 6.6) | 10.7 (5.8, 15.4) | 0.78 (0.67–0.89) | 2.71 (1.37–5.98) | 0.037 |
| BPE:FGT ratio (%) | 59.3 (53.7, 68.0) | 62.3 (55.5, 75.0) | 0.59 (0.45–0.74) | 1.19 (0.68–2.06) | >0.9 |
| BPE:vessel ratio | 8.3 (5.8, 12.1) | 19.5 (10.2, 53.0) | 0.79 (0.68–0.90) | 4.59 (1.75–15.79) | 0.037 |
| Integrated intensity (cm3) | 36 (20, 49) | 63 (31, 123) | 0.66 (0.52–0.80) | 0.69 (0.22–1.15) | 0.66 |
| Diffusion-weighted measures | |||||
| Median ADC (× 10−3 mm2/s) | 1.56 (1.38, 1.66) | 1.61 (1.44, 1.79) | 0.61 (0.47–0.76) | 1.34 (0.79–2.37) | 0.57 |
| ADC interquartile range (× 10−3 mm2/s) | 0.48 (0.44, 0.56) | 0.49 (0.44, 0.57) | 0.51 (0.36–0.66) | 1.32 (0.73–2.58) | 0.57 |
| ADC skewness (mm2/s) | 0.12 (−0.07, 0.33) | −0.06 (−0.31, 0.30) | 0.59 (0.45–0.73) | 0.66 (0.37–1.15) | 0.44 |
| Tyrer–Cuzick Risk Group | |||
|---|---|---|---|
| Marker | Overall Cohort (n = 77) | Low Risk (n = 20) | High Risk (n = 57) |
| BPE:breast ratio | 52 (67%) | 14 (70%) | 38 (67%) |
| FGT:breast ratio | 54 (70%) | 14 (70%) | 40 (70%) |
| BPE:vessel ratio | 53 (69%) | 15 (75%) | 38 (67%) |
| Qualitative BPE | 52 (67%) | 13 (65%) | 39 (68%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surento, W.; Fischer, R.; Biswas, D.; Hippe, D.S.; Kazerouni, A.S.; Kim, J.Y.; Li, I.; Gennari, J.H.; Rahbar, H.; Partridge, S.C. Multiparametric MRI Markers Associated with Breast Cancer Risk in Women with Dense Breasts. Cancers 2025, 17, 3771. https://doi.org/10.3390/cancers17233771
Surento W, Fischer R, Biswas D, Hippe DS, Kazerouni AS, Kim JY, Li I, Gennari JH, Rahbar H, Partridge SC. Multiparametric MRI Markers Associated with Breast Cancer Risk in Women with Dense Breasts. Cancers. 2025; 17(23):3771. https://doi.org/10.3390/cancers17233771
Chicago/Turabian StyleSurento, Wesley, Romy Fischer, Debosmita Biswas, Daniel S. Hippe, Anum S. Kazerouni, Jin You Kim, Isabella Li, John H. Gennari, Habib Rahbar, and Savannah C. Partridge. 2025. "Multiparametric MRI Markers Associated with Breast Cancer Risk in Women with Dense Breasts" Cancers 17, no. 23: 3771. https://doi.org/10.3390/cancers17233771
APA StyleSurento, W., Fischer, R., Biswas, D., Hippe, D. S., Kazerouni, A. S., Kim, J. Y., Li, I., Gennari, J. H., Rahbar, H., & Partridge, S. C. (2025). Multiparametric MRI Markers Associated with Breast Cancer Risk in Women with Dense Breasts. Cancers, 17(23), 3771. https://doi.org/10.3390/cancers17233771






