Risk Stratification for Cardiotoxicity in Childhood Cancer Survivors: State-of-the-Art Review and a Novel Two-Step Approach
Simple Summary
Abstract
1. Introduction
2. Risk Prediction Strategies for Cardiotoxicity in Childhood Cancer Survivors
2.1. International Guidelines and Recommendations
2.2. The Risk Prediction Models
2.3. How to Perform the Cardiologic Evaluation in Childhood Cancer Survivors
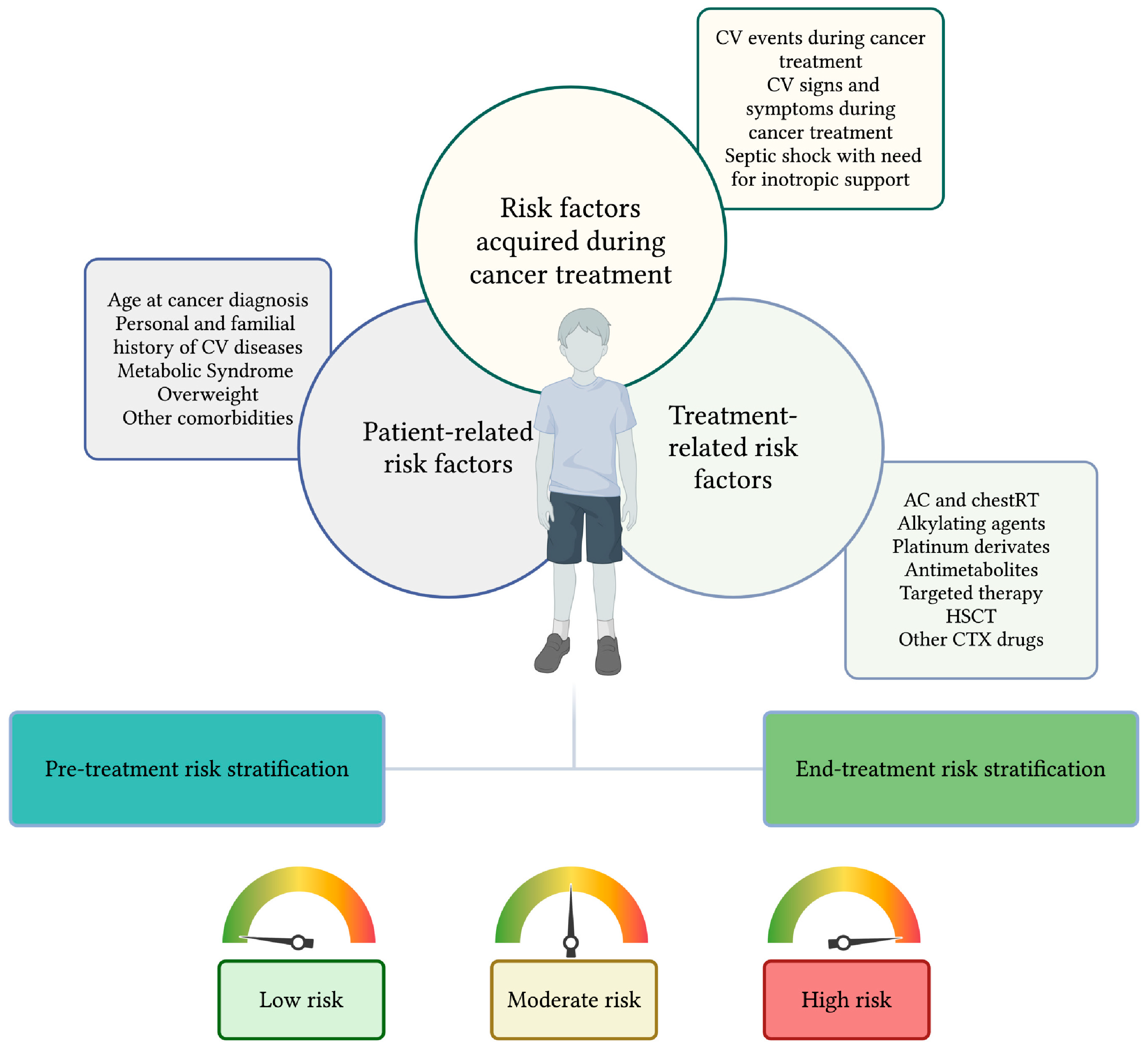
3. Risk Factors for Cardiotoxicity
3.1. Patient-Related Risk Factors for Cardiotoxicity
3.1.1. Age at Cancer Diagnosis
3.1.2. Personal and Familial History of Cardiovascular Diseases
3.1.3. Metabolic Syndrome and Cardiometabolic Risk Factors
3.1.4. Other Risk Factors
3.2. Treatment-Related Risk Factors
3.2.1. Anthracyclines and Chest Radiation Therapy
3.2.2. Alkylating Agents
3.2.3. Platinum Derivates
3.2.4. Antimetabolites
3.2.5. Hematopoietic Stem Cell Transplantation
3.2.6. Targeted Therapy
3.2.7. Other Cardiotoxic Drugs
3.3. Risk Factors Acquired During Cancer Treatment
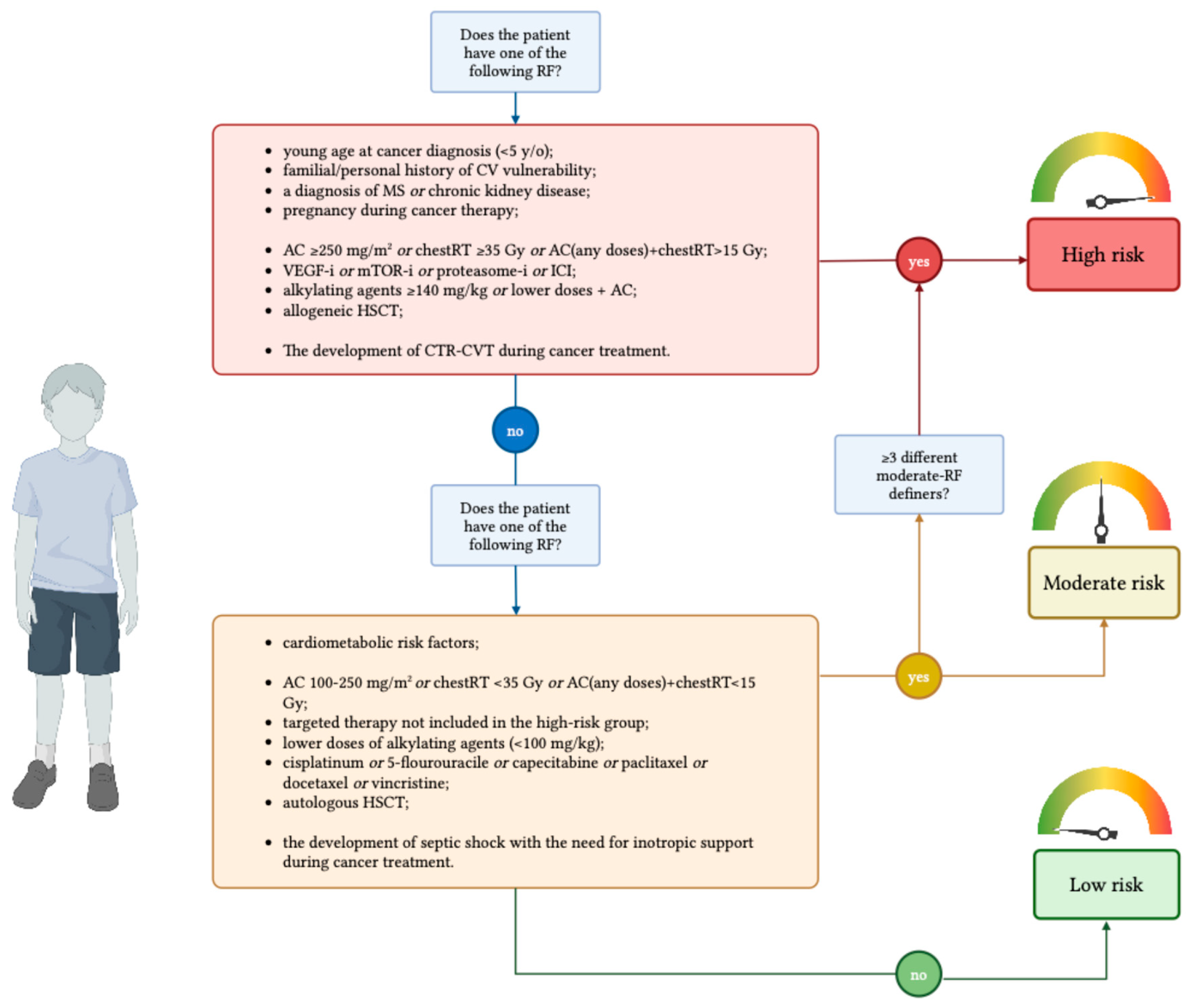
4. A Practical Tool for a Two-Step Risk Stratification: Our Proposal
4.1. Definition of Risk Groups
- •
- young age at cancer diagnosis (<5 y/o);
- •
- previously diagnosed left systolic ventricular dysfunction or CVD and congenital heart disease that can determine systolic dysfunction;
- •
- a familial history of genetic disorders that impact cardiac structure, or storage disorders, excluding non-congenital or acquired diseases;
- •
- a diagnosis of MS;
- •
- chronic kidney disease;
- •
- pregnancy during cancer therapy;
- •
- high doses of AC (≥250 mg/m2 of doxorubicin equivalents), or high doses of chestRT (≥35 Gy), or a combination of any doses of AC and chestRT (>15 Gy);
- •
- the administration of VEGF inhibitors, mTOR inhibitors, proteasome inhibitors, or immune checkpoint inhibitors;
- •
- high dose of alkylating agents (≥140 mg/kg of cyclophosphamide equivalent dose) or lower doses (<140 mg/kg of cyclophosphamide equivalent doses) administered with AC;
- •
- allogeneic HSCT;
- •
- The development of CTR-CVT during cancer treatment.
- •
- the presence and/or the development of cardiometabolic risk factors (obesity, hypertension, diabetes, and dyslipidemia);
- •
- AC at 100–250 mg/m2 of doxorubicin equivalent doses, the exposure to chest radiotherapy at lower doses (<35 Gy), or a combination of low doses of chestRT (<15 Gy) and AC;
- •
- the administration of targeted therapy not included in the high-risk group;
- •
- lower doses of alkylating agents (< 100 mg/kg);
- •
- the administration of other cardiotoxic drugs (cisplatinum, 5-flourouracile, capecitabine, paclitaxel, docetaxel, vincristine);
- •
- autologous HSCT;
- •
- the development of septic shock with the need for inotropic support during cancer treatment.
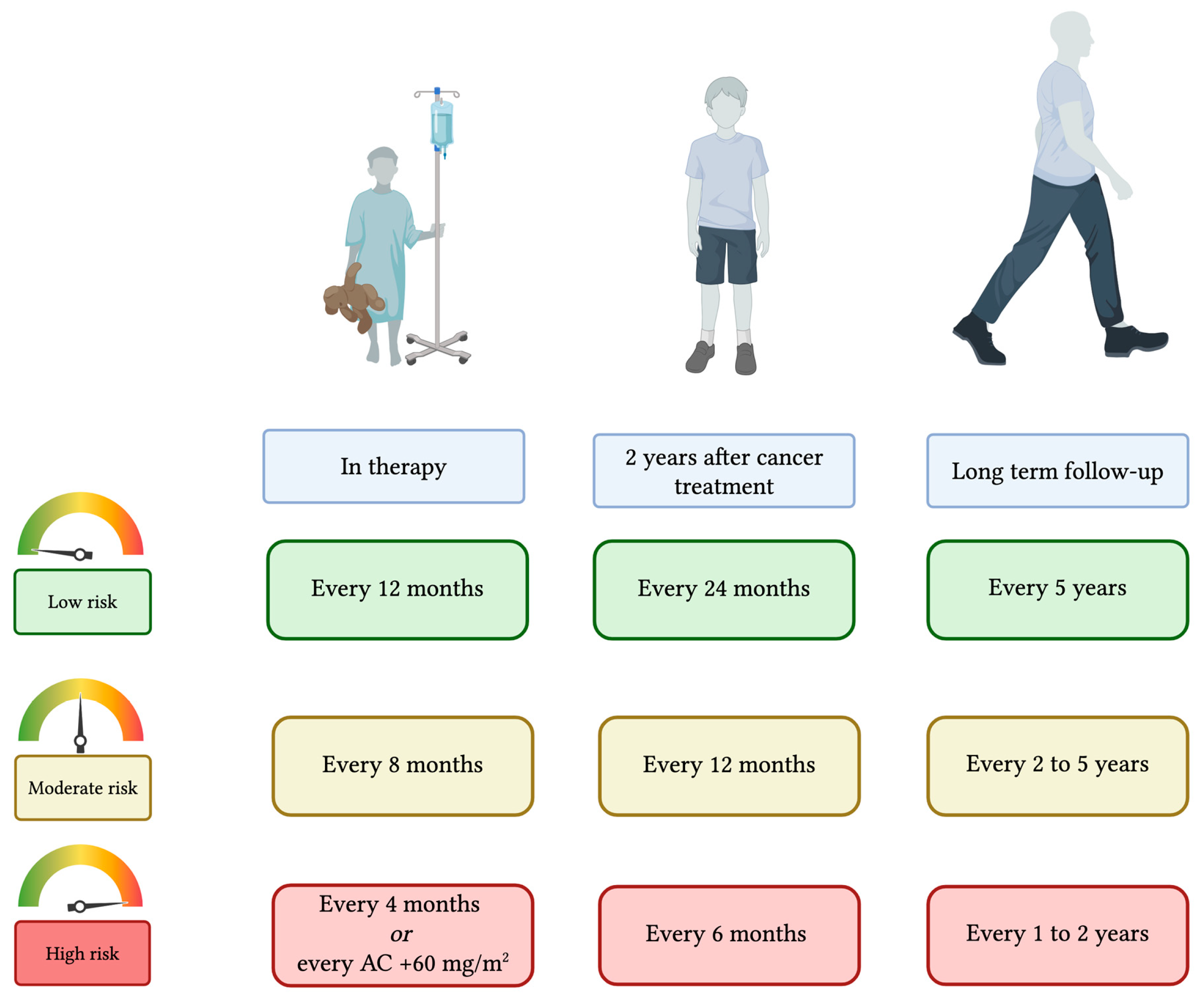
4.2. Tailored Approach for the In-Treatment and Long-Term Follow-Up
4.3. Future Directions and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Anthracyclines |
| CCS | Childhood cancer survivor |
| chestRT | Chest radiotherapy |
| CTRCD | Cancer therapy–related cardiac dysfunction |
| CTR-CVT | sCancer therapy-related cardiovascular toxicities |
| CTX | Cardiotoxicity |
| CV | Cardiovascular |
| CVDs | Cardiovascular diseases |
| HF | Heart failure |
| HSCT | Hematopoietic stem cell transplantation |
| RF | Risk factor |
Appendix A
| Risk Factors | Round 1 Median | Round 2 Median | Final Risk Category |
|---|---|---|---|
| Age at cancer diagnosis <5 y/o | 8 | 8 | High |
| Previous cardiovascular disease | 10 | 10 | High |
| Familial history of genetic disorders affecting cardiac structure or storage disorders | 8 | 9 | High |
| Diagnosis of metabolic syndrome | 8 | 8 | High |
| Chronic kidney disease | 9 | 9 | High |
| Pregnancy during cancer therapy | 8 | 9 | High |
| High-dose anthracyclines (≥250 mg/m2) | 10 | 10 | High |
| High-dose chest radiotherapy (≥35 Gy) | 10 | 10 | High |
| Any combination of anthracyclines + chestRT (>15 Gy) | 10 | 10 | High |
| Administration of VEGF, mTOR, proteasome inhibitors, or immune checkpoint inhibitors | 8 | 9 | High |
| High-dose of alkylating agents (≥140 mg/kg) | 8 | 9 | High |
| Lower doses of alkylating agents (<140 mg/kg) combined with anthracyclines | 8 | 9 | High |
| Allogeneic HSCT | 9 | 10 | High |
| Development of CTR-CVT during treatment | 10 | 10 | High |
| Overweight / Obesity | 5 | 6 | Moderate |
| Anthracyclines 100–250 mg/m2 or low-dose chest RT (<35 Gy) or combination <15 Gy | 6 | 7 | Moderate |
| Targeted therapies not included in high-risk group | 6 | 7 | Moderate |
| Lower doses of alkylating agents (<100 mg/kg) | 6 | 7 | Moderate |
| Other cardiotoxic drugs (cisplatin, 5-FU, capecitabine, paclitaxel, docetaxel, vincristine) | 6 | 7 | Moderate |
| Autologous HSCT | 5 | 6 | Moderate |
| Septic shock requiring inotropic support during treatment | 6 | 7 | Moderate |
| ≥3 moderate-risk RFs present simultaneously | 8 | 9 | High |
| Female gender | 2 | 2 | Not included |
| Familial history for adult-type CV diseases (arterial hypertension, stroke, ischemic heart disease) | 2 | 2 | Not included |
| Risk Class | Timing of Cardio-Oncological Evaluation | Round 1 Median | Round 2 Median | Final RAM Interpretation | |
|---|---|---|---|---|---|
| During cancer treatment | High risk | Every 4 months from the start of antineoplastic treatment | 8 | 9 | Strong agreement |
| or to be performed at cumulative AC dose 120 mg/m2, then every additional 60 mg/m2 | 8 | 9 | Strong agreement | ||
| Moderate risk | Every 8 months | 7 | 8 | Strong agreement | |
| Low risk | Every 12 months | 8 | 9 | Strong agreement | |
| In the first two years after the end of cancer treatment | High risk | Every 6 months | 6 | 7 | Strong agreement |
| Moderate risk | Every 12 months | 7 | 9 | Strong agreement | |
| Low risk | Every 24 months | 9 | 10 | Strong agreement | |
| Long-term follow-up | High risk | if normal echocardiography: every 2 years | 7 | 8 | Strong agreement |
| if echochardiographic anomalies: every year | 8 | 9 | Strong agreement | ||
| Moderate risk | if echochardiographic anomalies: every 2 years | 6 | 7 | Strong agreement | |
| If normal echocardiography: every 5 years | 5 | 6 | Strong agreement | ||
| Low risk | every 5 years | 5 | 5 | Strong agreement |
References
- National Cancer Institute. Definitions. Office of Cancer Survivorship, Division of Cancer Control and Population Sciences. Available online: https://cancercontrol.cancer.gov/ocs/definitions (accessed on 8 November 2025).
- Silva, N.D.P.; Gini, A.; Dolya, A.; Colombet, M.; Soerjomataram, I.; Youlden, D.; Stiller, C.; Steliarova-Foucher, E.; Aitken, J.; Bray, F.; et al. Prevalence of childhood cancer survivors in Europe: A scoping review. EJC Paediatr. Oncol. 2024, 3, 100155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammoud, R.A.; Liu, Q.; Dixon, S.B.; Onerup, A.; Mulrooney, D.A.; Huang, I.-C.; Jefferies, J.L.; Rhea, I.B.; Ness, K.K.; Ehrhardt, M.J.; et al. The burden of cardiovascular disease and risk for subsequent major adverse cardiovascular events in survivors of childhood cancer: A prospective, longitudinal analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2024, 25, 811–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feijen, E.A.M.; van Dalen, E.C.; van der Pal, H.J.H.; Reulen, R.C.; Winter, D.L.; Kuehni, C.E.; Morsellino, V.; Alessi, D.; Allodji, R.S.; Byrne, J.; et al. Increased risk of cardiac ischaemia in a pan-European cohort of 36 205 childhood cancer survivors: A PanCareSurFup study. Heart 2020, 107, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Hinz, A.; Brähler, E.; Merzenich, H.; Faber, J.; Wild, P.S.; Beutel, M.E. Quality of life after pediatric cancer: Comparison of long-term childhood cancer survivors’ quality of life with a representative general population sample and associations with physical health and risk indicators. Health Qual. Life Outcomes 2023, 21, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Dalen, E.C.; Mulder, R.L.; Suh, E.; Ehrhardt, M.J.; Aune, G.J.; Bardi, E.; Benson, B.J.; Bergler-Klein, J.; Chen, M.H.; Frey, E.; et al. Coronary artery disease surveillance among childhood, adolescent and young adult cancer survivors: A systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Eur. J. Cancer 2021, 156, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Noyd, D.H.; Liu, Q.; Yasui, Y.; Chow, E.J.; Bhatia, S.; Nathan, P.C.; Landstrom, A.P.; Tonorezos, E.; Casillas, J.; Berkman, A.; et al. Cardiovascular Risk Factor Disparities in Adult Survivors of Childhood Cancer Compared with the General Population. J. Am. Coll. Cardiol. CardioOncol. 2023, 5, 489–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361, Erratum in Eur. Heart J. 2023, 44, 1621. https://doi.org/10.1093/eurheartj/ehad196. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, E.R.; Chow, E.J.; Doody, D.R.; Armenian, S.H.; Asselin, B.L.; Baker, K.S.; Bhatia, S.; Constine, L.S.; Freyer, D.R.; Kopp, L.M.; et al. Cardiometabolic Risk in Childhood Cancer Survivors: A Report from the Children’s Oncology Group. Cancer Epidemiol. Biomark. Prev. 2022, 31, 536–542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayek, S.; Li, J.; Dai, J.; Milam, J.; Jiang, L. Cardiometabolic factors’ and cardiovascular risk in young adult cancer survivors: Evidence from real-world data. Eur. J. Prev. Cardiol. 2025; in press. [Google Scholar]
- Singh, A.; Bruemmer, D. Cardiometabolic Risk: Shifting the Paradigm Toward Comprehensive Assessment. JACC Adv. 2024, 3, 100867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammoud, R.A.; Mulrooney, D.A.; Rhea, I.B.; Yu, C.; Johnson, J.N.; Chow, E.J.; Ehrhardt, M.J.; Hudson, M.M.; Ness, K.K.; Armstrong, G.T.; et al. Modifiable Cardiometabolic Risk Factors in Survivors of Childhood Cancer: JACC: CardioOncology State-of-the-Art Review. J. Am. Coll. Cardiol. CardioOnc. 2024, 6, 16–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Chow, E.J.; Oeffinger, K.C.; Border, W.L.; Leisenring, W.M.; Meacham, L.R.; Mulrooney, D.A.; Sklar, C.A.; Stovall, M.; Robison, L.L.; et al. Traditional Cardiovascular Risk Factors and Individual Prediction of Cardiovascular Events in Childhood Cancer Survivors. JNCI J. Natl. Cancer Inst. 2019, 112, 256–265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raisi-Estabragh, Z.; Murphy, A.C.; Ramalingam, S.; Scherrer-Crosbie, M.; Lopez-Fernandez, T.; Reynolds, K.L.; Aznar, M.; Lin, A.E.; Libby, P.; Cordoba, R.; et al. Cardiovascular Considerations Before Cancer Therapy: Gaps in Evidence and JACC: CardioOncology Expert Panel Recommendations. J. Am. Col.l Cardiol. CardioOnc. 2024, 6, 631–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armenian, S.H.; Lacchetti, C.; Lenihan, D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2017, 13, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F.; et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, vii155–vii166. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995, Erratum in Circulation 2013, 128, e394. [Google Scholar] [CrossRef] [PubMed]
- Bennati, E.; Girolami, F.; Spaziani, G.; Calabri, G.B.; Favre, C.; Parrini, I.; Lucà, F.; Tamburini, A.; Favilli, S. Cardio-Oncology in Childhood: State of the Art. Curr. Oncol. Rep. 2022, 24, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Simbre, V.C., II; Duffy, S.A.; Dadlani, G.H.; Miller, T.L.; Lipshultz, S.E. Cardiotoxicity of Cancer Chemotherapy: Implications for children. Pediatr. Drugs 2005, 7, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.; Felmingham, B.; Jessop, S.; Celermajer, D.S.; Kotecha, R.S.; Govender, D.; Hanna, D.M.T.; O’COnnor, M.; Manudhane, R.; Ayer, J.; et al. Cardio-Oncology Recommendations for Pediatric Oncology Patients: An Australian and New Zealand Delphi Consensus. J. Am. Coll. Cardiol. Adv. 2022, 1, 100155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehrhardt, M.J.; Leerink, J.M.; Mulder, R.L.; Mavinkurve-Groothuis, A.; Kok, W.; Nohria, A.; Nathan, P.C.; Merkx, R.; de Baat, E.; A Asogwa, O.; et al. Systematic review and updated recommendations for cardiomyopathy surveillance for survivors of childhood, adolescent, and young adult cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2023, 24, e108–e120. [Google Scholar] [CrossRef]
- DeVine, A.; Landier, W.; Hudson, M.M.; Constine, L.S.; Bhatia, S.; Armenian, S.H.; Gramatges, M.M.; Chow, E.J.; Friedman, D.N.; Ehrhardt, M.J. The Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. JAMA Oncol. 2025, 11, 544, Erratum in JAMA Oncol. 2025, 11, 570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kesting, S.; Giordano, U.; Weil, J.; McMahon, C.J.; Albert, D.C.; Berger, C.; Budts, W.; Fritsch, P.; Hidvégi, E.V.; Oberhoffer-Fritz, R.; et al. Association of European Paediatric and Congenital Cardiology practical recommendations for surveillance and prevention of cardiac disease in childhood cancer survivors: The importance of physical activity and lifestyle changes From the Association of European Paediatric and Congenital Cardiology Working Group Sports Cardiology, Physical Activity and Prevention, Working Group Adult Congenital Heart Disease, Working Group Imaging and Working Group Heart Failure. Cardiol. Young 2024, 34, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Children’s Cancer and Leukaemia Group (CCLG). Late Effects Therapy-Based Long-Term Follow-Up Practice Statement; Version 3.0; CCLG: Leicester, UK, 2023; Available online: https://media.gosh.nhs.uk/documents/Appendix48-Late_Effects_Therapy_Base_Long-term_Follow-up_Practice_Statement_CCLG.pdf (accessed on 8 November 2025).
- Sieswerda, E.; Postma, A.; van Dalen, E.C.; van der Pal, H.J.H.; Tissing, W.J.E.; Rammeloo, L.A.J.; Kok, W.E.M.; van Leeuwen, F.E.; Caron, H.N.; Kremer, L.C.M. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann. Oncol. 2012, 23, 2191–2198. [Google Scholar] [CrossRef]
- Wong-Siegel, J.R.; Kim, Y.; Stitziel, N.O.; Javaheri, A. Genetic Testing in Evaluating Risk of Anthracycline Cardiomyopathy: Are We There Yet? J. Am. Coll. Cardiol. CardioOnc. 2023, 5, 406–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leerink, J.M.; de Baat, E.C.; Feijen, E.A.; Bellersen, L.; van Dalen, E.C.; Grotenhuis, H.B.; Kapusta, L.; Kok, W.E.; Loonen, J.; van der Pal, H.J.; et al. Cardiac Disease in Childhood Cancer Survivors: JACC CardioOncology State-of-the-Art Review. J. Am. Coll. Cardiol. CardioOnc. 2020, 2, 363–378. [Google Scholar] [CrossRef]
- Chow, E.J.; Chen, Y.; Kremer, L.C.; Breslow, N.E.; Hudson, M.M.; Armstrong, G.T.; Border, W.L.; Feijen, E.A.; Green, D.M.; Meacham, L.R.; et al. Individual Prediction of Heart Failure Among Childhood Cancer Survivors. J. Clin. Oncol. 2015, 33, 394–402. [Google Scholar] [CrossRef]
- Chow, E.J.; Chen, Y.; Hudson, M.M.; Feijen, E.A.; Kremer, L.C.; Border, W.L.; Green, D.M.; Meacham, L.R.; Mulrooney, D.A.; Ness, K.K.; et al. Prediction of Ischemic Heart Disease and Stroke in Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Athanasopoulou, S.G.; Kampaktsis, P.N.; Kokkinidis, D.G.; Papanastasiou, C.A.; Feher, A.; Steingart, R.M.; Oeffinger, K.C.; Gupta, D. Development and Validation of a Clinical Score for Cardiovascular Risk Stratification of Long-Term Childhood Cancer Survivors. Oncologist 2018, 23, 965–973. [Google Scholar] [CrossRef]
- Aziz-Bose, R.; Margossian, R.; Ames, B.L.; Moss, K.; Ehrhardt, M.J.; Armenian, S.H.; Yock, T.I.; Nekhlyudov, L.; Williams, D.; Hudson, M.; et al. Delphi Panel Consensus Recommendations for Screening and Managing Childhood Cancer Survivors at Risk for Cardiomyopathy. J Am. Coll. Cardiol. CardioOnc. 2022, 4, 354–367. [Google Scholar] [CrossRef]
- CCSS Cardiovascular Risk Calculator. St. Jude Children’s Research Hospital, Childhood Cancer Survivor Study (CCSS). Available online: https://ccss.stjude.org/tools-documents/calculators-other-tools/ccss-cardiovascular-risk-calculator.html (accessed on 31 October 2025).
- Ryan, T.D.; Border, W.L.; Baker-Smith, C.; Barac, A.; Bock, M.J.; Canobbio, M.M.; Choueiter, N.F.; Chowdhury, D.; Gambetta, K.E.; Glickstein, J.S.; et al. The landscape of cardiovascular care in pediatric cancer patients and survivors: A survey by the ACC Pediatric Cardio-Oncology Work Group. Cardio-Oncol. 2019, 5, 16. [Google Scholar] [CrossRef]
- Hernandez, N.B.; Shliakhtsitsava, K.; Tolani, D.; Cochran, C.; Butts, R.; Bonifacio, J.; Journey, E.; Oppenheim, J.N.; Pennant, S.G.; Arnold, K.; et al. A comprehensive pediatric cardio-oncology program: A single institution approach to cardiovascular care for pediatric patients with cancer and childhood cancer survivors. Cardio-Oncol. 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karazisi, C.; Dellborg, M.; Mellgren, K.; Giang, K.W.; Skoglund, K.; Eriksson, P.; Mandalenakis, Z. Heart failure in patients with congenital heart disease after a cancer diagnosis. ESC Heart Fail. 2024, 11, 3388–3394. [Google Scholar] [CrossRef]
- Goldberg, J.F.; Ness, K.K.; Chi, X.; Santucci, A.K.; Plana, J.C.; Joshi, V.M.; Luepker, R.V.; Durand, J.-B.; Partin, R.E.; Howell, R.M.; et al. Cardiovascular Family History Increases Risk for Late-Onset Adverse Cardiovascular Outcomes in Childhood Cancer Survivors: A St. Jude Lifetime Cohort Report. Cancer Epidemiol. Biomark. Prev. 2020, 30, 123–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; E Tissing, W.J.; Shankar, S.; Sieswerda, E.; et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015, 16, e123–e136. [Google Scholar] [CrossRef]
- Nieto, Y.; Cagnoni, P.J.; Bearman, S.I.; Shpall, E.J.; Matthes, S.; Jones, R.B. Cardiac toxicity following high-dose cyclophosphamide, cisplatin, and BCNU (STAMP-I) for breast cancer. Biol. Blood Marrow Transplant. 2000, 6, 198–203. [Google Scholar] [CrossRef]
- Steinherz, L.J.; Steinherz, P.G.; Mangiacasale, D.; O’Reilly, R.; Allen, J.; Sorell, M.; Miller, D.R. Cardiac changes with cyclophosphamide. Med. Pediatr. Oncol. 1981, 9, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Yanagi, N.; Maruyama, T.; Gondo, H.; Okamura, T.; Kaji, Y.; Harada, M. Left Ventricular Diastolic Dysfunction Induced by Cyclophosphamide in Blood Stem Cell Transplantation. Jpn. Heart J. 2002, 43, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Gottdiener, J.S.; Appelbaum, F.R.; Ferrans, V.J.; Deisseroth, A.; Ziegler, J. Cardiotoxicity Associated with High-Dose Cyclophosphamide Therapy. Arch. Intern. Med. 1981, 141, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of Chemotherapeutic Agents: Incidence, treatment and prevention. Drug Saf. 2000, 22, 263–302. [Google Scholar] [CrossRef] [PubMed]
- Alizadehasl, A.; Shahrami, B.; Rahbarghazi, R.; Aliabadi, A.Y.; Jebelli, S.F.H.; Zonooz, Y.A.; Hakimian, H.; Fathi, F.; Forati, S.; Rezabakhsh, A. Post-transplant cyclophosphamide-induced cardiotoxicity: A comprehensive review. J. Cardiovasc. Thorac. Res. 2024, 16, 211–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheppard, R.J.; Berger, J.; Sebag, I.A. Cardiotoxicity of Cancer Therapeutics: Current Issues in Screening, Prevention, and Therapy. Front. Pharmacol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Mu, K.; Zhang, J.; Chen, H.; Huang, G. Association between global longitudinal strain and occurrence of cardiovascular events among pediatric patients following high-dose cyclophosphamide chemotherapy: A prospective cohort study. Transl. Pediatr. 2024, 13, 1061–1070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quezado, Z.M.; Wilson, W.H.; Cunnion, R.E.; Parker, M.M.; Reda, D.; Bryant, G.; Ognibene, F.P. High-Dose Ifosfamide Is Associated with Severe, Reversible Cardiac Dysfunction. Ann. Intern. Med. 1993, 118, 31–36. [Google Scholar] [CrossRef]
- Hanchate, L.P.; Sharma, S.R.; Madyalkar, S. Cisplatin Induced Acute Myocardial Infarction and Dyslipidemia. J. Clin. Diagn. Res. 2017, 11, OD05–OD07. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Dwyer, P.J.; Johnson, S.W.; Hamilton, T.C. Cisplatin and its analogues. In Cancer: Principles and Practice of Oncology; DeVita, V.T., Jr., Hellman, S., Rosenberg, S.A., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 418–432. [Google Scholar]
- Mudd Jr, T.W.; Khalid, M.; Guddati, A.K. Cardiotoxicity of chemotherapy and targeted agents. Am. J. Cancer Res. 2021, 11, 1132–1147. [Google Scholar] [PubMed] [PubMed Central]
- Depetris, I.; Marino, D.; Bonzano, A.; Cagnazzo, C.; Filippi, R.; Aglietta, M.; Leone, F. Fluoropyrimidine-induced cardiotoxicity. Crit. Rev. Oncol. Hematol. 2018, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Jain, D.; Aronow, W.S.; Frishman, W.H. Cardiotoxicity of Cancer Therapies. Cardiol. Rev. 2019, 27, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Brell, J.M. 5-Fluorouracil Cardiotoxicity: Known but Unknown. J. Am. Coll. Cardiol. CardioOnc. 2021, 3, 110–112. [Google Scholar] [CrossRef]
- Lenneman, C.G.; Sawyer, D.B. Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ. Res. 2016, 118, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Hiraide, M. Cardiotoxicities of 5-Fluorouracil and Other Fluoropyrimidines. Curr. Treat. Options Oncol. 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Forni, M.; Malet-Martino, M.C.; Jaillais, P.; Shubinski, R.E.; Bachaud, J.M.; Lemaire, L.; Canal, P.; Chevreau, C.; Carrié, D.; Soulié, P. Cardiotoxicity of high-dose continuous infusion fluorouracil: A prospective clinical study. J. Clin. Oncol. 1992, 10, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.; Kloner, R.A.; Ensley, J.; Al-Sarraf, M.; Revels, S.; Olivenstein, A.; Bhasin, S.; Kerpel-Fronious, S.; Turi, Z.G. Continuous ambulatory ECG monitoring during fluorouracil therapy: A prospective study. J. Clin. Oncol. 1989, 7, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. Cardio-oncology and transplantation for acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2023, 36, 101465. [Google Scholar] [CrossRef] [PubMed]
- Rotz, S.J.; Ryan, T.D.; Hlavaty, J.; George, S.A.; El-Bietar, J.; Dandoy, C.E. Cardiotoxicity and cardiomyopathy in children and young adult survivors of hematopoietic stem cell transplant. Pediatr. Blood Cancer 2017, 64, e26600. [Google Scholar] [CrossRef] [PubMed]
- Uderzo, C.; Pillon, M.; Corti, P.; Tridello, G.; Tana, F.; Zintl, F.; Nysom, K.; Galambrun, C.; Fagioli, F.; Varotto, S.; et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: A prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007, 39, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Alizadehasl, A.; Ghadimi, N.; Hosseinifard, H.; Roudini, K.; Emami, A.H.; Ghavamzadeh, A.; Khoda-Amorzideh, D. Cardiovascular diseases in patients after hematopoietic stem cell transplantation: Systematic review and Meta-analysis. Curr. Res. Transl. Med. 2022, 71, 103363. [Google Scholar] [CrossRef] [PubMed]
- Gent, D.G.; Saif, M.; Dobson, R.; Wright, D.J. Cardiovascular Disease After Hematopoietic Stem Cell Transplantation in Adults JACC: CardioOncology State-of-the-Art Review. J. Am. Coll. Cardiol. CardioOnc. 2024, 6, 475–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bangeas, A.; Tragiannidis, A. Cardiotoxicity of Targeted Therapies in Children with Haematological Malignancies and Solid Tumors. Anti-Cancer Agents Med. Chem. 2023, 23, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, S.J.; Petrie, M.C.; Myles, R.C.; Touyz, R.M.; Lang, N.N. Cardiotoxic effects of angiogenesis inhibitors. Clin. Sci. 2021, 135, 71–100. [Google Scholar] [CrossRef]
- Wong-Siegel, J.R.; Hayashi, R.J.; Foraker, R.; Mitchell, J.D. Cardiovascular toxicities after anthracycline and VEGF-targeted therapies in adolescent and young adult cancer survivors. Cardio-Oncol. 2023, 9, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgiopoulos, G.; Makris, N.; Laina, A.; Theodorakakou, F.; Briasoulis, A.; Trougakos, I.P.; Dimopoulos, M.-A.; Kastritis, E.; Stamatelopoulos, K. Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: CardioOncology State-of-the-Art Review. J. Am. Coll. Cardiol. CardioOnc. 2023, 5, 1–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Ewer, M.S.; Suter, T.M.; Lenihan, D.J.; Niculescu, L.; Breazna, A.; Demetri, G.D.; Motzer, R.J. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: A comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur. J. Cancer 2014, 50, 2162–2170. [Google Scholar] [CrossRef]
- Jain, D.; Russell, R.R.; Schwartz, R.G.; Panjrath, G.S.; Aronow, W. Cardiac Complications of Cancer Therapy: Pathophysiology, Identification, Prevention, Treatment, and Future Directions. Curr. Cardiol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Chaar, M.; Kamta, J.; Ait-Oudhia, S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. OncoTargets Ther. 2018, 11, 6227–6237. [Google Scholar] [CrossRef] [PubMed]
- Love, V.A.; Grabie, N.; Duramad, P.; Stavrakis, G.; Sharpe, A.; Lichtman, A. CTLA-4 Ablation and Interleukin-12–Driven Differentiation Synergistically Augment Cardiac Pathogenicity of Cytotoxic T Lymphocytes. Circ. Res. 2007, 101, 248–257. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: A meta-analysis. Eur. J. Heart Fail. 2021, 23, 1739–1747. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Darvishi, B.; Farahmand, L.; Jalili, N.; Majidzadeh-A, K. Blinatumomab provoked fatal heart failure. Int. Immunopharmacol. 2016, 41, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef]
- Pennesi, E.; Michels, N.; Brivio, E.; van der Velden, V.H.J.; Jiang, Y.; Thano, A.; Ammerlaan, A.J.C.; Boer, J.M.; Beverloo, H.B.; Sleight, B.; et al. Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: Results from a phase II trial. Leukemia 2022, 36, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of Immune Therapy. Cardiol. Clin. 2019, 37, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Pathan, N.; Hemingway, C.A.; Alizadeh, A.A.; Stephens, A.C.; Boldrick, J.C.; E Oragui, E.; McCabe, C.; Welch, S.B.; Whitney, A.; O’Gara, P.; et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 2004, 363, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated with Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Kang, Y.; Smith, A.M.; Frey, N.V.; Carver, J.R.; Scherrer-Crosbie, M. Cardiovascular Effects of CAR T Cell Therapy: A Retrospective Study. J. Am. Coll. Cardiol. CardioOnc. 2020, 2, 193–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowinsky, E.K.; McGuire, W.P.; Guarnieri, T.; Fisherman, J.S.; Christian, M.C.; Donehower, R.C. Cardiac disturbances during the administration of taxol. J. Clin. Oncol. 1991, 9, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Mayer, E.L.; Burstein, H.J.; Winer, E.P.; Goldhirsch, A. Cardiac Toxicity from Systemic Cancer Therapy: A Comprehensive Review. Prog. Cardiovasc. Dis. 2010, 53, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Roca, E.; Bruera, E.; Politi, P.; Barugel, M.; Cedaro, L.; Carraro, S.; Chacon, R. Vinca Alkaloid-Induced Cardiovascular Autonomic Neuropathy. Cancer Treat. Rep. 1985, 69, 149–151. [Google Scholar] [PubMed]
- Merkx, R.; Leerink, J.M.; de Baat, E.C.; Feijen, E.A.M.; Kok, W.E.M.; Mavinkurve-Groothuis, A.M.C.; Loonen, J.; van der Pal, H.J.H.; Bellersen, L.; de Korte, C.L.; et al. Asymptomatic systolic dysfunction on contemporary echocardiography in anthracycline-treated long-term childhood cancer survivors: A systematic review. J. Cancer Surviv. 2021, 16, 338–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merkx, R.; Feijen, E.L.A.M.; Leerink, J.M.; de Baat, E.C.; Bellersen, L.; van Dalen, E.C.; van Dulmen-den Broeder, E.; van der Heiden-van der Loo, M.; van den Heuvel-Eibrink, M.M.; de Korte, C.L.; et al. Cardiac function in childhood cancer survivors treated with vincristine: Echocardiographic results from the DCCSS LATER 2 CARD study. Int. J. Cardiol. 2022, 369, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Meacham, L.R.; Chow, E.J.; Ness, K.K.; Kamdar, K.Y.; Chen, Y.; Yasui, Y.; Oeffinger, K.C.; Sklar, C.A.; Robison, L.L.; Mertens, A.C. Cardiovascular Risk Factors in Adult Survivors of Pediatric Cancer—A Report from the Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 170–181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Göbel, S.; Wingerter, A.; Prochaska, J.H.; Schulz, A.; Neu, M.A.; Henninger, N.; Spix, C.; Beutel, M.; Lackner, K.; Münzel, T.; et al. Development and Phenotype of Heart Failure in Long-Term Survivors of Childhood Cancer: The CVSS Study. J. Am. Heart Assoc. 2023, 12, e030020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knoester, H.; Sol, J.J.; Ramsodit, P.; Kuipers, I.M.; Clur, S.-A.B.; Bos, A.P. Cardiac Function in Pediatric Septic Shock Survivors. Arch. Pediatr. Adolesc. Med. 2008, 162, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.; Ransom, J.; Evans, W. Age-Related Adriamycin Cardiotoxicity in Children. Cancer Treat. Rep. 1978, 62, 1381–1385. [Google Scholar] [PubMed]
- Screever, E.M.; Meijers, W.C.; Moslehi, J.J. Age-Related Considerations in Cardio-Oncology. J. Cardiovasc. Pharmacol. Ther. 2020, 26, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female Sex and Higher Drug Dose as Risk Factors for Late Cardiotoxic Effects of Doxorubicin Therapy for Childhood Cancer. N. Engl. J. Med. 1995, 332, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and Meta-Analysis of Incidence and Clinical Predictors of Anthracycline Cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801, Erratum in Eur. Heart J. 2018, 39, 839. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, A.L.; Lobo, R.S.; Valencia, J.C.V.; Leal, O.A.; Hernández, D.C.P.; Barrios, N.A.; Ramírez, I.P. Early-onset Cardiotoxicity assessment related to anthracycline in children with leukemia. A Prospective Study. Colomb. Medica 2021, 52, e2034542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heemelaar, J.C.; Speetjens, F.M.; al Jaff, A.A.; Evenhuis, R.E.; Polomski, E.A.; Mertens, B.J.; Jukema, J.W.; Gelderblom, H.; van de Sande, M.A.; Antoni, M.L. Impact of Age at Diagnosis on Cardiotoxicity in High-Grade Osteosarcoma and Ewing Sarcoma Patients. J. Am. Coll. Cardiol. CardioOnc. 2023, 5, 117–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kouwenberg, T.W.; van Dalen, E.C.; Feijen, E.A.M.; Netea, S.A.; Bolier, M.; Slieker, M.G.; Hoesein, F.A.A.M.; Kremer, L.C.M.; Grotenhuis, H.B.; Mavinkurve-Groothuis, A.M.C. Acute and early-onset cardiotoxicity in children and adolescents with cancer: A systematic review. BMC Cancer 2023, 23, 866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Getz, K.D.; Sung, L.; Ky, B.; Gerbing, R.B.; Leger, K.J.; Leahy, A.B.; Sack, L.; Woods, W.G.; Alonzo, T.; Gamis, A.; et al. Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2019, 37, 12–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samosir, S.M.; Utamayasa, I.K.A.; Andarsini, M.R.; Rahman, M.A.; Ontoseno, T.; Hidayat, T.; Ugrasena, I.D.G.; Larasati, M.C.S.; Cahyadi, A. Risk Factors of Daunorubicine Induced Early Cardiotoxicity in Childhood Acute Lymphoblastic Leukemia: A Retrospective Study. Asian Pac. J. Cancer Prev. 2021, 22, 1407–1412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.J.; Levy, D.; Benjamin, E.J.; Vasan, R.S. The Epidemiology of Asymptomatic Left Ventricular Systolic Dysfunction: Implications for Screening. Ann. Intern. Med. 2003, 138, 907–916. [Google Scholar] [CrossRef]
- Hunt, S.C.; Williams, R.R.; Barlow, G.K. A comparison of positive family history definitions for defining risk of future disease. J. Chronic Dis. 1986, 39, 809–821. [Google Scholar] [CrossRef]
- Silberberg, J.S.; Wlodarczyk, J.; Fryer, J.; Robertson, R.; Hensley, M.J. Risk Associated with Various Definitions of Family History of Coronary Heart Disease: The Newcastle Family History Study II. Am. J. Epidemiol. 1998, 147, 1133–1139. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Lee, I.-M.; Gaziano, J.M.; Rexrode, K.M.; Glynn, R.J.; Buring, J.E. Maternal and Paternal History of Myocardial Infarction and Risk of Cardiovascular Disease in Men and Women. Circulation 2001, 104, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Al Rifai, M.; Scheuner, M.T.; Shea, S.; Blumenthal, R.S.; Nasir, K.; Blaha, M.J.; McEvoy, J.W. Basic vs More Complex Definitions of Family History in the Prediction of Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. Mayo Clin. Proc. 2018, 93, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berkman, A.M.; Andersen, C.R.; Landstrom, A.P.; Hildebrandt, M.A.; Gilchrist, S.C.; Roth, M.E. Cardiovascular Disease in Childhood, Adolescent, and Young Adult Cancer Survivors: The Impact of Family History of Premature Heart Disease. J. Adolesc. Young-Adult Oncol. 2024, 13, 548–556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Follin, C.; Gabery, S.; Petersén, Å.; Sundgren, P.C.; Björkman-Burtcher, I.; Lätt, J.; Mannfolk, P.; Erfurth, E.M. Associations between Metabolic Risk Factors and the Hypothalamic Volume in Childhood Leukemia Survivors Treated with Cranial Radiotherapy. PLoS ONE 2016, 11, e0147575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morel, S.; Léveillé, P.; Samoilenko, M.; Franco, A.; England, J.; Malaquin, N.; Tu, V.; Cardin, G.B.; Drouin, S.; Rodier, F.; et al. Biomarkers of cardiometabolic complications in survivors of childhood acute lymphoblastic leukemia. Sci. Rep. 2020, 10, 21507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Joshi, V.M.; Ness, K.K.; Marwick, T.H.; Zhang, N.; Srivastava, D.; Griffin, B.P.; Grimm, R.A.; Thomas, J.; Phelan, D.; et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results from the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 2015, 65, 2511–2522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zareifar, S.; Haghpanah, S.; Shorafa, E.; Shakibazad, N.; Karamizadeh, Z. Evaluation of Metabolic Syndrome and Related Factors in Children Affected by Acute Lymphoblastic Leukemia. Indian J. Med. Paediatr. Oncol. 2017, 38, 97–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oudin, C.; Simeoni, M.-C.; Sirvent, N.; Contet, A.; Coroller, A.B.-L.; Bordigoni, P.; Curtillet, C.; Poirée, M.; Thuret, I.; Play, B.; et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood 2011, 117, 4442–4448. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Masetti, R.; Andreozzi, L.; Zama, D.; Fabi, M.; Meli, M.; Prete, A.; Lanari, M. The Role of Nutrition in Primary and Secondary Prevention of Cardiovascular Damage in Childhood Cancer Survivors. Nutrients 2022, 14, 3279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, C.; Friedenreich, C.M.; Yang, L. Association of Daily Sitting Time and Leisure-Time Physical Activity with Survival Among US Cancer Survivors. JAMA Oncol. 2022, 8, 395–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nghiem, V.T.; Jin, J.; Mennemeyer, S.T.; Wong, F.L. Health-related risk behaviors among U.S. childhood cancer survivors: A nationwide estimate. BMC Cancer 2024, 24, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, C.-C.; Wen, P.-C.; Yu, W.-C.; Hu, Y.-W.; Yang, C.-C. Pre-existing chronic kidney disease and hypertension increased the risk of cardiotoxicity among colorectal cancer patients treated with anticancer drugs. J. Chin. Med. Assoc. 2021, 84, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-B.; Zhang, D.; Meng, T.-T.; Zhang, Y.; Tian, P.; Chen, J.-L.; Zheng, Y.; Su, G.-H. Association of Chronic Kidney Disease with Cardiovascular Disease in Cancer Patients: A Cross-Sectional Study. Cardiorenal Med. 2023, 13, 344–353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lubas, M.M.; Wang, M.; Jefferies, J.L.; Ness, K.K.; Ehrhardt, M.J.; Krull, K.R.; Mulrooney, D.A.; Srivastava, D.K.; Howell, R.M.; Robison, L.L.; et al. The Contribution of Stress and Distress to Cardiovascular Health in Adult Survivors of Childhood Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 30, 286–294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camilli, M.; Ferdinandy, P.; Salvatorelli, E.; Menna, P.; Minotti, G. Anthracyclines, Diastolic Dysfunction and the road to Heart Failure in Cancer survivors: An untold story. Prog. Cardiovasc. Dis. 2024, 86, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.L.; Mazur, W.M.; Howell, C.R.; Plana, J.C.; Ness, K.K.; Li, Z.; Joshi, V.M.; Green, D.M.; Mulrooney, D.A.; Towbin, J.A.; et al. Cardiac remodeling after anthracycline and radiotherapy exposure in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Cancer 2021, 127, 4646–4655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldman, D.R.; Schaffer, W.L.; Steingart, R.M. Late Cardiovascular Toxicity Following Chemotherapy for Germ Cell Tumors. J. Natl. Compr. Cancer Netw. 2012, 10, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Abu Zaid, M.; Hanna, N.; El-Kouri, N.; Einhorn, L.; Albany, C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann. Oncol. 2017, 28, 2670–2679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willemse, P.-P.M.; van der Meer, R.W.; Burggraaf, J.; van Elderen, S.G.C.; de Kam, M.L.; de Roos, A.; Lamb, H.J.; Osanto, S. Abdominal visceral and subcutaneous fat increase, insulin resistance and hyperlipidemia in testicular cancer patients treated with cisplatin-based chemotherapy. Acta Oncol. 2013, 53, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hjelle, L.V.; Gundersen, P.O.M.; Oldenburg, J.; Brydøy, M.; Tandstad, T.; Wilsgaard, T.; Fosså, S.D.; Bremnes, R.M.; Haugnes, H.S. Long-term platinum retention after platinum-based chemotherapy in testicular cancer survivors: A 20-year follow-up study. Anticancer Res. 2015, 35, 1619–1625. [Google Scholar] [PubMed]
- Hjelle, L.V.; Bremnes, R.M.; Gundersen, P.O.; Sprauten, M.; Brydøy, M.; Tandstad, T.; Wilsgaard, T.; Fosså, S.D.; Oldenburg, J.; Haugnes, H.S. Associations between long-term serum platinum and neurotoxicity and ototoxicity, endocrine gonadal function, and cardiovascular disease in testicular cancer survivors. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 487.e13–487.e20. [Google Scholar] [CrossRef] [PubMed]
- Hjelle, L.V.; Gundersen, P.O.M.; Hellesnes, R.; Sprauten, M.; Brydøy, M.; Tandstad, T.; Wilsgaard, T.; Fosså, S.D.; Oldenburg, J.; Bremnes, R.M.; et al. Long-term serum platinum changes and their association with cisplatin-related late effects in testicular cancer survivors. Acta Oncol. 2018, 57, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Rothenberg, M.L.; Amiri-Kordestani, L.; Sullivan, R.J.; Steingart, R.M.; Gregory, W.; Hariharan, S.; Hammad, T.A.; Lindenfeld, J.; Murphy, M.J.; et al. Myocarditis Associated with Immune Checkpoint Inhibitors: An Expert Consensus on Data Gaps and a Call to Action. OncolOGIST 2018, 23, 874–878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivendran, S.; Agarwal, N.; Gartrell, B.; Ying, J.; Boucher, K.M.; Choueiri, T.K.; Sonpavde, G.; Oh, W.K.; Galsky, M.D. Metabolic complications with the use of mTOR inhibitors for cancer therapy. Cancer Treat. Rev. 2014, 40, 190–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lobenwein, D.; Kocher, F.; Dobner, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Cardiotoxic mechanisms of cancer immunotherapy—A systematic review. Int. J. Cardiol. 2021, 323, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Goggin, K.P.; Lu, L.; Lee, D.E.; Howell, C.R.; Srivastava, D.; Brinkman, T.M.; Armstrong, G.T.; Bhakta, N.; Robison, L.L.; Ehrhardt, M.J.; et al. Severe Sepsis During Treatment for Childhood Leukemia and Sequelae Among Adult Survivors. JAMA Netw. Open 2024, 7, e242727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D.; Burnand, B.; LaCalle, J.R.; Lázaro, P.; van het Loo, M.; McDonnell, J.; Vader, J.P.; Kahan, J.P. The RAND/UCLA Appropriateness Method User’s Manual; RAND Corporation: Santa Monica, CA, USA, 2001; Available online: https://www.rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf (accessed on 31 October 2025).
- Sparks, J.B.; Klamerus, M.L.; Caverly, T.J.; E Skurla, S.; Hofer, T.P.; A Kerr, E.; Bernstein, S.J.; Damschroder, L.J. Planning and Reporting Effective Web-Based RAND/UCLA Appropriateness Method Panels: Literature Review and Preliminary Recommendations. J. Med. Internet Res. 2022, 24, e33898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez, L.; Saurers, D.L.; Barker, P.C.; Cohen, M.S.; Colan, S.D.; Dwyer, J.; Forsha, D.; Friedberg, M.K.; Lai, W.W.; Printz, B.F.; et al. Guidelines for Performing a Comprehensive Pediatric Transthoracic Echocardiogram: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2024, 37, 119–170. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.; Singh, G.; Armenian, S.; Chen, M.-H.; Dorfman, A.L.; Garg, R.; Husain, N.; Joshi, V.; Leger, K.J.; Lipshultz, S.E.; et al. Multimodality Imaging for Cardiac Surveillance of Cancer Treatment in Children: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 1227–1253. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Skinner, R.; Iannaccone, G.; La Vecchia, G.; Montone, R.A.; Lanza, G.A.; Natale, L.; Crea, F.; Cameli, M.; Del Buono, M.G.; et al. Cardiac Imaging in Childhood Cancer Survivors: A State-of-the-Art Review. Curr. Probl. Cardiol. 2022, 48, 101544. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.D.; Bates, J.E.; Kinahan, K.E.; Leger, K.J.; Mulrooney, D.A.; Narayan, H.K.; Ness, K.; Okwuosa, T.M.; Rainusso, N.C.; Steinberger, J.; et al. Cardiovascular Toxicity in Patients Treated for Childhood Cancer: A Scientific Statement from the American Heart Association. Circulation 2025, 151, e926–e943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Delphi Consensus [22] | IGHG [23] | COG [24] | AEPC [25] | UKCCSG [26] | DCOG [27] | |
|---|---|---|---|---|---|---|
| Which kind of CTR-CVT? | Acute onset CTR-CVT | Late onset “cardiomyopathy” | Late onset “cardiomyopathy” | Late onset CTR-CVT * | Late onset HF and ischemic heart disease | Asymptomatic cardiac dysfunction |
| When to start monitoring? | During cancer treatment | After the completion of treatment ^ | After the completion of treatment ^1 | After the completion of treatment ^2 | After the completion of treatment ^3 | After the completion of treatment ^4 |
| Which cardiotoxic treatment? | AC, chestRT, VEGF-I, mTOR-I, proteasomal inhibitors, ICI | AC, chestRT, AC plus chestRT | AC, chestRT, AC plus chestRT; Others § | AC, chestRT, AC plus chestRT | AC, chestRT, AC plus chestRT; Others §1 | AC, chestRT |
| Other RFs considered | Age; CVD; familial history of CVD (not adult type); MS; CKD; Pregnancy | Pregnancy | Age, diabetes, hypertension | Age, nutritional habits, physical activity | Pregnancy | Pregnancy |
| Timing for in-treatment evaluations | Provided for VEGF-I, mTOR-I, proteasomal inhibitors, ICI | Not provided | Not provided | Not provided | Not provided | Not provided |
| How to perform cardio-oncological evaluation | For all patients: ECG, TTE For selected patients: cardiac MRI, CK-MB ° For research purpose: cardiac biomarkers | For all patients: ECG, TTE For selected patients: cardiac biomarkers # | For all patients: ECG, TTE | For all patients: ECG, TTE For selected patients: cardiac biomarkers † | For all patients: ECG, TTE | For all patients: ECG, TTE For research purpose: cardiac biomarkers |
| Risk Stratification | |||
|---|---|---|---|
| Low Risk | Moderate Risk | High Risk | |
| Delphi Consensus [22] | Cumulative AC exposure < 100 mg/m2 Level of evidence ∋: A | Not provided Level of evidence ∋: A | Defined by one of the following: − Cumulative AC exposure − ≥250 mg/m2 − Any AC dose combined with chestRT ≥15 Gy − chestRT ≥ 35 Gy − Pre-existing cardiac vulnerability * − Current treatment with VEGF-I, mTOR-I, proteasome inhibitors, ICI − Diagnosis of MS during or after cancer therapy − Chronic kidney disease − Pregnancy during cancer therapy Level of evidence ∋: A |
| IGHG [23] | Cumulative AC exposure <100 mg/m2 or RT < 15 Gy or both § Level of evidence ∋: A | Cumulative AC exposure 100 to <250 mg/m2 or RT ≥ 15 to <35 Gy Level of evidence ∋: A | Cumulative AC exposure ≥250 mg/m2 or RT ≥ 35 Gy or combined exposure of AC ≥100 mg/m2 and RT ≥ 15 Gy ^ Level of evidence ∋: A |
| COG [24] | Cumulative AC exposure < 100 mg/m2 and RT < 15 Gy § Level of evidence ∋: A | Cumulative AC exposure <250 mg/m2 and RT < 15 Gy or none # or Cumulative AC exposure 100 to <250 mg/m2 and RT ≥15 Gy † Level of evidence ∋: A | Cumulative AC exposure >250 mg/m2 with any Gy doses or no RT † Level of evidence ∋: A |
| AEPC [25] | Cumulative AC exposure <100 mg/m2 or RT < 15 Gy § Level of evidence ∋: A | Cumulative AC exposure 100 to <250 mg/m2 or RT ≥ 15 to <35 Gy ^ Level of evidence ∋: B | Cumulative AC exposure ≥250 mg/m2 or RT ≥ 35 Gy or combined exposure of AC ≥ 100 mg/m2 and RT ≥ 15 Gy ^ Level of evidence ∋: A |
| UKCCSG ° [26] | Cumulative AC exposure <250 mg/m2 # | Not provided | Cumulative AC exposure >250 mg/m2 † |
| DCOG [27] | Cumulative AC exposure <300 mg/m2 # or Exposure to AC and chestRT (regardless of doses) # or chestRT < 30 Gy # or Mitoxantrone ≥ 40 mg/m2 # Level of evidence ∋: A | Not provided Level of evidence ∋: A | Cumulative AC exposure >300 mg/m2 † or chestRT ≥30 Gy † Level of evidence ∋: A |
| Ri sk Factors for Developing CTR-CVT | |||
|---|---|---|---|
| Patient-related RFs | Age at diagnosis | Below five years of age [16,18] | |
| Personal and familial history of CV diseases | CHD [37] | ||
| impaired left ventricular systolic function [22,34] | |||
| familial history of genetic disorders that impact cardiac structure; storage disorders [22] | |||
| Familial history of non-congenital or acquired CV diseases [38] | |||
| Cardiometabollic risk factors | Metabolic syndrome [22] obesity, hypertension, diabetes, and dyslipidemia [12] | ||
| Other risk factors | Chronic kidney disease [22] Pregnancy [22,23,26,27] | ||
| Treatment related RFs | Anthracyclines [22,23,24,25,26,27] | From acute to late-onset CTX | |
| Chest radiotherapy [22,23,24,25,26,27] | From acute to late-onset CTX | ||
| Anthracyclines plus chest radiotherapy [22,25,26,39] | From acute to late-onset CTX | ||
| Alkylating agents | Cyclophosphamide >140 mg/kg or Cyclophosphamide 120–140 mg/kg plus AC [40,41,42,43,44,45,46,47] | From acute to late-onset CTX | |
| Ifosfamide [48] | Acute or early-onset CTX | ||
| Platinum derivates | Cisplatin [49,50,51] | ||
| Antimetabolites | 5-flourouracil, capecitabine [52,53,54,55,56,57,58] | Acute or early-onset CTX | |
| HSCT | Allogeneic HSCT [59,60,61,62,63] | From acute to late-onset CTX | |
| Autologous HSCT [62,63] | |||
| Targeted therapy | mTOR inhibitors [22] | Acute or early-onset CTX | |
| VEGF inhibitors [22,64,65,66] | From acute to late-onset CTX | ||
| proteasomal inhibitors [67] | |||
| Tyrosine kinase inhibitors [68,69,70,71] | |||
| Immune checkpoint inhibitors [72,73,74] | |||
| Blinatumomab [75,76] | Acute or early-onset CTX | ||
| Inotuzumab ozogamicin [77,78] | |||
| CAR-T [79,80,81,82] | |||
| Other cardiotoxic drugs | Paclitaxel [83,84] | Acute or early-onset CTX | |
| Docetaxel [19] | |||
| Vincristine [85,86,87] | From acute to late-onset CTX | ||
| RFs acquired during cancer treatment | Symptomatic or asymptomatic CTR-CVT [88] | ||
| Septic shock with need for inotropic support [89,90] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guida, F.; Fabi, M.; Balducci, A.; Zama, D.; Masetti, R.; Mercolini, F.; Belotti, T.; Cantarini, M.E.; Facchini, E.; Legnani, E.L.; et al. Risk Stratification for Cardiotoxicity in Childhood Cancer Survivors: State-of-the-Art Review and a Novel Two-Step Approach. Cancers 2025, 17, 3740. https://doi.org/10.3390/cancers17233740
Guida F, Fabi M, Balducci A, Zama D, Masetti R, Mercolini F, Belotti T, Cantarini ME, Facchini E, Legnani EL, et al. Risk Stratification for Cardiotoxicity in Childhood Cancer Survivors: State-of-the-Art Review and a Novel Two-Step Approach. Cancers. 2025; 17(23):3740. https://doi.org/10.3390/cancers17233740
Chicago/Turabian StyleGuida, Fiorentina, Marianna Fabi, Anna Balducci, Daniele Zama, Riccardo Masetti, Federico Mercolini, Tamara Belotti, Maria Elena Cantarini, Elena Facchini, Elena Lara Legnani, and et al. 2025. "Risk Stratification for Cardiotoxicity in Childhood Cancer Survivors: State-of-the-Art Review and a Novel Two-Step Approach" Cancers 17, no. 23: 3740. https://doi.org/10.3390/cancers17233740
APA StyleGuida, F., Fabi, M., Balducci, A., Zama, D., Masetti, R., Mercolini, F., Belotti, T., Cantarini, M. E., Facchini, E., Legnani, E. L., Melchionda, F., Bartolacelli, Y., Ciuca, C., Gesuete, V., Prete, A., Donti, A., & Lanari, M. (2025). Risk Stratification for Cardiotoxicity in Childhood Cancer Survivors: State-of-the-Art Review and a Novel Two-Step Approach. Cancers, 17(23), 3740. https://doi.org/10.3390/cancers17233740









