Evaluation of Risk Factors Associated with Expectant Management in CIN 1/2: A Multicenter Real-World Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Variables
2.3. Progression and Regression
2.4. Definitions of Evaluated Risk Factors
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of Published Literature
4.2.1. Findings from the Expectant Management Group
4.2.2. Findings from the Immediate Surgery Group
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANC | Absolute neutrophil count |
| ASC-H | Atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion |
| ASC-US | Atypical squamous cells of undetermined significance |
| CIN | Cervical intraepithelial neoplasia |
| CI | Confidence interval |
| DNA | Deoxyribonucleic acid |
| HPV | Human papillomavirus |
| HR | Hazard ratio |
| HSIL | High-grade squamous intraepithelial lesion |
| IRB | Institutional Review Board |
| LSIL | Low-grade squamous intraepithelial lesion |
| p16 | Cyclin-dependent kinase inhibitor 2A |
| ASCCP | American Society for Colposcopy and Cervical Pathology |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kang, M.J.; Jung, K.-W.; Bang, S.H.; Choi, S.H.; Park, E.H.; Yun, E.H.; Kim, H.-J.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2020. Cancer Res. Treat. 2023, 55, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef]
- Results of the Korean Cervical Cancer Screening Test. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=350&tblId=DT_35007_N019&vw_cd=MT_ZTITLE&list_id=350_35007_A002&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=MT_ZTITLE&path=%252FstatisticsList%252FstatisticsListIndex.do (accessed on 1 September 2024).
- Conner, S.N.; Frey, H.A.; Cahill, A.G.; Macones, G.A.; Colditz, G.A.; Tuuli, M.G. Loop electrosurgical excision procedure and risk of preterm birth: A systematic review and meta-analysis. Obstet. Gynecol. 2014, 123, 752–761. [Google Scholar] [CrossRef]
- Lycke, K.D.; Kahlert, J.; Eriksen, D.O.; Omann, C.; Pedersen, L.H.; Sundtoft, I.; Landy, R.; Petersen, L.K.; Hammer, A. Preterm birth following active surveillance vs loop excision for cervical intraepithelial neoplasia grade 2. JAMA Netw. Open 2024, 7, e242309. [Google Scholar] [CrossRef]
- Pinto, A.P.; Crum, C.P. Natural history of cervical neoplasia: Defining progression and its consequence. Clin. Obstet. Gynecol. 2000, 43, 352–362. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: Background and consensus recommendations. Int. J. Gynecol. Pathol. 2012, 16, 205–242. [Google Scholar] [CrossRef]
- Arbyn, M.; Castellsagué, X.; de Sanjosé, S.; Bruni, L.; Saraiya, M.; Bray, F.; Ferlay, J. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011, 22, 2675–2686. [Google Scholar] [CrossRef]
- de Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus vaccine: Updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar] [PubMed]
- Dempster-Rivett, K.; Innes, C.R.; Simcock, B.J.; Harker, D.; Williman, J.A.; Van Der Griend, R.A.; Whitehead, M.; Hibma, M.; Lawton, B.A.; Fitzgerald, P.; et al. Evaluation of guidelines for observational management of cervical intraepithelial neoplasia 2 in young women. Am. J. Obstet. Gynecol. 2020, 223, 408.e1–408.e11. [Google Scholar] [CrossRef] [PubMed]
- Tainio, K.; Athanasiou, A.; A O Tikkinen, K.; Aaltonen, R.; Hernándes, C.J.; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; Louvanto, K.; et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: Systematic review and meta-analysis. BMJ 2018, 360, k499. [Google Scholar] [CrossRef]
- Loopik, D.L.; Bentley, H.A.; Eijgenraam, M.N.; IntHout, J.; Bekkers, R.L.; Bentley, J.R. The natural history of cervical intraepithelial neoplasia grades 1, 2, and 3: A systematic review and meta-analysis. J. Low Genit. Tract Dis. 2021, 25, 221–231. [Google Scholar] [CrossRef]
- Sykes, P.H.; Simcock, B.J.; Innes, C.R.; Harker, D.; Williman, J.A.; Whitehead, M.; van der Griend, R.A.; Lawton, B.A.; Hibma, M.; Fitzgerald, P.; et al. Predicting regression of cervical intraepithelial neoplasia grade 2 in women under 25 years. Am. J. Obstet. Gynecol. 2022, 226, 222.e1–222.e13. [Google Scholar] [CrossRef]
- Solomon, D.; Schiffman, R.; Tarone, R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: Baseline results from a randomized trial. J. Natl. Cancer Inst. 2001, 93, 293–299. [Google Scholar] [CrossRef]
- A So, K.; Kim, S.A.; Lee, Y.K.; Lee, I.H.; Lee, K.H.; Rhee, J.E.; Kee, M.K.; Cho, C.H.; Hong, S.R.; Hwang, C.S.; et al. Risk factors for cytological progression in HPV 16 infected women with ASC-US or LSIL: The Korean HPV cohort. Obstet. Gynecol. Sci. 2018, 61, 662–668. [Google Scholar] [CrossRef]
- Korean Society of Gynecologic Oncology. Practice Guidelines for the Early Detection of Cervical Cancer. Available online: https://cdn.medsoft.co.kr/201/date/j_02.pdf (accessed on 1 September 2024).
- Su, Y.; Zheng, T.; Bi, Z.; Jia, X.; Li, Y.; Kuang, X.; Yang, Y.; Chen, Q.; Lin, H.; Huang, Y.; et al. Pattern of multiple human papillomavirus infection and type competition: An analysis in healthy Chinese women aged 18–45 years. Hum. Vaccin. Immunother. 2024, 20, 2334474. [Google Scholar] [CrossRef]
- Seong, J.; Ryou, S.; Lee, J.; Yoo, M.; Hur, S.; Choi, B.S.; Korea HPV Cohort Study. Enhanced disease progression due to persistent HPV-16/58 infections in Korean women: A systematic review and the Korea HPV cohort study. Virol. J. 2021, 18, 188. [Google Scholar] [CrossRef]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Gynecol. Cancer 2007, 17, 979–983. [Google Scholar] [CrossRef] [PubMed]
- So, K.A.; Lee, I.H.; Lee, K.H.; Hong, S.R.; Kim, Y.J.; Seo, H.H.; Kim, T.J. Human papillomavirus genotype-specific risk in cervical carcinogenesis. J. Gynecol. Oncol. 2019, 30, e52. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, H.; Yan, Y.; Bian, R.; Huang, W.; Zhang, X.; Nie, J. Distribution of HPV types among women with HPV-related diseases and exploration of lineages and variants of HPV 52 and 58 in China: A systematic literature review. Virol. J. 2023, 20, 254. [Google Scholar] [CrossRef]
- Ouh, Y.-T.; Park, J.J.; Kang, M.; Kim, M.; Song, J.Y.; Shin, S.J.; Shim, S.-H.; Yoo, H.J.; Lee, M.; Lee, S.-J.; et al. Discrepancy between cytology and histology in cervical cancer screening: A multicenter retrospective study (KGOG 1040). J. Korean Med. Sci. 2021, 36, e164. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J. Low Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Park, S.; Park, J.W.; Pitot, H.C.; Lambert, P.F. Loss of dependence on continued expression of the HPV16 e7 oncogene in cervical cancers and precancerous lesions arising in Fanconi anemia pathway-deficient mice. mBio 2016, 7, e00628-16. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Chen, Y.; Lin, W.; Gao, H.; Chen, M.; Osafo, K.S.; Mao, X.; Kang, Y.; Huang, L.; et al. Peripheral Blood Lymphocytes Influence Human Papillomavirus Infection and Clearance: A Retrospective Cohort Study. Virol. J. 2023, 20, 80. [Google Scholar] [CrossRef]
- Boulesteix, A.; Hoffmann, S. To adjust or not to adjust: It is not the tests performed that count, but how they are reported and interpreted. BMJ Med. 2024, 3, e000783. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, X.X.; Li, Z.; Peppelenbosch, M.P.; Ma, Z.; Pan, Q. The burden of human papillomavirus and Chlamydia trachomatis coinfection in women: A large cohort study in Inner Mongolia, China. J. Infect. Dis. 2019, 219, 206–214. [Google Scholar] [CrossRef]
- Bellaminutti, S.; Seraceni, S.; De Seta, F.; Gheit, T.; Tommasino, M.; Comar, M. HPV and Chlamydia trachomatis co-detection in young asymptomatic women from a high-incidence area for cervical cancer. J. Med. Virol. 2014, 86, 1920–1925. [Google Scholar] [CrossRef]
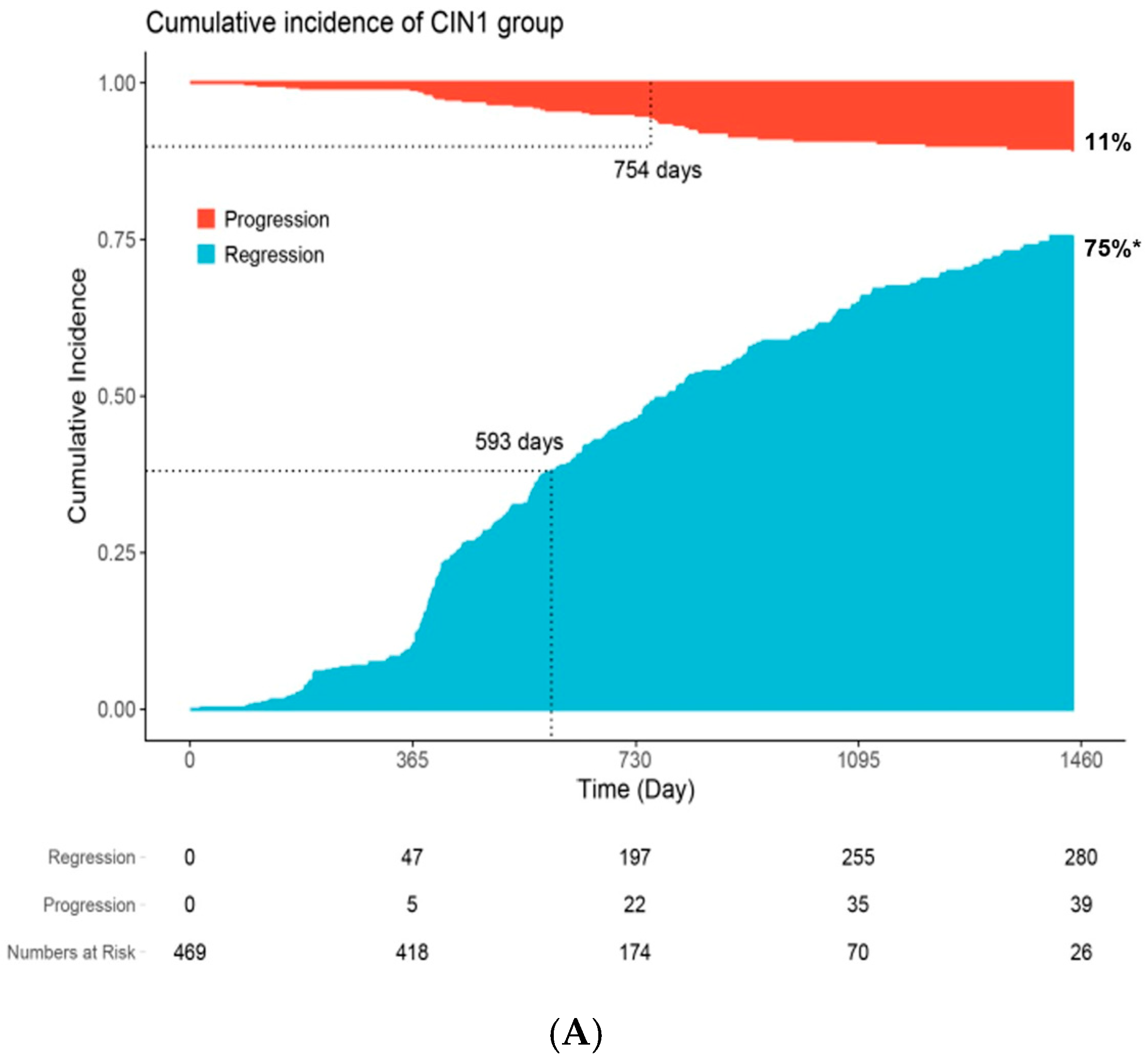
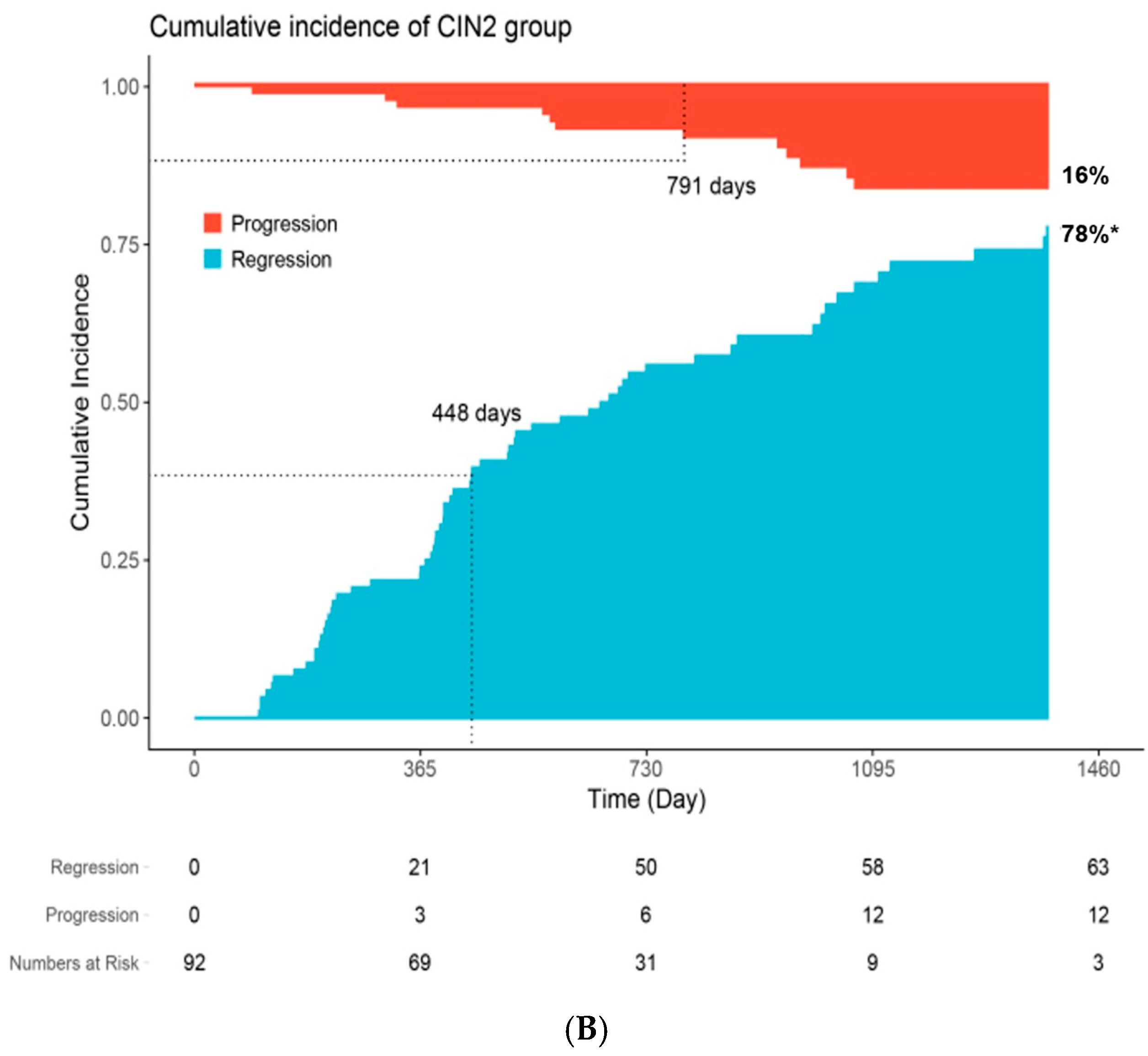
| Characteristic | N | Overall N = 561 a | Persistent n = 151 a | Progression n = 53 a | Regression n = 357 a | p-Value b |
|---|---|---|---|---|---|---|
| Diagnosed age, years | 561 | 0.3 | ||||
| 20s | 138 (25%) | 36 (24%) | 15 (28%) | 87 (24%) | ||
| 30s | 149 (27%) | 41 (27%) | 17 (32%) | 91 (25%) | ||
| 40s | 127 (23%) | 27 (18%) | 8 (15%) | 92 (26%) | ||
| over 50s | 147 (26%) | 47 (31%) | 13 (25%) | 87 (24%) | ||
| Time to clinical outcome c | 561 | 568 (18, 3382) | 661 (367, 3382) | 656 (91, 1575) | 520 (18, 1987) | <0.001 |
| HPV virus type | 531 | <0.001 | ||||
| Low-risk | 89 (17%) | 32 (22%) | 7 (14%) | 50 (15%) | ||
| High-risk | 324 (61%) | 85 (59%) | 43 (84%) | 196 (58%) | ||
| Negative | 118 (22%) | 26 (18%) | 1 (2.0%) | 91 (27%) | ||
| High-risk HPV virus | ||||||
| 16 | 531 | 45 (8.5%) | 10 (7.0%) | 10 (20%) | 25 (7.4%) | 0.021 |
| 18 | 531 | 13 (2.4%) | 2 (1.4%) | 2 (3.9%) | 9 (2.7%) | 0.5 |
| 52 | 531 | 27 (5.1%) | 6 (4.2%) | 2 (3.9%) | 19 (5.6%) | 0.9 |
| 58 | 531 | 38 (7.2%) | 10 (7.0%) | 9 (18%) | 19 (5.6%) | 0.015 |
| 31 | 531 | 8 (1.5%) | 4 (2.8%) | 2 (3.9%) | 2 (0.6%) | 0.029 |
| 33 | 531 | 6 (1.1%) | 2 (1.4%) | 2 (3.9%) | 2 (0.6%) | 0.083 |
| 45 | 531 | 7 (1.3%) | 2 (1.4%) | 1 (2.0%) | 4 (1.2%) | 0.7 |
| 35 | 531 | 10 (1.9%) | 2 (1.4%) | 2 (3.9%) | 6 (1.8%) | 0.4 |
| 39 | 531 | 16 (3.0%) | 3 (2.1%) | 1 (2.0%) | 12 (3.6%) | 0.8 |
| 51 | 531 | 31 (5.8%) | 9 (6.3%) | 3 (5.9%) | 19 (5.6%) | >0.9 |
| 56 | 531 | 27 (5.1%) | 12 (8.4%) | 3 (5.9%) | 12 (3.6%) | 0.068 |
| 59 | 531 | 7 (1.3%) | 3 (2.1%) | 0 (0%) | 4 (1.2%) | 0.5 |
| 66 | 531 | 23 (4.3%) | 7 (4.9%) | 3 (5.9%) | 13 (3.9%) | 0.6 |
| 68 | 531 | 32 (6.0%) | 5 (3.5%) | 3 (5.9%) | 24 (7.1%) | 0.3 |
| Multiple HPV infection | 531 | 73 (14%) | 23 (16%) | 13 (25%) | 37 (11%) | 0.012 |
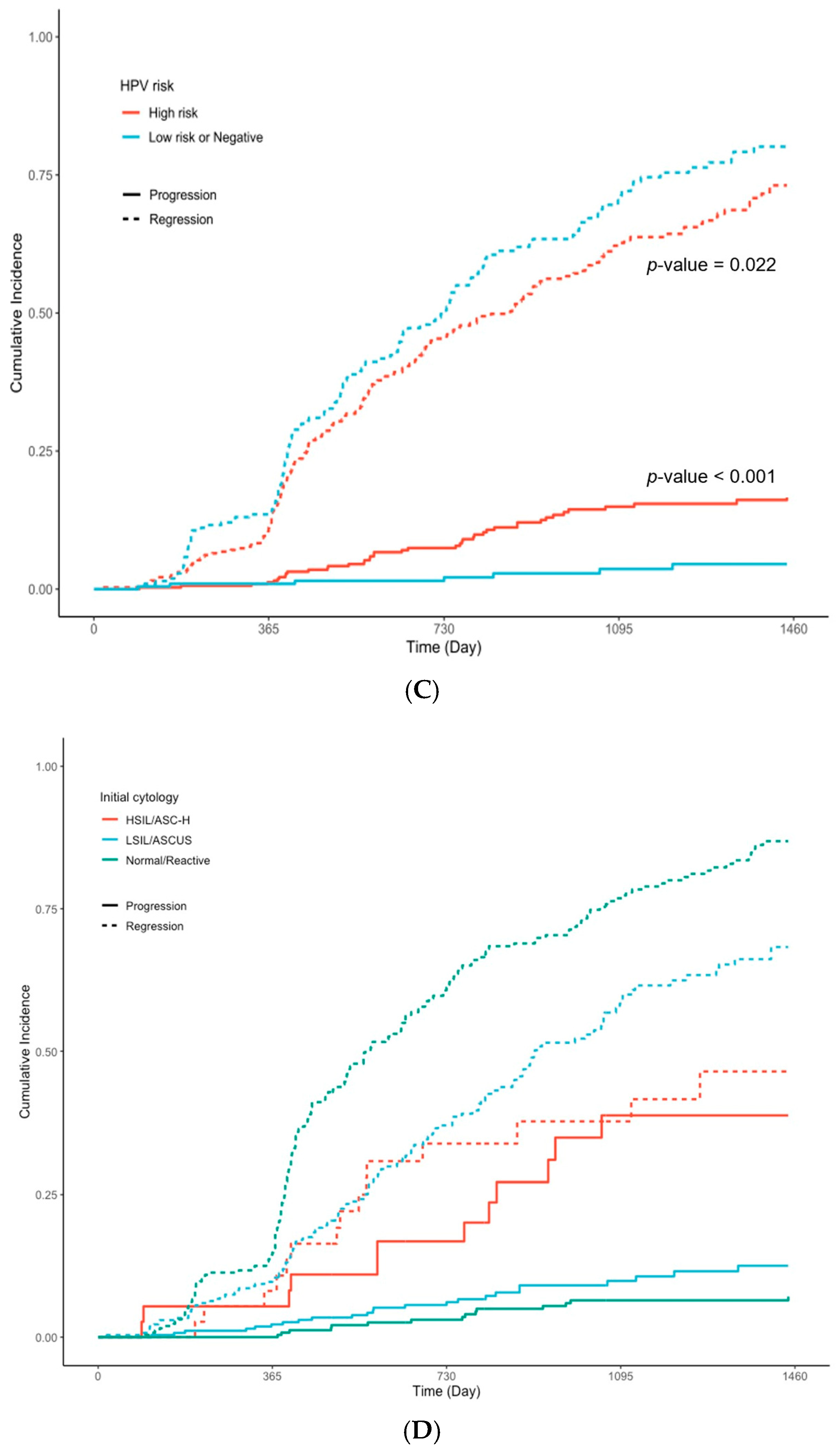
| Initial Cytology | 561 | <0.001 | ||||
| ASCUS/LSIL | 268 (48%) | 102 (68%) | 24 (45%) | 142 (40%) | ||
| ASC-H/HSIL | 37 (6.6%) | 8 (5.3%) | 13 (25%) | 16 (4.5%) | ||
| Normal/RCC | 256 (46%) | 41 (27%) | 16 (30%) | 199 (56%) | ||
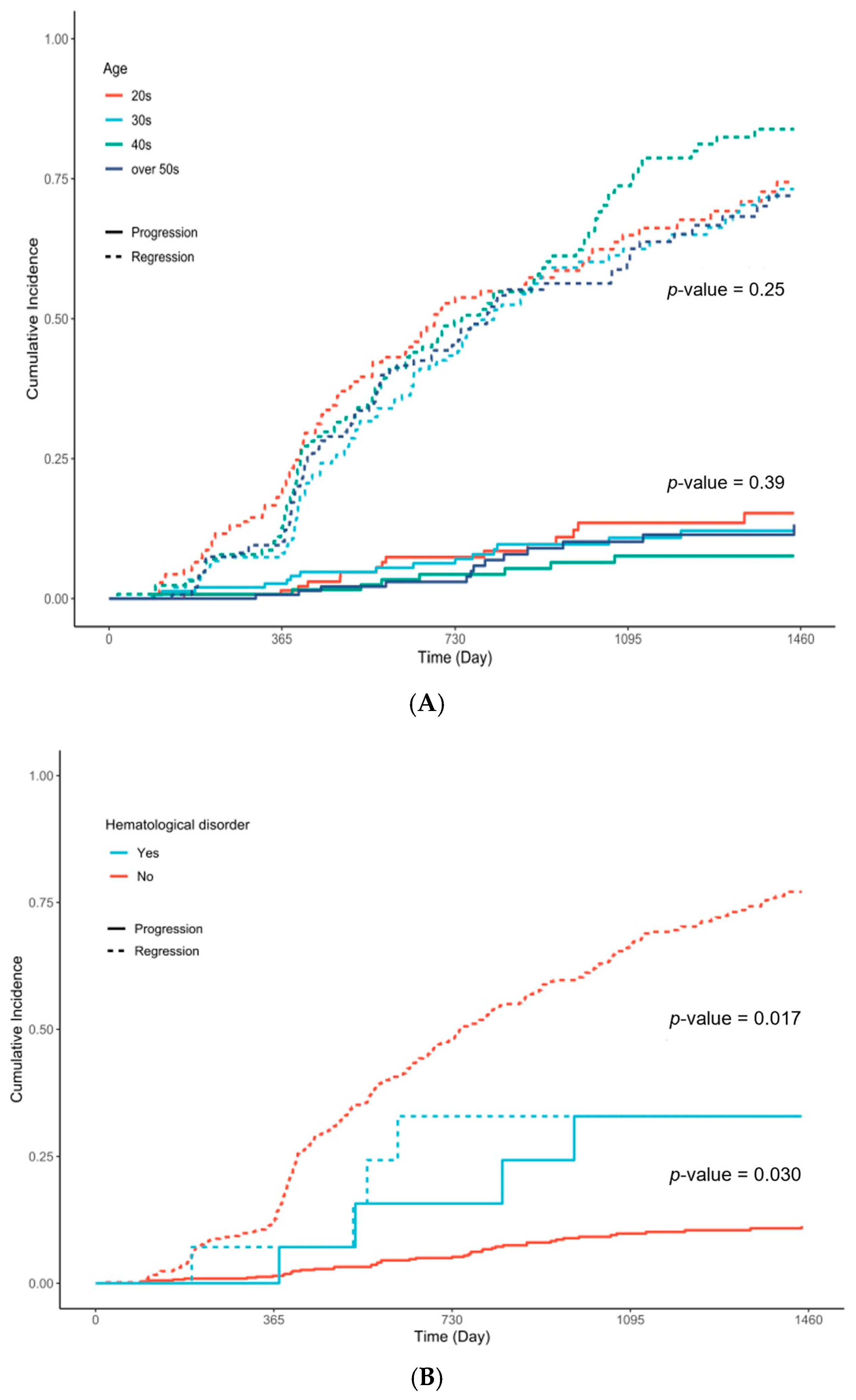
| Hematological Disorder | 561 | 14 (2.5%) | 5 (3.3%) | 4 (7.5%) | 5 (1.4%) | 0.022 |
| Pelvic inflammatory disease | 561 | 260 (46%) | 73 (48%) | 26 (49%) | 161 (45%) | 0.7 |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Crude HR | 95% CI | p-Value | N | Adjusted HR | 95% CI | p-Value |
| Initial CIN state | 561 | 531 | ||||||
| CIN 1 | 1.00 | Ref. | 1.00 | Ref. | ||||
| CIN 2 | 1.16 | 0.86, 1.57 | 0.3 | 1.47 | 1.07, 2.01 | 0. 016 | ||
| Diagnosed age | 561 | 531 | ||||||
| 20s | 1.00 | Ref. | 1.00 | Ref. | ||||
| 30s | 0.83 | 0.62, 1.11 | 0.2 | 0.87 | 0.63, 1.21 | 0.4 | ||
| 40s | 1.09 | 0.82, 1.45 | 0.6 | 1.03 | 0.76, 1.40 | 0.9 | ||
| over 50s | 0.87 | 0.64, 1.17 | 0.4 | 0.92 | 0.67, 1.27 | 0.6 | ||
| Initial Cytology | 561 | 531 | ||||||
| Normal/RCC | 1.00 | Ref. | 1.00 | Ref. | ||||
| ASCUS/LSIL | 0.54 | 0.44, 0.67 | <0.001 | 0.54 | 0.44, 0.68 | <0.001 | ||
| ASC-H/HSIL | 0.30 | 0.17, 0.52 | <0.001 | 0.30 | 0.17, 0.55 | <0.001 | ||
| Hematological Disorder | 561 | 531 | ||||||
| No | 1.00 | Ref. | 1.00 | Ref. | ||||
| Yes | 0.39 | 0.16, 0.94 | 0.035 | 0.39 | 0.16, 0.98 | 0.045 | ||
| Pelvic inflammatory disease | 561 | 531 | ||||||
| No | 1.00 | Ref. | 1.00 | Ref. | ||||
| Yes | 0.91 | 0.74, 1.12 | 0.4 | 0.97 | 0.79, 1.21 | 0.8 | ||
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Crude HR a | 95% CI a | p-Value | N | Adjusted HR a | 95% CI a | p-Value |
| HPV virus type | 531 | 531 | ||||||
| Low risk or Negative | 1.00 | Ref. | 1.00 | Ref. | ||||
| High risk | 0.78 | 0.63, 0.96 | 0.020 | 0.78 | 0.63, 0.97 | 0.025 | ||
| HPV type a | ||||||||
| 16 | 531 | 0.86 | 0.55, 1.34 | 0.5 | 531 | 0.89 | 0.56, 1.39 | 0.6 |
| 18 | 531 | 0.82 | 0.44, 1.52 | 0.5 | 531 | 0.73 | 0.38, 1.43 | 0.4 |
| 52 | 531 | 1.42 | 0.86, 2.35 | 0.2 | 531 | 1.44 | 0.89, 2.32 | 0.14 |
| 58 | 531 | 0.65 | 0.41, 1.04 | 0.071 | 531 | 0.61 | 0.39, 0.96 | 0.032 |
| 31 | 531 | 0.43 | 0.11, 1.66 | 0.2 | 531 | NA b | ||
| 33 | 531 | 0.41 | 0.11, 1.56 | 0.2 | 531 | 0.47 | 0.13, 1.76 | 0.3 |
| 45 | 531 | 0.75 | 0.29, 1.96 | 0.6 | 531 | 0.98 | 0.39, 2.49 | >0.9 |
| 35 | 531 | 0.61 | 0.28, 1.32 | 0.2 | 531 | 0.71 | 0.34, 1.49 | 0.4 |
| 39 | 531 | 1.38 | 0.70, 2.74 | 0.4 | 531 | - c | - c | 0.2 c |
| 51 | 531 | 0.99 | 0.59, 1.65 | >0.9 | 531 | 0.89 | 0.52, 1.50 | 0.7 |
| 56 | 531 | 0.69 | 0.39, 1.22 | 0.2 | 531 | 0.72 | 0.40, 1.31 | 0.3 |
| 59 | 531 | 0.65 | 0.35, 1.24 | 0.2 | 531 | - c | - c | 0.4 c |
| 66 | 531 | 0.65 | 0.39, 1.08 | 0.10 | 531 | 0.67 | 0.40, 1.13 | 0.14 |
| 68 | 531 | 1.23 | 0.84, 1.79 | 0.3 | 531 | 1.02 | 0.72, 1.46 | 0.9 |
| HPV multiple infection | 531 | 531 | ||||||
| No | 1.00 | Ref. | 1.00 | Ref. | ||||
| Yes | 0.65 | 0.46, 0.91 | 0.011 | 0.73 | 0.52, 1.02 | 0.066 | ||
| Characteristic | Overall N = 359 | Expected n = 236 | Pathologic Upgrading at Surgery n = 123 | p-Value a | |
|---|---|---|---|---|---|
| Hidden CIN 2 | Hidden CIN 3 | ||||
| n = 31 | n = 92 | ||||
| Preoperative biopsy result | <0.001 | ||||
| CIN 1 | 108 (30%) | 61 (26%) | 25 (81%) | 22 (24%) | |
| CIN 2 | 236 (66%) | 167 (71%) | 0 (0%) | 69 (75%) | |
| HSIL | 6 (1.7%) | 6 (2.5%) | 0 (0%) | 0 (0%) | |
| Negative | 1 (0.3%) | 1 (0.4%) | 0 (0%) | 0 (0%) | |
| Not detected | 8 (2.2%) | 1 (0.4%) | 6 (19%) | 1 (1.0%) | |
| Diagnosed age | 0.016 | ||||
| 20s | 57 (16%) | 41 (17%) | 2 (6.5%) | 14 (15%) | |
| 30s | 90 (25%) | 50 (21%) | 5 (16%) | 35 (38%) | |
| 40s | 92 (26%) | 65 (28%) | 8 (26%) | 19 (21%) | |
| over 50s | 120 (33%) | 80 (34%) | 16 (52%) | 24 (26%) | |
| HPV virus type | 0.4 | ||||
| Negative | 32 (12%) | 23 (10%) | 2 (6.5%) | 7 (7.6%) | |
| Low risk | 33 (12%) | 17 (7.2%) | 2 (6.5%) | 14 (15%) | |
| High risk | 212 (77%) | 137 (58%) | 20 (65%) | 55 (60%) | |
| HPV type | |||||
| 16 | 39 (14%) | 16 (6.8%) | 5 (16%) | 18 (20%) | 0.005 |
| 18 | 8 (2.9%) | 6 (2.5%) | 1 (3.2%) | 1 (1.0%) | 0.5 |
| 52 | 28 (10%) | 16 (6.8%) | 4 (13%) | 8 (8.7%) | 0.4 |
| 58 | 17 (6.1%) | 11 (4.7%) | 0 (0%) | 6 (6.5%) | 0.5 |
| 31 | 9 (3.2%) | 5 (2.1%) | 0 (0%) | 4 (4.3%) | 0.5 |
| 33 | 2 (0.7%) | 2 (0.8%) | 0 (0%) | 0 (0%) | >0.9 |
| 35 | 6 (2.2%) | 6 (2.5%) | 0 (0%) | 0 (0%) | 0.3 |
| 39 | 4 (1.4%) | 4 (1.7%) | 0 (0%) | 0 (0%) | 0.5 |
| 51 | 11 (4.0%) | 8 (3.4%) | 2 (6.5%) | 1 (1.0%) | 0.2 |
| 56 | 3 (1.1%) | 1 (0.4%) | 0 (0%) | 2 (2.2%) | 0.4 |
| 66 | 2 (0.7%) | 2 (0.8%) | 0 (0%) | 0 (0%) | >0.9 |
| 68 | 3 (1.1%) | 3 (1.3%) | 0 (0%) | 0 (0%) | 0.7 |
| HPV multiple infection | 41 (15%) | 28 (12%) | 4 (13%) | 9 (9.8%) | 0.7 |
| Pelvic inflammatory disease | 248 (69%) | 167 (71%) | 24 (77%) | 57 (62%) | 0.2 |
| Hematological disorders | 8 (2.2%) | 6 (2.5%) | 0 (0%) | 2 (2.2%) | >0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Song, H.; Lee, H.Y.; Lee, S.; Lee, J.; Choi, S.; Hur, S.Y. Evaluation of Risk Factors Associated with Expectant Management in CIN 1/2: A Multicenter Real-World Cohort Study. Cancers 2025, 17, 3738. https://doi.org/10.3390/cancers17233738
Lee S, Song H, Lee HY, Lee S, Lee J, Choi S, Hur SY. Evaluation of Risk Factors Associated with Expectant Management in CIN 1/2: A Multicenter Real-World Cohort Study. Cancers. 2025; 17(23):3738. https://doi.org/10.3390/cancers17233738
Chicago/Turabian StyleLee, Sanha, Heekyoung Song, Hong Yeon Lee, Sujin Lee, Jeongyoon Lee, Suein Choi, and Soo Young Hur. 2025. "Evaluation of Risk Factors Associated with Expectant Management in CIN 1/2: A Multicenter Real-World Cohort Study" Cancers 17, no. 23: 3738. https://doi.org/10.3390/cancers17233738
APA StyleLee, S., Song, H., Lee, H. Y., Lee, S., Lee, J., Choi, S., & Hur, S. Y. (2025). Evaluation of Risk Factors Associated with Expectant Management in CIN 1/2: A Multicenter Real-World Cohort Study. Cancers, 17(23), 3738. https://doi.org/10.3390/cancers17233738






