Characterizing Overall Survival of Patients with Acute Myeloid Leukemia: A Competing Risk Analysis of SEER Data Covering 46 Years †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Extraction and Variables
2.3. Statistical Analysis
2.4. Ethical Considerations and Data Sharing
3. Results
3.1. Description of the Study Population
3.2. Historic Changes in Cofactors
3.3. Overall Survival and Early Death Rate
3.4. Competing Risk Analysis:
3.4.1. AML Related Mortality
3.4.2. Non-AML Reasons for Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| CRA | Competing risk analysis |
| COD | Cause of death |
| ED | Early death |
| FDA | U.S. Food and Drug Administration |
| HCT | Hematological cell transplantation |
| HR | Hazard ratio |
| KM | Kaplan–Meier |
| OS | Overall survival |
| PSI | Population stability index |
| SEER | Surveillance Epidemiology and End Results (SEER) Program |
| sHR | Hazard-ratio estimated from a sub-distribution Fine-Grey model |
References
- Kantarjian, H.; Borthakur, G.; Daver, N.; DiNardo, C.D.; Issa, G.; Jabbour, E.; Kadia, T.; Sasaki, K.; Short, N.J.; Yilmaz, M.; et al. Current status and research directions in acute myeloid leukemia. Blood Cancer J. 2024, 14, 163. [Google Scholar] [CrossRef]
- Dohner, H.; Pratz, K.W.; DiNardo, C.D.; Wei, A.H.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Recher, C.; Schuh, A.C.; Babu, S.; et al. Genetic risk stratification and outcomes among treatment-naive patients with AML treated with venetoclax and azacitidine. Blood 2024, 144, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Wei, A.H.; Roboz, G.J.; Montesinos, P.; Thol, F.R.; Ravandi, F.; Dombret, H.; Porkka, K.; Sandhu, I.; Skikne, B.; et al. Prognostic impact of NPM1 and FLT3 mutations in patients with AML in first remission treated with oral azacitidine. Blood 2022, 140, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Turkalj, S.; Radtke, F.A.; Vyas, P. An Overview of Targeted Therapies in Acute Myeloid Leukemia. Hemasphere 2023, 7, e914. [Google Scholar] [CrossRef]
- Qureshi, Z.; Jamil, A.; Altaf, F.; Siddique, R. Meta-analysis of Therapeutic Approaches in Acute Myeloid Leukemia: Unveiling Trends and Predictors of Treatment Response. Am. J. Clin. Oncol. 2025, 48, 242–256. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DiNardo, C.D.; Kadia, T.M.; Daver, N.G.; Altman, J.K.; Stein, E.M.; Jabbour, E.; Schiffer, C.A.; Lang, A.; Ravandi, F. Acute myeloid leukemia management and research in 2025. CA Cancer J. Clin. 2025, 75, 46–67. [Google Scholar] [CrossRef]
- Pulte, D.; Gondos, A.; Brenner, H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica 2008, 93, 594–600. [Google Scholar] [CrossRef]
- Pulte, D.; Redaniel, M.T.; Jansen, L.; Brenner, H.; Jeffreys, M. Recent trends in survival of adult patients with acute leukemia: Overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica 2013, 98, 222–229. [Google Scholar] [CrossRef]
- Vener, C.; Lillini, R.; De Angelis, R.; Bonfarnuzzo, S.; Poirel, H.A.; Trama, A.; Visser, O.; Troussard, X.; Marcos-Gragera, R.; Maynadie, M.; et al. Long-term survival for myeloid neoplasms and national health expenditure: A EUROCARE-6 retrospective, population-based study. Eur. J. Cancer 2025, 220, 115231. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y. Development and validation of a risk predictive nomogram for colon cancer-specific mortality: A competing risk model based on the SEER database. Discov. Oncol. 2024, 15, 621. [Google Scholar] [CrossRef]
- Zheng, S.; Xie, S.; Yu, H.; Duan, X.; He, Y.; Ho, C.; Wan, Y.; Hang, T.; Chen, W.; Lyu, J.; et al. Competing-risks analysis for evaluating the prognosis of patients with microinvasive cutaneous squamous cell carcinoma based on the SEER database. BMC Med. Res. Methodol. 2023, 23, 286. [Google Scholar] [CrossRef]
- Luo, X.; Niu, J.; Zhang, Y.; Jia, Y.; Wang, Q.; Li, Y.; Wang, Y.; Li, J.; Li, Z.; Yan, R.; et al. Cardiovascular disease mortality risk among patients with metastatic melanoma before and after the approval of immunotherapy in the United States: A Surveillance, Epidemiology, and End Results database-based study. Eur. J. Med. Res. 2025, 30, 977. [Google Scholar] [CrossRef]
- Tang, M.; Chen, Y.; Zhou, Y.; Zhuang, X.; Fu, Y.; Chen, J.; Wei, R.; Chen, Y. Long-term risks of cardiovascular-specific mortality among myeloproliferative neoplasms patients. Ther. Adv. Hematol. 2024, 15, 20406207241290886. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Görlich, D.; Lanwer, L.; Sauerland, C.; Faldum, A. Characterizing overall survival in AML patients: A competing risk analysis of SEER data covering 46 years. In Proceedings of the International Symposium ACUTE LEUKEMIAS XIX (ISALXIX), Munich, Germany, 16–19 March 2025. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric-Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Austin, P.C.; Fine, J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 2017, 36, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Putter, H.; Schumacher, M.; van Houwelingen, H.C. On the relation between the cause-specific hazard and the subdistribution rate for competing risks data: The Fine-Gray model revisited. Biom. J. 2020, 62, 790–807. [Google Scholar] [CrossRef]
- Lau, B.; Cole, S.R.; Gange, S.J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009, 170, 244–256. [Google Scholar] [CrossRef]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef]
- Wolbers, M.; Koller, M.T.; Stel, V.S.; Schaer, B.; Jager, K.J.; Leffondre, K.; Heinze, G. Competing risks analyses: Objectives and approaches. Eur. Heart J. 2014, 35, 2936–2941. [Google Scholar] [CrossRef]
- Loh, K.P.; Abdallah, M.; Kadambi, S.; Wells, M.; Kumar, A.J.; Mendler, J.H.; Liesveld, J.L.; Wittink, M.; O’Dwyer, K.; Becker, M.W.; et al. Treatment decision-making in acute myeloid leukemia: A qualitative study of older adults and community oncologists. Leuk. Lymphoma 2021, 62, 387–398. [Google Scholar] [CrossRef]
- Genc, E.E.; Sarac, I.S.; Arslan, H.; Eskazan, A.E. Diagnostic and Treatment Obstacles in Acute Myeloid Leukemia: Social, Operational, and Financial. Oncol. Ther. 2023, 11, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Meillon-Garcia, L.A.; Demichelis-Gomez, R. Access to Therapy for Acute Myeloid Leukemia in the Developing World: Barriers and Solutions. Curr. Oncol. Rep. 2020, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, C.; Wang, E.; Tu, N.; Salas, M.; Kamel, Y.M. Characteristics and outcomes of elderly patients with acute myeloid leukemia who receive no treatment in the Surveillance, Epidemiology and End Results-Medicare database. Future Oncol. 2023, 19, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Klepin, H.D.; Estey, E.; Kadia, T. More Versus Less Therapy for Older Adults With Acute Myeloid Leukemia: New Perspectives on an Old Debate. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 421–432. [Google Scholar] [CrossRef]
- Oran, B.; Weisdorf, D.J. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef]
- Hubscher, E.; Sikirica, S.; Bell, T.; Brown, A.; Welch, V.; Russell-Smith, A.; D’Amico, P. Patterns of undertreatment among patients with acute myeloid leukemia (AML): Considerations for patients eligible for non-intensive chemotherapy (NIC). J. Cancer Res. Clin. Oncol. 2021, 147, 3359–3368. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Satram-Hoang, S.; Hurst, D.; Hoang, K.Q.; Momin, F.; Reyes, C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann. Hematol. 2015, 94, 1127–1138. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, E.; Farrell, A.T.; Wang, Y.C.; Sridhara, R.; Pazdur, R. FDA drug approval summary: Azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist 2005, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.P.; Chou, W.C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland, S.A., Jr.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019, 33, 379–389. [Google Scholar] [CrossRef]
- Kadia, T.M.; Cortes, J.; Ravandi, F.; Jabbour, E.; Konopleva, M.; Benton, C.B.; Burger, J.; Sasaki, K.; Borthakur, G.; DiNardo, C.D.; et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: A phase 2 single-arm trial. Lancet Haematol. 2018, 5, e411–e421. [Google Scholar] [CrossRef]
- Maiti, A.; Qiao, W.; Sasaki, K.; Ravandi, F.; Kadia, T.M.; Jabbour, E.J.; Daver, N.G.; Borthakur, G.; Garcia-Manero, G.; Pierce, S.A.; et al. Venetoclax with decitabine vs intensive chemotherapy in acute myeloid leukemia: A propensity score matched analysis stratified by risk of treatment-related mortality. Am. J. Hematol. 2021, 96, 282–291. [Google Scholar] [CrossRef]
- Winer, E.S.; Stone, R.M. AML in the Elderly–When less may be more. Curr. Oncol. Rep. 2024, 26, 1502–1510. [Google Scholar] [CrossRef]
- Servais, S.; Beguin, Y.; Baron, F. Current Status and Perspectives of Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia. Stem. Cells Transl. Med. 2022, 11, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Muffly, L.; Pasquini, M.C.; Martens, M.; Brazauskas, R.; Zhu, X.; Adekola, K.; Aljurf, M.; Ballen, K.K.; Bajel, A.; Baron, F.; et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 2017, 130, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Labopin, M.; Moukalled, N.; Kroger, N.; Rautenberg, C.; Schetelig, J.; Finke, J.; Blau, I.W.; Blaise, D.; Stelljes, M.; et al. Improvements in Posttransplant Outcomes Over Two Decades in Older Patients with Acute Myeloid Leukemia in the EBMT ALWP Study. Clin. Cancer Res. 2024, 30, 1778–1787. [Google Scholar] [CrossRef]
- Devillier, R.; Forcade, E.; Garnier, A.; Guenounou, S.; Thepot, S.; Guillerm, G.; Ceballos, P.; Hicheri, Y.; Dumas, P.Y.; Peterlin, P.; et al. In-depth time-dependent analysis of the benefit of allo-HSCT for elderly patients with CR1 AML: A FILO study. Blood Adv. 2022, 6, 1804–1812. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Atlija, M.; Kleovoulou, I.; Witaszek, A.; Alexander, T.; Angelucci, E.; Averbuch, D.; Bazarbachi, A.; Ciceri, F.; et al. The 2023 EBMT report on hematopoietic cell transplantation and cellular therapies. Increased use of allogeneic HCT for myeloid malignancies and of CAR-T at the expense of autologous HCT. Bone Marrow Transplant. 2025, 60, 519–528. [Google Scholar] [CrossRef]
- Merrill, R.M.; Dearden, K.A. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. 2004, 15, 1027–1034. [Google Scholar] [CrossRef]
- Kuo, T.M.; Mobley, L.R. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control. 2016, 27, 1117–1126. [Google Scholar] [CrossRef]
- Lu, S.C.; Song, W.; Pfob, A.; Gibbons, C. Assessing the representativeness of large medical data using population stability index. BMC Med. Res. Methodol. 2025, 25, 44. [Google Scholar] [CrossRef] [PubMed]
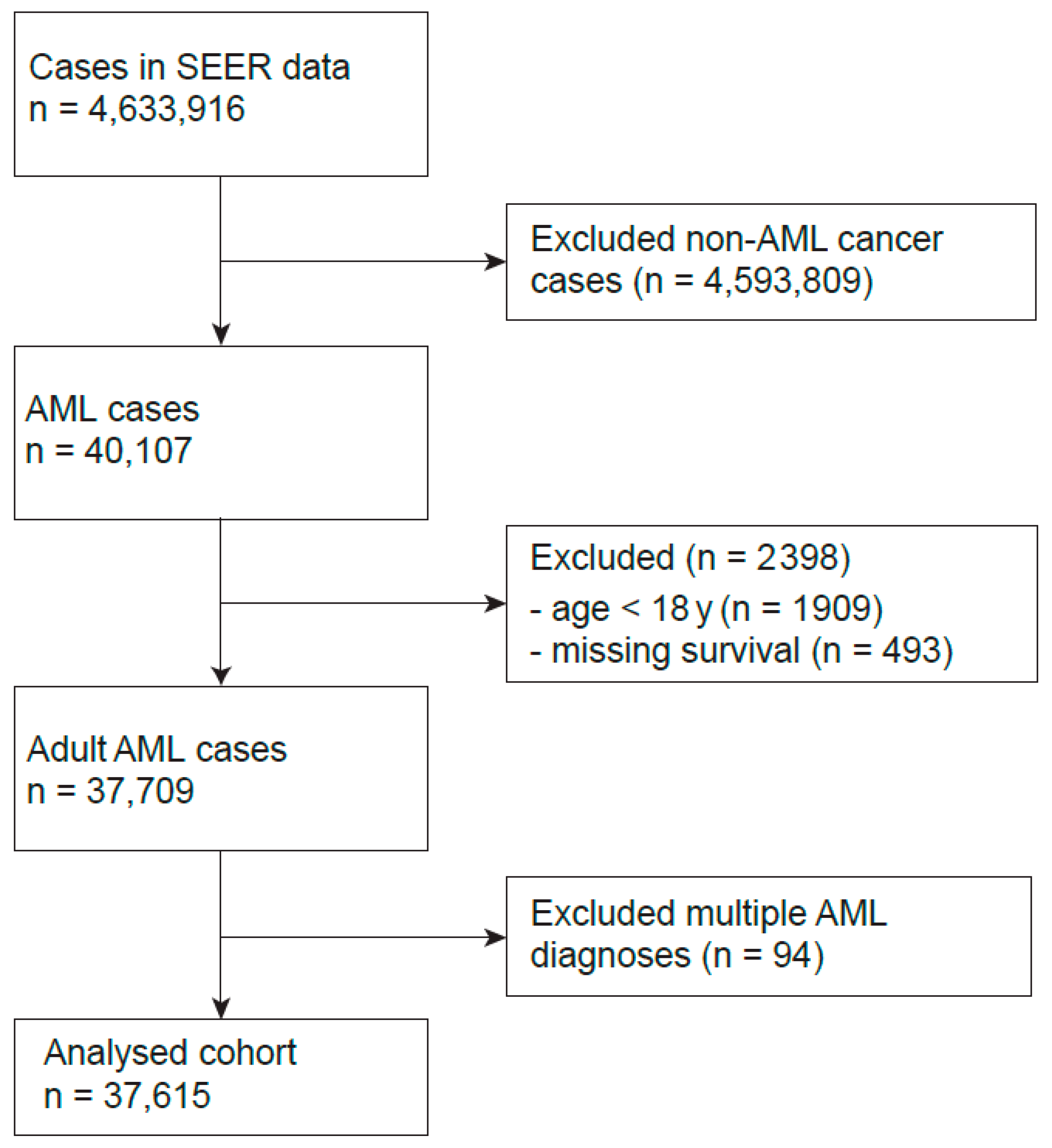

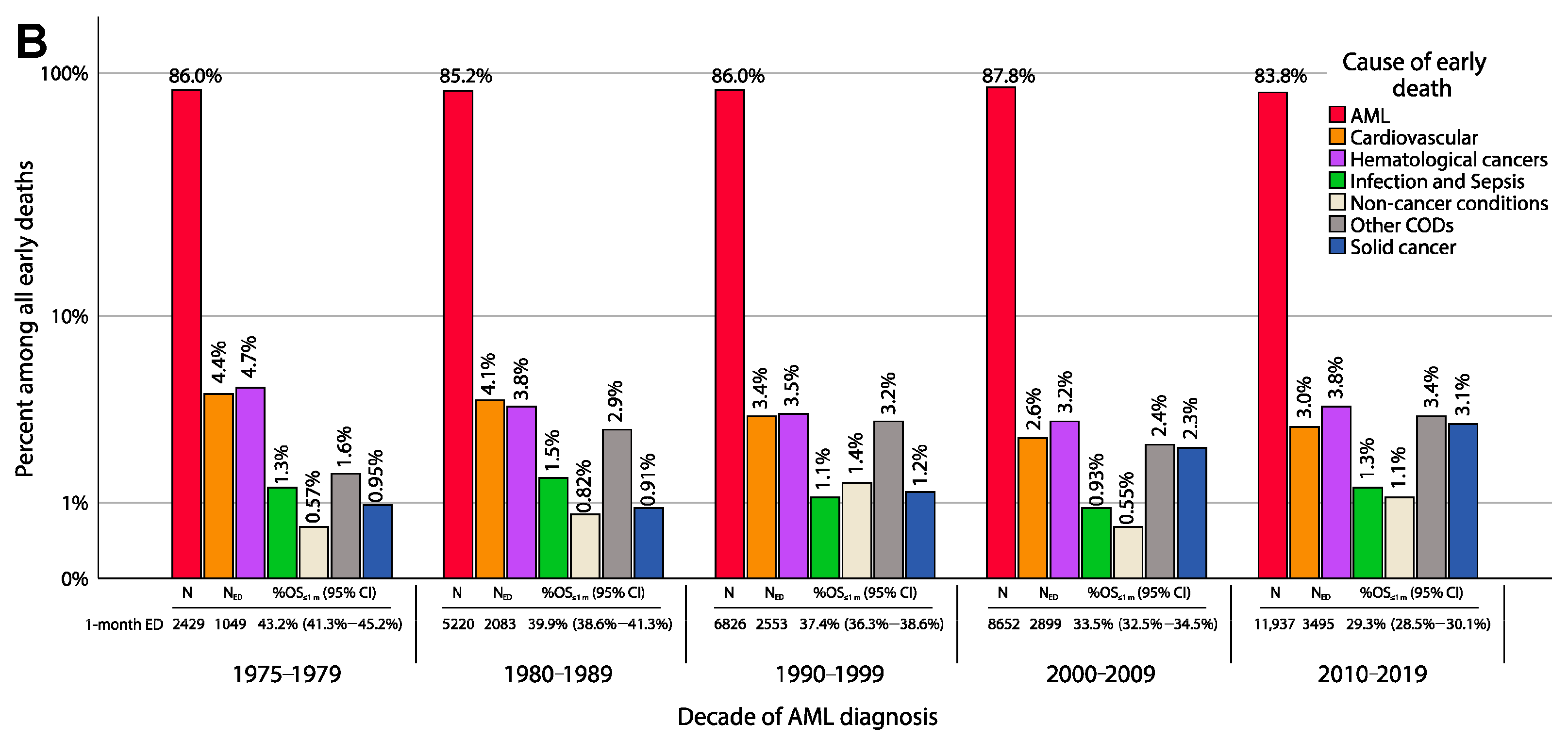
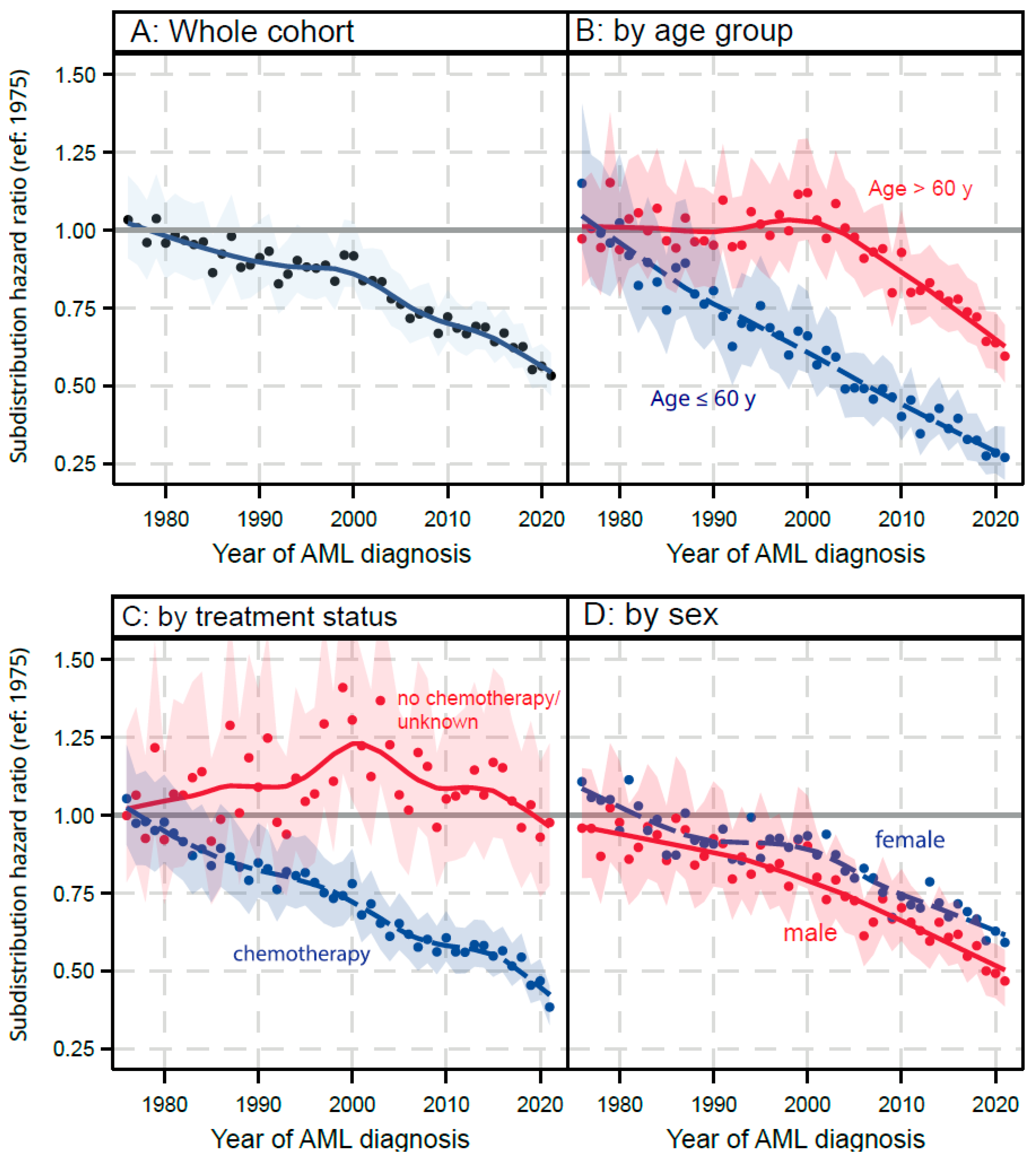

| N | Statistic | |
|---|---|---|
| Sex, n(%) | ||
| Female | 17,095 | (45.5%) |
| Male | 20,520 | (54.6%) |
| Age at diagnosis(y) mean(SD) | 37,615 | 64.8 (17.2) |
| Chemotherapy, n(%) | ||
| Yes | 25,214 | (67.0%) |
| No/unknown | 12,401 | (33.0%) |
| First malignant tumor, n(%) | 28,340 | (75.3%) |
| Race, n(%) | ||
| Black | 2225 | (5.9%) |
| White | 31,579 | (83.9%) |
| Other (American Indian, Native, Pacific Islander) | 3718 | (9.9%) |
| Unknown | 93 | (0.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Görlich, D.; Lanwer, L.; Sauerland, C.; Krug, U.; Faldum, A. Characterizing Overall Survival of Patients with Acute Myeloid Leukemia: A Competing Risk Analysis of SEER Data Covering 46 Years. Cancers 2025, 17, 3735. https://doi.org/10.3390/cancers17233735
Görlich D, Lanwer L, Sauerland C, Krug U, Faldum A. Characterizing Overall Survival of Patients with Acute Myeloid Leukemia: A Competing Risk Analysis of SEER Data Covering 46 Years. Cancers. 2025; 17(23):3735. https://doi.org/10.3390/cancers17233735
Chicago/Turabian StyleGörlich, Dennis, Leonas Lanwer, Cristina Sauerland, Utz Krug, and Andreas Faldum. 2025. "Characterizing Overall Survival of Patients with Acute Myeloid Leukemia: A Competing Risk Analysis of SEER Data Covering 46 Years" Cancers 17, no. 23: 3735. https://doi.org/10.3390/cancers17233735
APA StyleGörlich, D., Lanwer, L., Sauerland, C., Krug, U., & Faldum, A. (2025). Characterizing Overall Survival of Patients with Acute Myeloid Leukemia: A Competing Risk Analysis of SEER Data Covering 46 Years. Cancers, 17(23), 3735. https://doi.org/10.3390/cancers17233735







