Comparison of Standard of Care with or Without a PD-1/PD-L-1 Inhibitor for the Treatment of Multiple Myeloma: A Systematic Review and Meta-Analysis of Phase II and III Randomized Controlled Trials
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Search Criteria
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Search Results
3.2. Efficacy
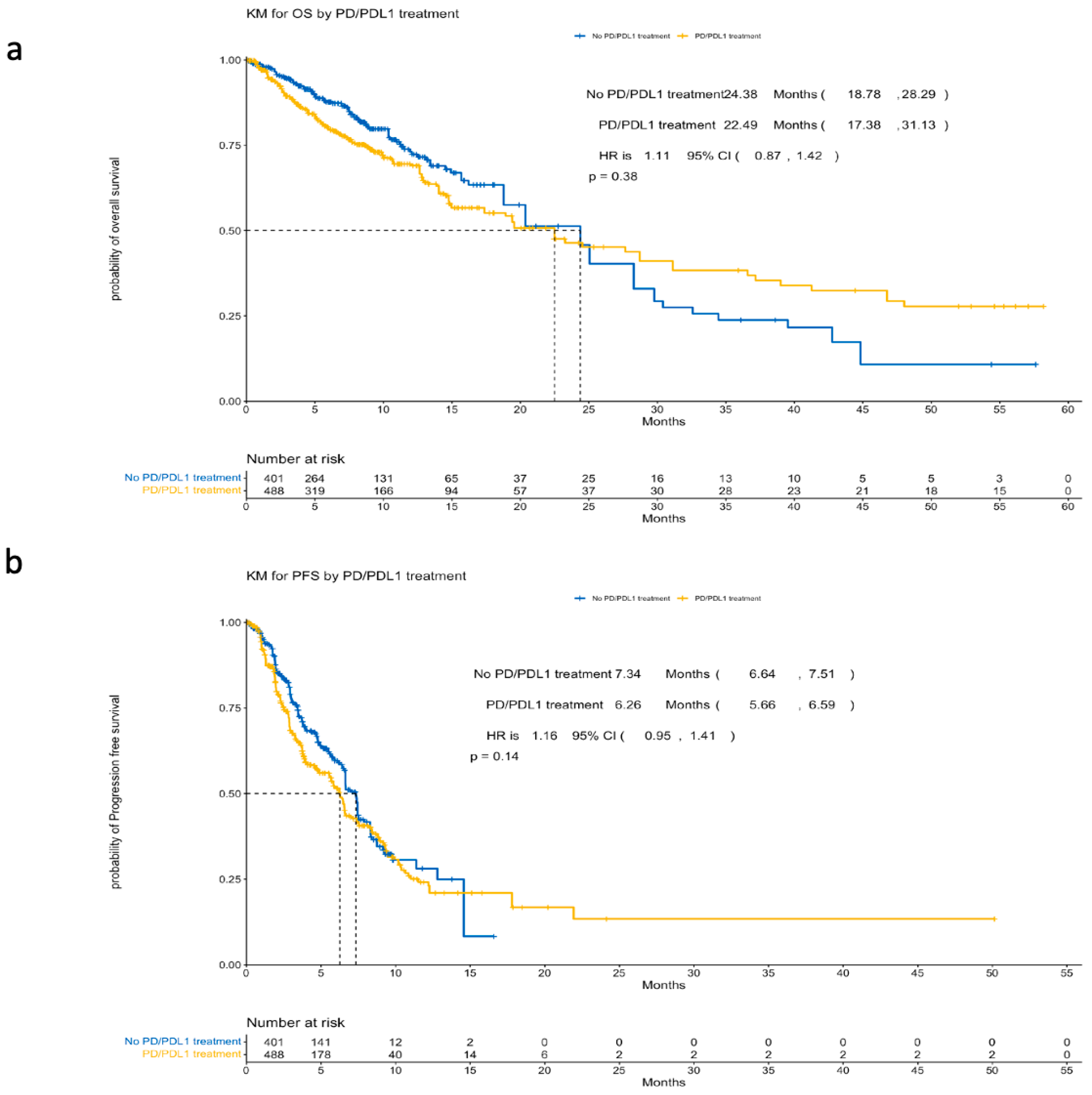
3.2.1. Survival Outcomes
3.2.2. Response Rate Outcomes
3.3. Safety
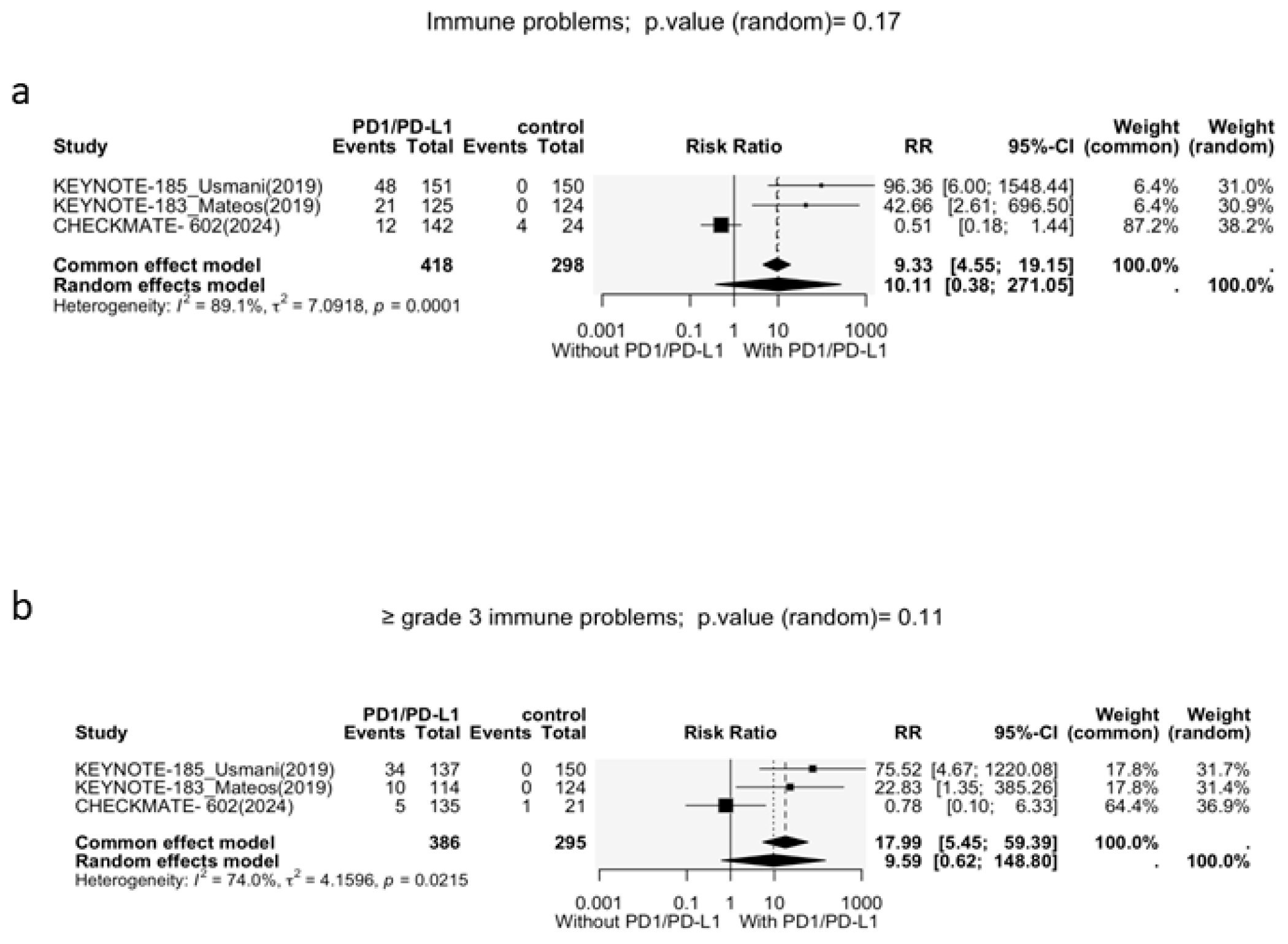
3.3.1. Immune-Related Adverse Events
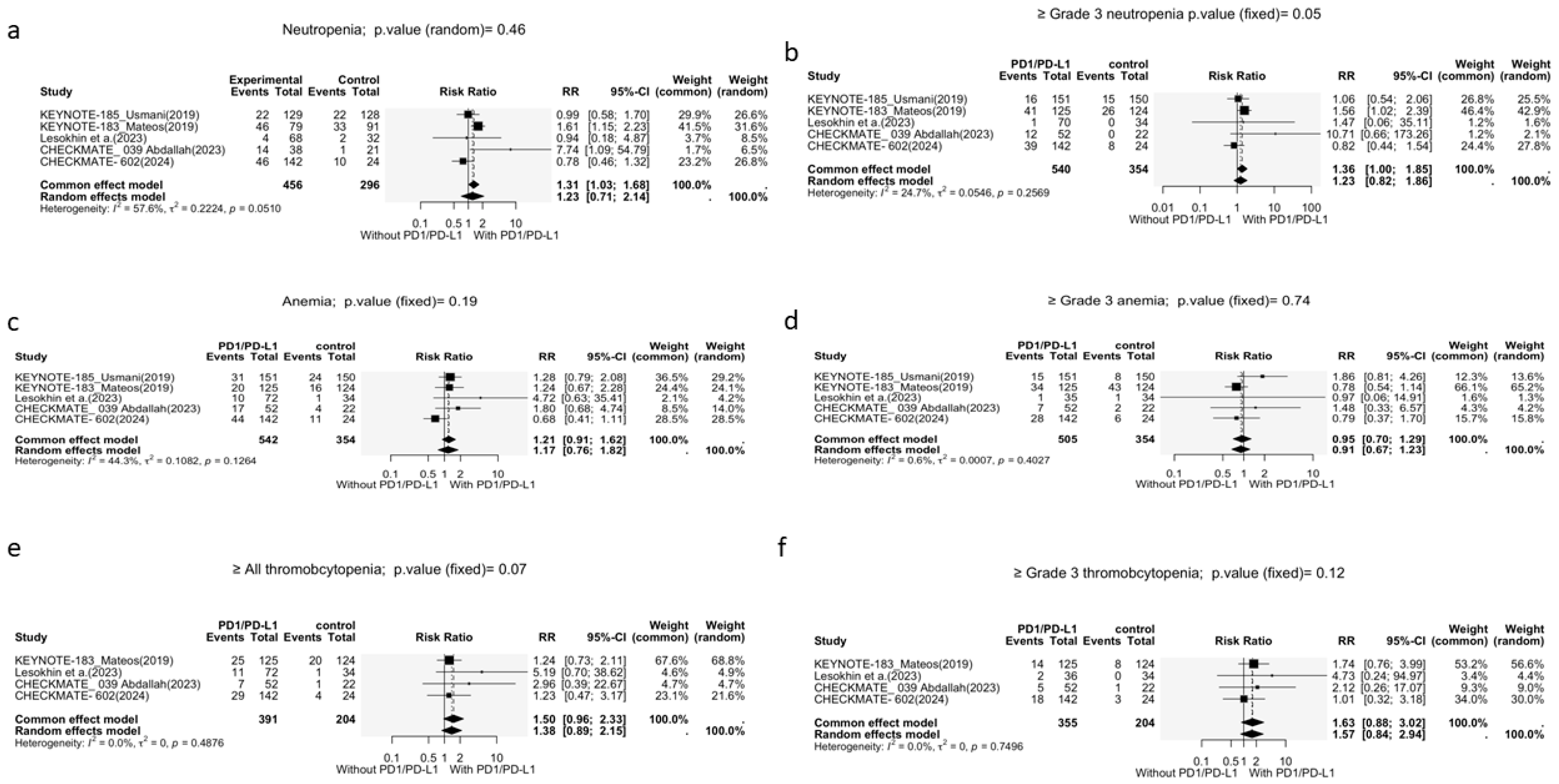
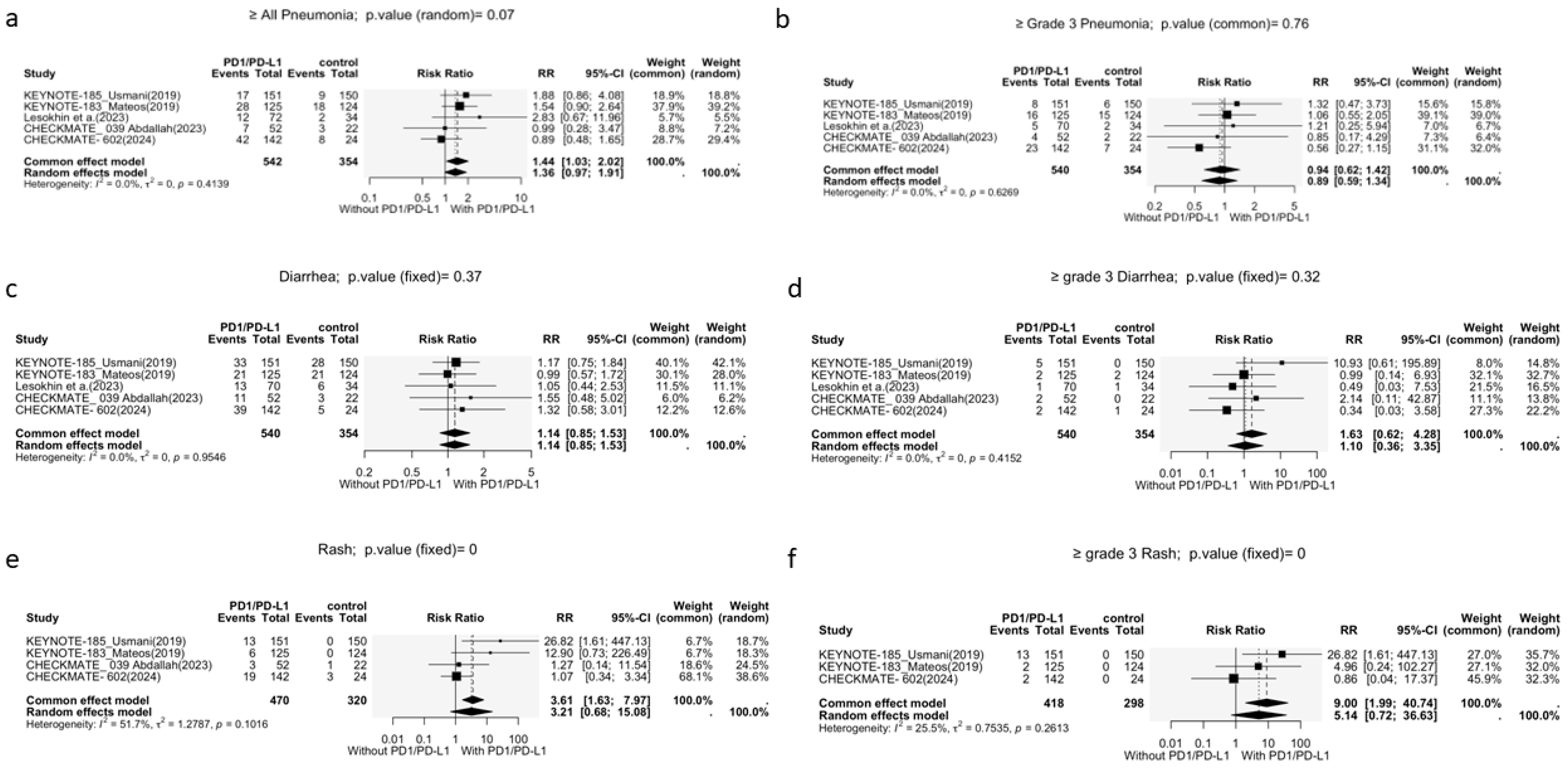
3.3.2. Other Adverse Events
Neutropenia
Anemia
Thrombocytopenia
Pneumonia
Diarrhea
Rash
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Vij, R.; Kuter, D.; Cella, D.; Durie, B.G.M.; Abonour, R.; Rifkin, R.M.; Ailawadhi, S.; Lee, H.C.; Cowan, A.J.; et al. Real-World Treatment Patterns and Clinical Outcomes in Patients with Multiple Myeloma Previously Treated with Lenalidomide and an Anti-CD38 Monoclonal Antibody. Clin. Lymphoma Myeloma Leuk. 2025, 25, 337–348.e2. [Google Scholar] [CrossRef] [PubMed]
- Girvan, A.; Yu, J.; Kamalakar, R.; Mearns, E.S.; Nixon, M.; Cornell, R.F. Treatment Patterns and Outcomes in Patients with Relapsed/Refractory Multiple Myeloma Receiving ≥3 Lines of Therapy: A Real-World Evaluation in the United States. Blood 2022, 140 (Suppl. S1), 5288–5290. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliva, S.; Troia, R.; D’Agostino, M.; Boccadoro, M.; Gay, F. Promises and Pitfalls in the Use of PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousef, S.; Marvin, J.; Steinbach, M.; Langemo, A.; Kovacsovics, T.; Binder, M.; Kröger, N.; Luetkens, T.; Atanackovic, D. Immunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma-propagating pre-plasma cells in the bone marrow of multiple myeloma patients. Blood Cancer J. 2015, 5, e285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ray, A.; Das, D.S.; Song, Y.; Richardson, P.; Munshi, N.C.; Chauhan, D.; Anderson, K.C. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia 2015, 29, 1441–1444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jelinek, T.; Paiva, B.; Hajek, R. Update on PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.; LeBlanc, R.; Dimopoulos, M.A.; Capra, M.; Carlo-Stella, C.; Karlin, L.; Castilloux, J.F.; Forsberg, P.; Parmar, G.; Tosikyan, A.; et al. Isatuximab in combination with cemiplimab in patients with relapsed/refractory multiple myeloma: A phase 1/2 study. Cancer Med. 2023, 12, 10254–10266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Usmani, S.Z.; Schjesvold, F.; Oriol, A.; Karlin, L.; Cavo, M.; Rifkin, R.M.; Yimer, H.A.; LeBlanc, R.; Takezako, N.; McCroskey, R.D.; et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e448–e458. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.O.; Lesokhin, A.; Wrobel, T.; Jamroziak, K.; Dytfeld, D.; Touzeau, C.; Suvannasankha, A.; Leleu, X.; Silbermann, R.; Khan, A.M.; et al. Nivolumab and daratumumab combination regimens for the treatment of relapsed and refractory multiple myeloma: Results of a randomized phase I/II clinical trial. Front. Hematol. 2023, 2, 1244494. [Google Scholar] [CrossRef]
- Oriol, A.; Hájek, R.; Spicka, I.; Sandhu, I.; Cohen, Y.C.; Gatt, M.E.; Mariz, J.; Cavo, M.; Berdeja, J.; Jin, K.; et al. Nivolumab, Pomalidomide, and Elotuzumab Combination Regimens for Treatment of Relapsed and Refractory Multiple Myeloma: Results from the Phase 3 CheckMate 602 Study. Clin. Lymphoma Myeloma Leuk. 2024, 24, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Chinai, J.M.; Janakiram, M.; Chen, F.; Chen, W.; Kaplan, M.; Zang, X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol. Sci. 2015, 36, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawano, Y.; Zavidij, O.; Park, J.; Moschetta, M.; Kokubun, K.; Mouhieddine, T.H.; Manier, S.; Mishima, Y.; Murakami, N.; Bustoros, M.; et al. Blocking IFNAR1 inhibits multiple myeloma-driven Treg expansion and immunosuppression. J. Clin. Investig. 2018, 128, 2487–2499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vuckovic, S.; Minnie, S.A.; Smith, D.; Gartlan, K.H.; Watkins, T.S.; Markey, K.A.; Mukhopadhyay, P.; Guillerey, C.; Kuns, R.D.; Locke, K.R.; et al. Bone marrow transplantation generates T cell-dependent control of myeloma in mice. J. Clin. Investig. 2019, 129, 106–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landowski, T.H.; Olashaw, N.E.; Agrawal, D.; Dalton, W.S. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene 2003, 22, 2417–2421. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, L.A.; Damiano, J.S.; Buyuksal, I.; Pledger, W.J.; Dalton, W.S. Adhesion to fibronectin via beta1 integrins regulates p27kip1 levels and contributes to cell adhesion mediated drug resistance (CAM-DR). Oncogene 2000, 19, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Scott, M.J.; Allen, J.S.; Yang, X.; Cui, G.; Pan, D.; Yanaba, N.; Fiala, M.A.; O’Neal, J.; Schmieder-Atteberry, A.H.; et al. VLA4-Targeted Nanoparticles Hijack Cell Adhesion-Mediated Drug Resistance to Target Refractory Myeloma Cells and Prolong Survival. Clin. Cancer Res. 2021, 27, 1974–1986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastenmuller, W.; Gasteiger, G.; Subramanian, N.; Sparwasser, T.; Busch, D.H.; Belkaid, Y.; Drexler, I.; Germain, R.N. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J. Immunol. 2011, 187, 3186–3197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alrasheed, N.; Lee, L.; Ghorani, E.; Henry, J.Y.; Conde, L.; Chin, M.; Galas-Filipowicz, D.; Furness, A.J.S.; Chavda, S.J.; Richards, H.; et al. Marrow-Infiltrating Regulatory T Cells Correlate with the Presence of Dysfunctional CD4+PD-1+ Cells and Inferior Survival in Patients with Newly Diagnosed Multiple Myeloma. Clin. Cancer Res. 2020, 26, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Singh, A.V.; Brahmandam, M.; Carrasco, R.; Bandi, M.; Hideshima, T.; Bianchi, G.; Podar, K.; Tai, Y.T.; Mitsiades, C.; et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: A therapeutic target. Cancer Cell 2009, 16, 309–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Tang, P.; Liu, L.; Wang, F.; Xing, H.; Sun, L.; Jiang, Z. Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle 2018, 17, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Barut, B.A.; Mohrbacher, A.F.; Chauhan, D.; Anderson, K.C. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood 1993, 82, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Gunn, W.G.; Conley, A.; Deininger, L.; Olson, S.D.; Prockop, D.J.; Gregory, C.A. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: A potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 2006, 24, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, C.P.M.; O’Neill, C.A.; Broekmans, M.E.C.; Frerichs, K.A.; Bruins, W.S.C.; Duetz, C.; Kruyswijk, S.; Baglio, S.R.; Skerget, S.; Montes de Oca, R.; et al. T-Cell Characteristics Impact Response and Resistance to T-Cell-Redirecting Bispecific Antibodies in Multiple Myeloma. Clin. Cancer Res. 2024, 30, 3006–3022. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, K.M.; Cohen, A.D.; Kaushal, A.; Garfall, A.L.; Manalo, R.J.; Carr, A.R.; McCachren, S.S.; Stadtmauer, E.A.; Lacey, S.F.; Melenhorst, J.J.; et al. Changes in Bone Marrow Tumor and Immune Cells Correlate with Durability of Remissions Following BCMA CAR T Therapy in Myeloma. Blood Cancer Discov. 2022, 3, 490–501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, A.K.; Schmidt, T.M.; Martell, E.B.; Chen, A.S.; Dogru, R.E.; Hematti, P.; Callander, N.S. PD1+TIGIT+2B4+KLRG1+ Cells Might Underlie T Cell Dysfunction in Patients Treated with BCMA-Directed Chimeric Antigen Receptor T Cell Therapy. Transplant. Cell Ther. 2024, 30, 191–202. [Google Scholar] [CrossRef] [PubMed]


| Studies | Treatment Arms | Control Arm | Number of Patients | Year of Publication |
|---|---|---|---|---|
| Lesokhin et al. [11] | Isatuximab + cemipilimab Q2W OR Isatuximab + cemipilimab Q4W | Isatuximab | 106 | 2023 |
| KEYNOTE-185 [12] | Pembrolizumab + Lenalidomide + Dexamethasone | Lenalidomide + Dexamethasone | 301 | 2019 |
| KEYNOTE-183 [13] | Pembrolizumab + Pomalidomide + Dexamethasone | Pomalidomide + Dexamethasone | 249 | 2019 |
| CHECKMATE- 039 [14] | Nivolumab + Daratumumab (Cohort A and B) OR Nivolumab + Daratumumab + Pomalidomide + dexamethasone (Cohort A) | Daratumumab | 74 | 2023 |
| CHECKMATE- 602 [15] | Nivolumab + Elotuzumab + Pomalidomide + Dexamethasone OR Nivolumab + Pomalidomide + dexamethasone | Pomalidomide + dexamethasone | 170 | 2024 |
| Group | Sample Size (N) | Median OS (Months) | 95% CI (Months) | HR | 95% CI (HR) | p-Value |
|---|---|---|---|---|---|---|
| No PD/PDL1 treatment | 401 | 24.38 | 18.78–28.29 | 1.11 | 0.87–1.42 | 0.38 |
| PD/PDL1 treatment | 488 | 22.49 | 17.38–31.13 |
| Study | Arm | PFS-6 Months (%) | OS-6 Months (%) | Median OS (Months, 95% CI) | Median PFS (Months, 95% CI) |
|---|---|---|---|---|---|
| Study1 | isa | - | - | NR (8.936–NR) | 2.89 (1.97–3.81) |
| Study1 | isa/cemi Q2W | - | - | 18.96 (6.932–NR) | 3.75 (1.97–5.88) |
| Study1 | isa/cemi Q4W | - | - | 14.75 (9.04–NR) | 3.02 (2.79–5.16) |
| Keynote-185 | Pem/len/dex | 82 (73.2–88.1) | 87.2 (79.9–92) | Not reached | Not reached |
| Keynote-185 | Len/dex | 85 (76.8–90.5) | 93.9 (88.1–96.9) | Not reached | Not reached |
| Keynote-183 | Pem/Pom/dex | 48 (37–58) | 82 (74–88) | Not reached (12.9–NR) | 5.6 (3.7–7.5) |
| Keynote-183 | Pom/dex | 60 (49–69) | 90 (82–95) | 15.2 (12.7–NR) | 8.4 (5.9–NR) |
| Checkmate-039 | Nivo/Dara (Cohort A) | - | - | - | 7.6 (3.2–NA) |
| Checkmate-039 | Nivo/Dara/Pom/dex (Cohort A) | - | - | - | 17.0 (NA–NA) |
| Checkmate-039 | Nivo/Dara (Cohort B) | - | - | - | 6.6 (4.7–10.3) |
| Checkmate-039 | Dara (Cohort B) | - | - | - | 6.6 (3.0–12.8) |
| Checkmate 602 | Nivo/Pom/dex | 27 (37.5%) | 18 (25%) | - | - |
| Checkmate 602 | Pom/dex | 20 (28.6%) | 11 (15.7%) | - | - |
| Checkmate 602 | Nivo/Elo/Pom/dex | 11 (45.8%) | 4 (16.7%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaddi, Z.; Younis, O.; Khasawneh, G.; Shatnawi, Y.; Ahmed, N.; Jafari, Z.M.; Mushtaq, M.U.; Abdallah, A.-O.; Atrash, S.; Paul, B. Comparison of Standard of Care with or Without a PD-1/PD-L-1 Inhibitor for the Treatment of Multiple Myeloma: A Systematic Review and Meta-Analysis of Phase II and III Randomized Controlled Trials. Cancers 2025, 17, 3730. https://doi.org/10.3390/cancers17233730
Alsaddi Z, Younis O, Khasawneh G, Shatnawi Y, Ahmed N, Jafari ZM, Mushtaq MU, Abdallah A-O, Atrash S, Paul B. Comparison of Standard of Care with or Without a PD-1/PD-L-1 Inhibitor for the Treatment of Multiple Myeloma: A Systematic Review and Meta-Analysis of Phase II and III Randomized Controlled Trials. Cancers. 2025; 17(23):3730. https://doi.org/10.3390/cancers17233730
Chicago/Turabian StyleAlsaddi, Zain, Osama Younis, Ghena Khasawneh, Yara Shatnawi, Nausheen Ahmed, Zahra Mahmoud Jafari, Muhammad Umair Mushtaq, Al-Ola Abdallah, Shebli Atrash, and Barry Paul. 2025. "Comparison of Standard of Care with or Without a PD-1/PD-L-1 Inhibitor for the Treatment of Multiple Myeloma: A Systematic Review and Meta-Analysis of Phase II and III Randomized Controlled Trials" Cancers 17, no. 23: 3730. https://doi.org/10.3390/cancers17233730
APA StyleAlsaddi, Z., Younis, O., Khasawneh, G., Shatnawi, Y., Ahmed, N., Jafari, Z. M., Mushtaq, M. U., Abdallah, A.-O., Atrash, S., & Paul, B. (2025). Comparison of Standard of Care with or Without a PD-1/PD-L-1 Inhibitor for the Treatment of Multiple Myeloma: A Systematic Review and Meta-Analysis of Phase II and III Randomized Controlled Trials. Cancers, 17(23), 3730. https://doi.org/10.3390/cancers17233730







