Exosomal Thomsen–Friedenreich Glycoantigen as a Sensitive and Specific Biomarker for Colon, Ovarian and Prostate Cancer Diagnosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Human Serum Sample Collection and Processing
2.3. Fabrication of SPR Biochip
2.4. Surface Modification of SPR Biochip
2.5. Detection of Exosomal TF-Ag-α by the SPR Biosensor
2.6. Characterization of Exosomes by Nanoparticle Tracking Analysis
2.7. Characterization of TF-Ag-α-Positive Tumor-Derived Exosomes
2.8. Statistical Data Analysis
3. Results
3.1. SPR-Based Detection and Characterization of Exosomal TF-Ag-α in Cancer Patient Serum
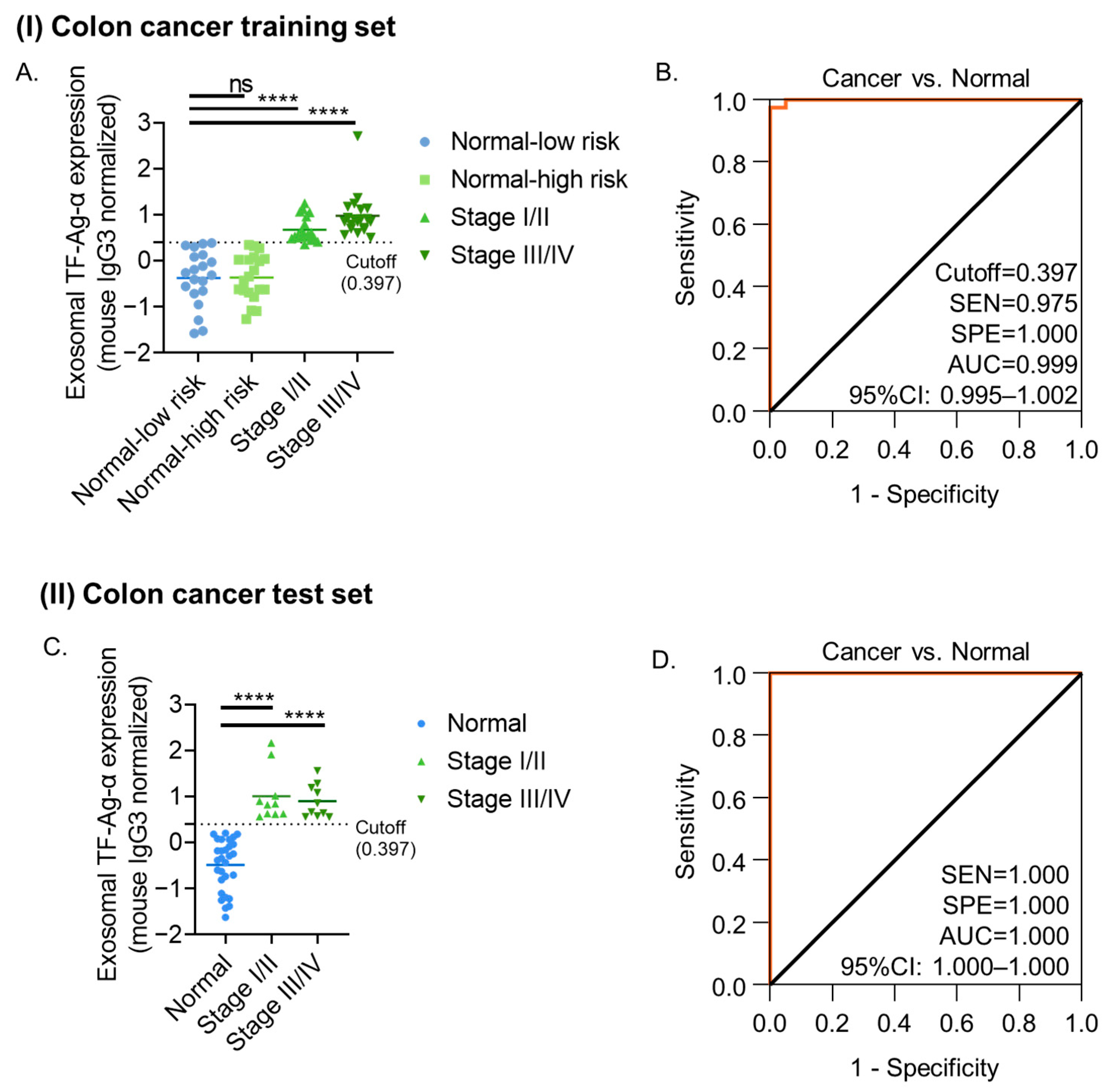
3.2. Evaluation of Exosomal TF-Ag-α in Colon Cancer Diagnosis
3.3. Diagnostic Evaluation of Exosomal TF-Ag-α in Ovarian Cancer
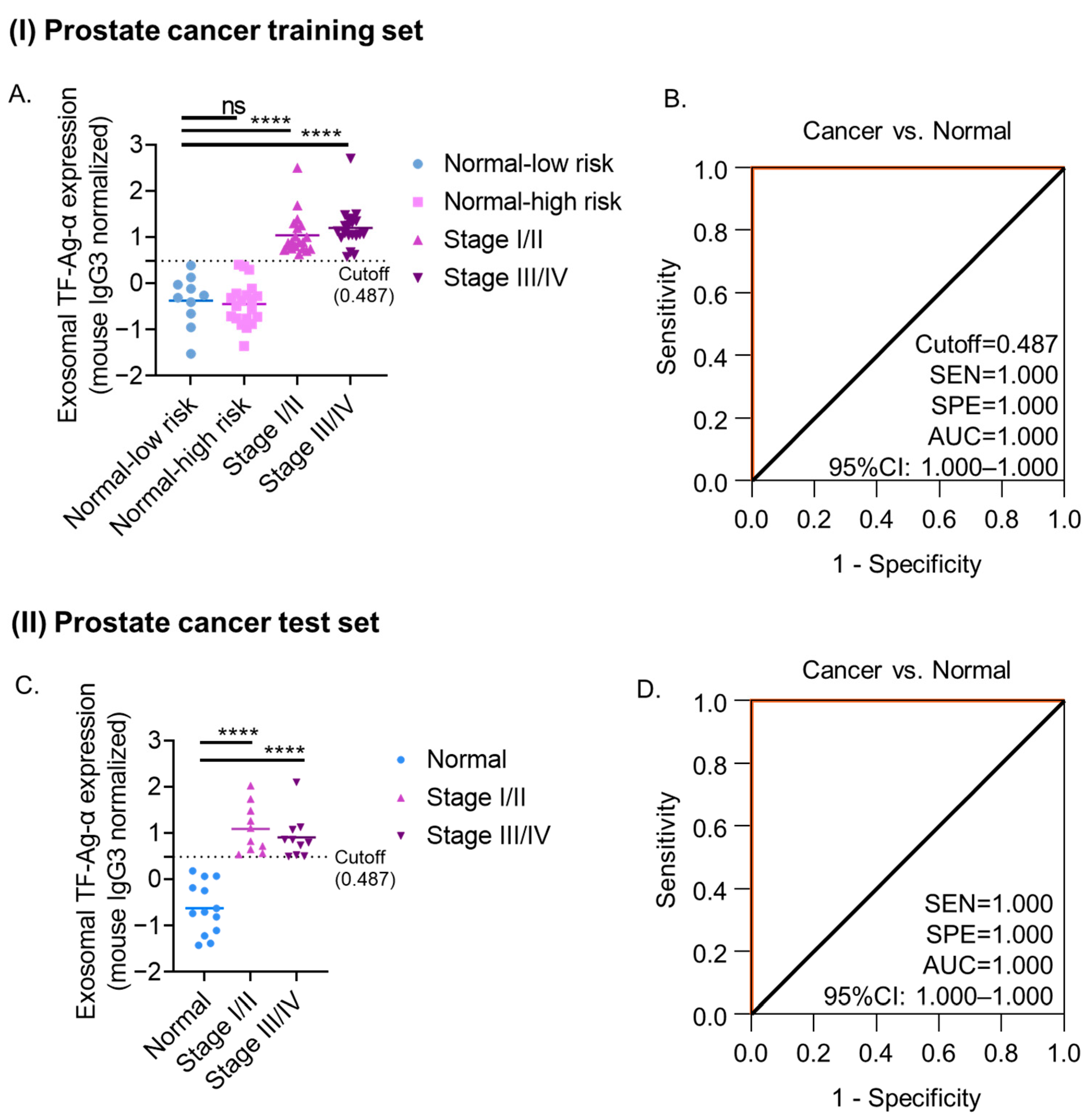
3.4. Evaluation of Exosomal TF-Ag-α in Prostate Cancer Diagnosis
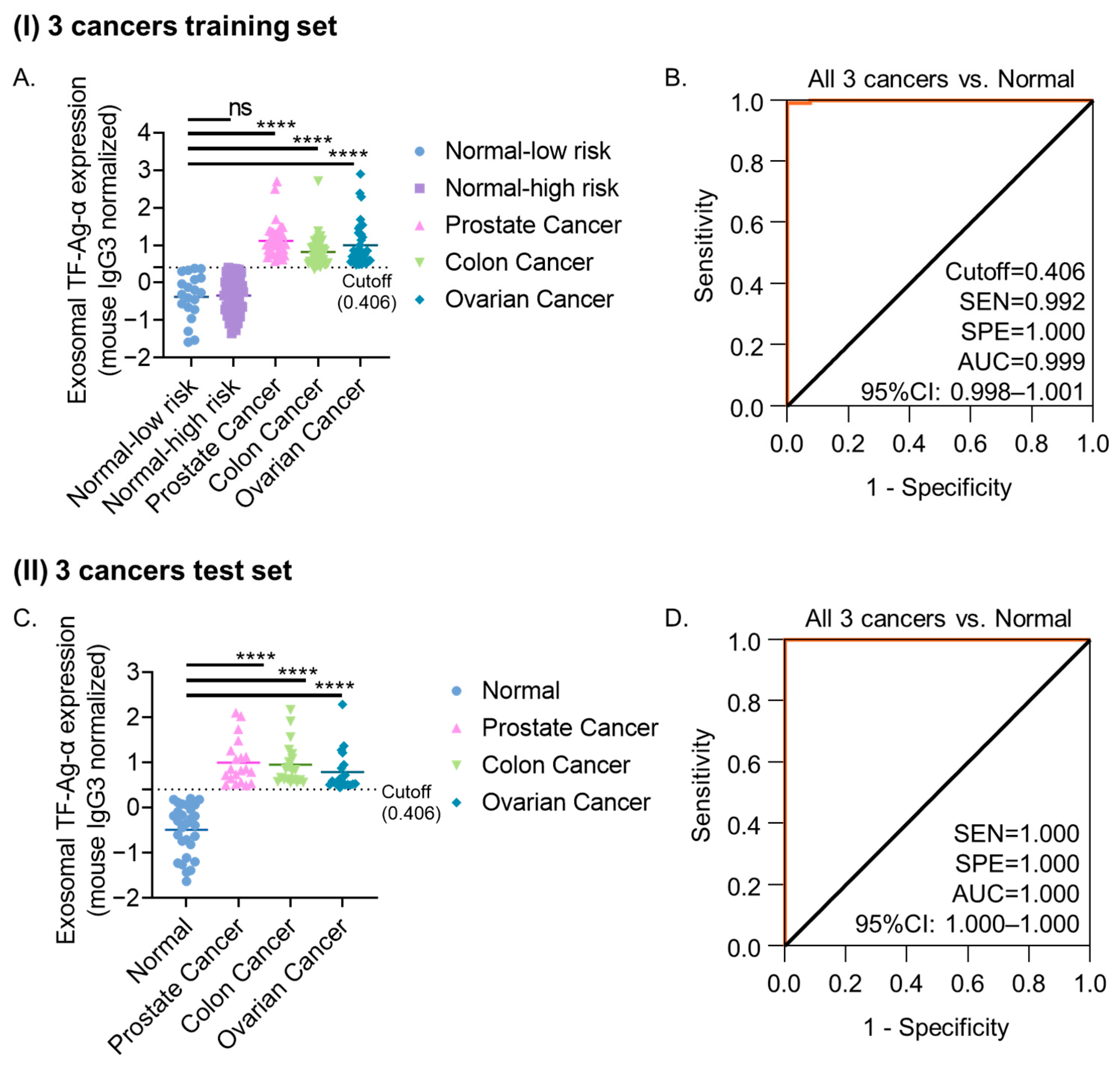
3.5. Evaluation of Exosomal TF-Ag-α Across Three Cancer Types (Colon, Ovarian, and Prostate)
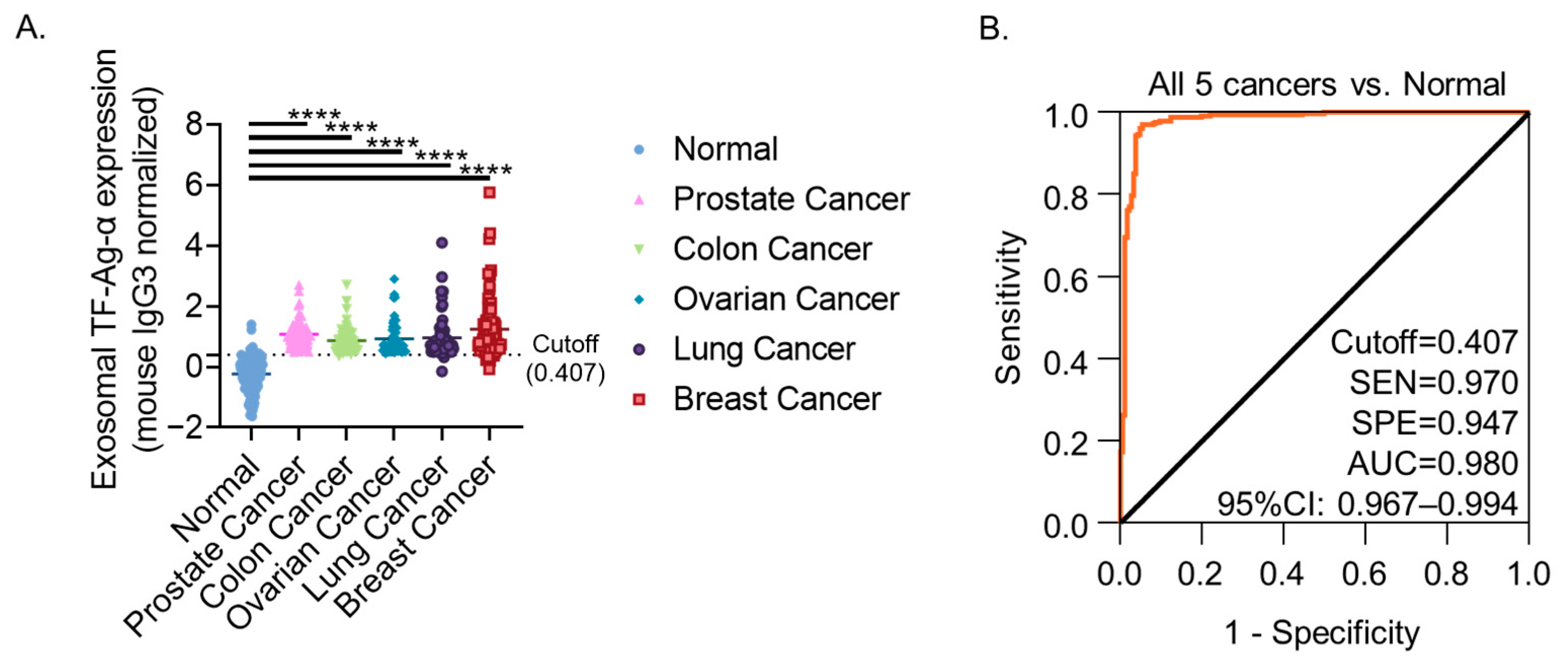
3.6. Evaluation of Exosomal TF-Ag-α Across Five Cancer Types (Colon, Ovarian, Prostate, Lung and Breast)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Overall accuracy |
| AUC | Area under the curve |
| BPH | Benign prostatic hyperplasia |
| CEA | Carcinoembryonic antigen |
| ctDNA | Circulating tumor DNA |
| DRE | Digital rectal exam |
| FIT | Fecal immunochemical tests |
| FOBT | Fecal occult blood test |
| HGPIN | High-grade prostatic intraepithelial neoplasia |
| HNPCC | Hereditary nonpolyposis colorectal cancer |
| MCED | Multi-cancer early detection |
| MRI | Magnetic resonance imaging |
| NPV | Negative predictive value |
| NTA | Nanoparticle tracking analysis |
| PEG | Polyethylene glycol |
| PPV | Positive predictive value |
| PSA | Prostate-specific antigen |
| ROC | Receiver operating characteristic |
| SEM | Scanning electron microscopy |
| SEN | Sensitivity |
| sEV | Small extracellular vesicle |
| SPE | Specificity |
| SPR | Surface plasmon resonance |
| TF-Ag-α | Alpha-linked Thomsen–Friedenreich glycoantigen |
| USPSTF | United States Preventive Services Task Force |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Struwe, W.B.; Wynne, K.; Elia, G.; Duffy, M.J.; Rudd, P.M. Exploring the glycosylation of serum CA125. Int. J. Mol. Sci. 2013, 14, 15636–15654. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef]
- Jayasinghe, M.; Prathiraja, O.; Caldera, D.; Jena, R.; Coffie-Pierre, J.A.; Silva, M.S.; Siddiqui, O.S. Colon Cancer Screening Methods: 2023 Update. Cureus 2023, 15, e37509. [Google Scholar] [CrossRef] [PubMed]
- Mohl, J.T.; Ciemins, E.L.; Miller-Wilson, L.A.; Gillen, A.; Luo, R.; Colangelo, F. Rates of Follow-up Colonoscopy After a Positive Stool-Based Screening Test Result for Colorectal Cancer Among Health Care Organizations in the US, 2017-2020. JAMA Netw. Open 2023, 6, e2251384. [Google Scholar] [CrossRef] [PubMed]
- Liberto, J.M.; Chen, S.Y.; Shih, I.M.; Wang, T.H.; Wang, T.L.; Pisanic, T.R., II. Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review. Cancers 2022, 14, 2885. [Google Scholar] [CrossRef]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Parnes, H. Screening for Prostate Cancer. N. Engl. J. Med. 2023, 388, 1405–1414. [Google Scholar] [CrossRef]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tidd-Johnson, A.; Sebastian, S.A.; Co, E.L.; Afaq, M.; Kochhar, H.; Sheikh, M.; Mago, A.; Poudel, S.; Fernandez, J.A.; Rodriguez, I.D.; et al. Prostate cancer screening: Continued controversies and novel biomarker advancements. Curr. Urol. 2022, 16, 197–206. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Tamura, T.; Yoshioka, Y.; Sakamoto, S.; Ichikawa, T.; Ochiya, T. Extracellular vesicles as a promising biomarker resource in liquid biopsy for cancer. Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 148–174. [Google Scholar] [CrossRef]
- Yu, J.; Ostowari, A.; Gonda, A.; Mashayekhi, K.; Dayyani, F.; Hughes, C.C.W.; Senthil, M. Exosomes as a Source of Biomarkers for Gastrointestinal Cancers. Cancers 2023, 15, 1263. [Google Scholar] [CrossRef]
- Wang, J.; Yan, F.; Zhao, Q.; Zhan, F.; Wang, R.; Wang, L.; Zhang, Y.; Huang, X. Circulating exosomal miR-125a-3p as a novel biomarker for early-stage colon cancer. Sci. Rep. 2017, 7, 4150. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Klimczyk, K.; Michalczewska, I.; Slomka, M.; Kubiak-Tomaszewska, G.; Olejarz, W. Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2118. [Google Scholar] [CrossRef]
- Krishn, S.R.; Singh, A.; Bowler, N.; Duffy, A.N.; Friedman, A.; Fedele, C.; Kurtoglu, S.; Tripathi, S.K.; Wang, K.; Hawkins, A.; et al. Prostate cancer sheds the alphavbeta3 integrin in vivo through exosomes. Matrix Biol. 2019, 77, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yoo, J.; Ho, J.Y.; Jung, Y.; Lee, S.; Hur, S.Y.; Choi, Y.J. Plasma-derived exosomal miR-4732-5p is a promising noninvasive diagnostic biomarker for epithelial ovarian cancer. J. Ovarian Res. 2021, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–8826. [Google Scholar] [CrossRef]
- Karacosta, L.G.; Fisk, J.C.; Jessee, J.; Tati, S.; Turner, B.; Ghazal, D.; Ludwig, R.; Johnson, H.; Adams, J.; Sajjad, M.; et al. Preclinical Analysis of JAA-F11, a Specific Anti-Thomsen-Friedenreich Antibody via Immunohistochemistry and In Vivo Imaging. Transl. Oncol. 2018, 11, 450–466. [Google Scholar] [CrossRef]
- Hsu, C.C.; Su, Y.; Rittenhouse-Olson, K.; Attwood, K.M.; Mojica, W.; Reid, M.E.; Dy, G.K.; Wu, Y. Exosomal Thomsen-Friedenreich Glycoantigen: A New Liquid Biopsy Biomarker for Lung and Breast Cancer Diagnoses. Cancer Res. Commun. 2024, 4, 1933–1945. [Google Scholar] [CrossRef]
- Siu, A.L.; U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar]
- U.S. Preventive Services Task Force; Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [PubMed]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; Bussetti, M.; Panteghini, M. Serum Prostate-Specific Antigen Testing for Early Detection of Prostate Cancer: Managing the Gap between Clinical and Laboratory Practice. Clin. Chem. 2021, 67, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Takanami, I. Expression of Thomsen-Friedenreich antigen as a marker of poor prognosis in pulmonary adenocarcinoma. Oncol. Rep. 1999, 6, 341–344. [Google Scholar] [CrossRef]
- Smith, D.; Sepehr, S.; Karakatsanis, A.; Strand, F.; Valachis, A. Yield of Surveillance Imaging After Mastectomy With or Without Reconstruction for Patients With Prior Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2244212. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Khatcheressian, J.L.; Hurley, P.; Bantug, E.; Esserman, L.J.; Grunfeld, E.; Halberg, F.; Hantel, A.; Henry, N.L.; Muss, H.B.; Smith, T.J.; et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef]


| Characteristics n (%) | ||||
|---|---|---|---|---|
| Training set | Test set | |||
| Total patients | 80 | 49 | ||
| Normal controls (low risk) | 20 (25) | 29 (59) | ||
| Normal controls (high risk) | 20 (25) | 0 (0) | ||
| Cancer | 40 (50) | 20 (41) | ||
| Normal controls (both low-risk and high-risk patients) | Patients with colon cancer | Normal controls (low-risk patients) | Patients with colon cancer | |
| Gender | ||||
| Female | 20 (25) | 20 (25) | 16 (33) | 10 (20) |
| Male | 20 (25) | 20 (25) | 13 (27) | 10 (20) |
| Age (years) | ||||
| Mean | 60 | 65 | 64 | 61 |
| Median | 60 | 66 | 64 | 58 |
| Range | 30–79 | 44–91 | 50–79 | 45–79 |
| Stage | ||||
| I | 10 (25) | 5 (25) | ||
| II | 10 (25) | 5 (25) | ||
| III | 10 (25) | 5 (25) | ||
| IV | 10 (25) | 5 (25) | ||
| Characteristics n (%) | ||||
|---|---|---|---|---|
| Training set | Test set | |||
| Total patients | 70 | 36 | ||
| Normal controls (low risk) | 10 (14) | 16 (44) | ||
| Normal controls (high risk) | 20 (29) | 0 (0) | ||
| Cancer | 40 (57) | 20 (56) | ||
| Normal controls (both low-risk and high-risk patients) | Patients with ovarian cancer | Normal controls (low-risk patients) | Patients with ovarian cancer | |
| Gender | ||||
| Female | 30 (43) | 40 (57) | 16 (44) | 20 (56) |
| Age (years) | ||||
| Mean | 58 | 65 | 64 | 59 |
| Median | 57 | 65 | 66 | 60 |
| Range | 38–85 | 40–89 | 50–79 | 47–76 |
| Stage | ||||
| I | 10 (25) | 5 (25) | ||
| II | 10 (25) | 5 (25) | ||
| III | 10 (25) | 5 (25) | ||
| IV | 10 (25) | 5 (25) | ||
| Characteristics n (%) | ||||
|---|---|---|---|---|
| Training set | Test set | |||
| Total patients | 70 | 33 | ||
| Normal controls (low risk) | 10 (14) | 13 (39) | ||
| Normal controls (high risk) | 20 (29) | 0 (0) | ||
| Cancer | 40 (57) | 20 (61) | ||
| Normal controls (both low-risk and high-risk patients) | Patients with prostate cancer | Normal controls (low-risk patients) | Patients with prostate cancer | |
| Gender | ||||
| Male | 30 (43) | 40 (57) | 13 (39) | 20 (61) |
| Age (years) | ||||
| Mean | 64 | 60 | 63 | 62 |
| Median | 64 | 60 | 61 | 59 |
| Range | 51–78 | 45–81 | 51–77 | 53–74 |
| Stage | ||||
| I | 10 (25) | 5 (25) | ||
| II | 10 (25) | 5 (25) | ||
| III | 10 (25) | 5 (25) | ||
| IV | 10 (25) | 5 (25) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Qi, M.; Vasini, S.; Reid, M.E.; Rittenhouse-Olson, K.; Dy, G.K.; Wu, Y. Exosomal Thomsen–Friedenreich Glycoantigen as a Sensitive and Specific Biomarker for Colon, Ovarian and Prostate Cancer Diagnosis. Cancers 2025, 17, 3729. https://doi.org/10.3390/cancers17233729
Su Y, Qi M, Vasini S, Reid ME, Rittenhouse-Olson K, Dy GK, Wu Y. Exosomal Thomsen–Friedenreich Glycoantigen as a Sensitive and Specific Biomarker for Colon, Ovarian and Prostate Cancer Diagnosis. Cancers. 2025; 17(23):3729. https://doi.org/10.3390/cancers17233729
Chicago/Turabian StyleSu, Yafei, Man Qi, Shoaib Vasini, Mary E. Reid, Kate Rittenhouse-Olson, Grace K. Dy, and Yun Wu. 2025. "Exosomal Thomsen–Friedenreich Glycoantigen as a Sensitive and Specific Biomarker for Colon, Ovarian and Prostate Cancer Diagnosis" Cancers 17, no. 23: 3729. https://doi.org/10.3390/cancers17233729
APA StyleSu, Y., Qi, M., Vasini, S., Reid, M. E., Rittenhouse-Olson, K., Dy, G. K., & Wu, Y. (2025). Exosomal Thomsen–Friedenreich Glycoantigen as a Sensitive and Specific Biomarker for Colon, Ovarian and Prostate Cancer Diagnosis. Cancers, 17(23), 3729. https://doi.org/10.3390/cancers17233729






