SBFI Inhibitors Reprogram Transcriptomic Landscape of Prostate Cancer Cells Leading to Cell Death
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Cell Proliferation
2.3. Cell Cycle Analysis
2.4. Apoptosis Assay
2.5. RNA-Sequencing
2.6. Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR)
2.7. ChIP-X Enrichment Analysis 3 (ChEA3)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
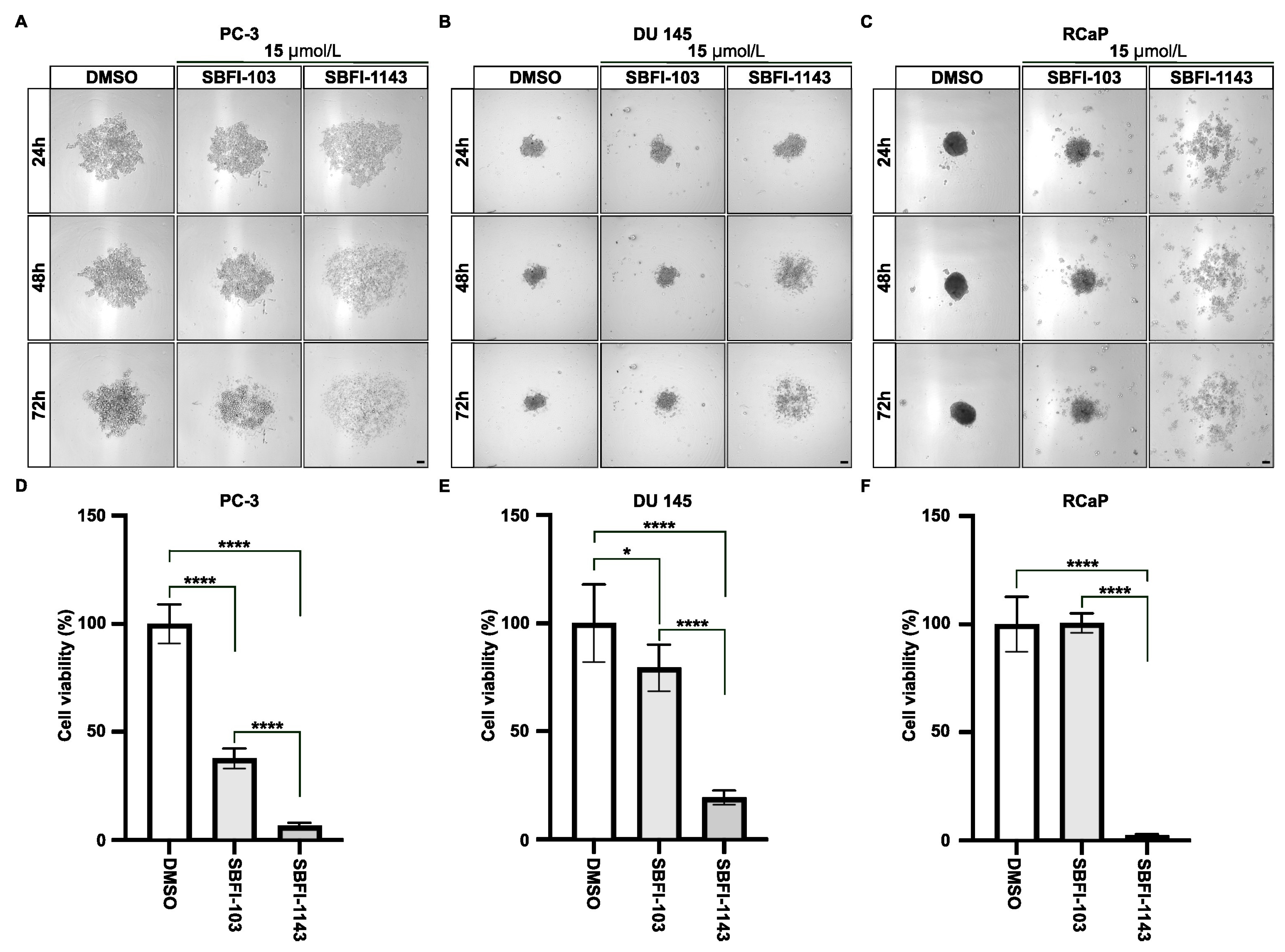
3.1. The Third-Generation FABP5 Inhibitor (SBFI-1143) Significantly Reduced the Proliferation of Prostate Cancer Cells
3.2. SBFI-1143 Alters the Cell Cycle Progression and Induces Cell Death of PCa Cells
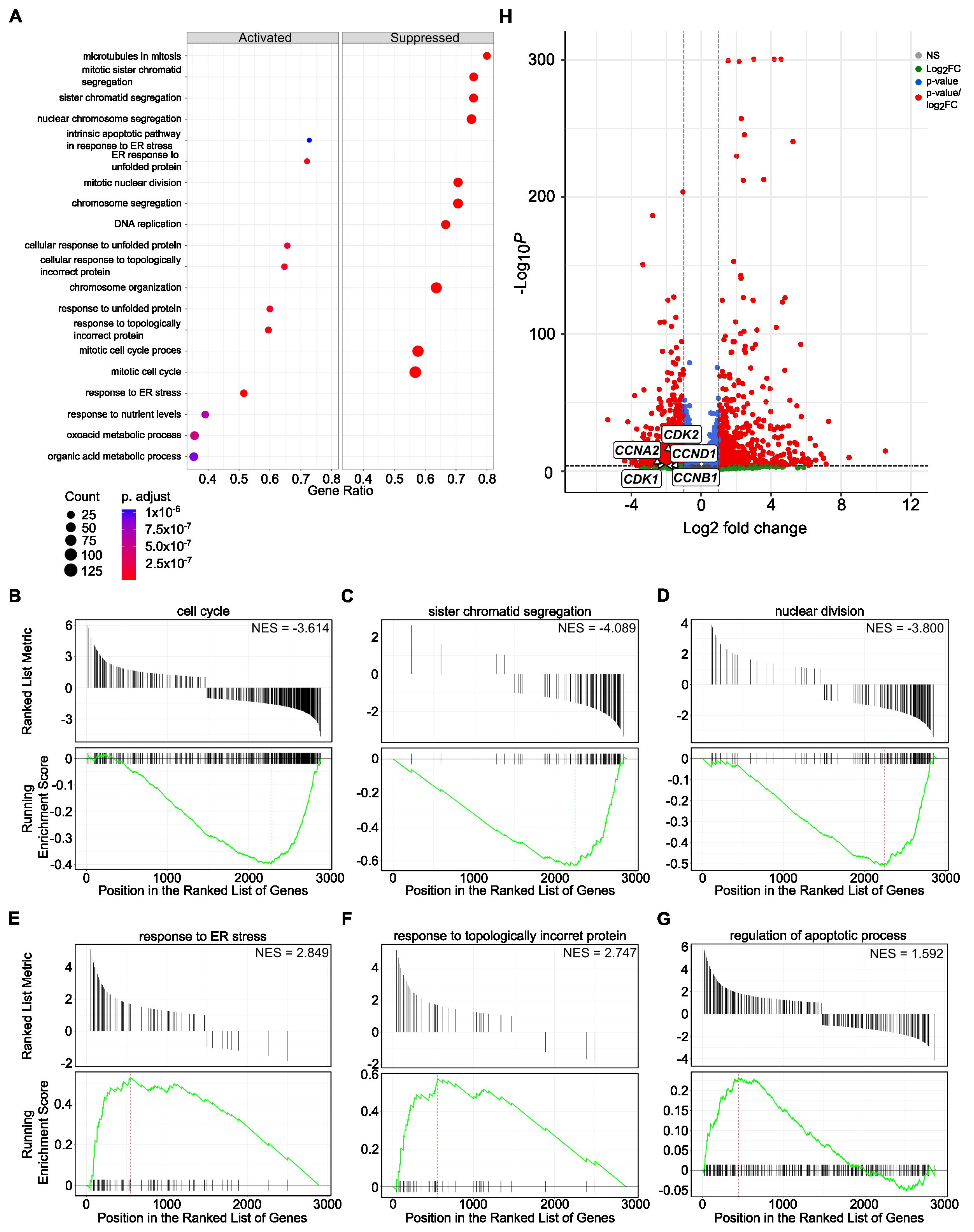
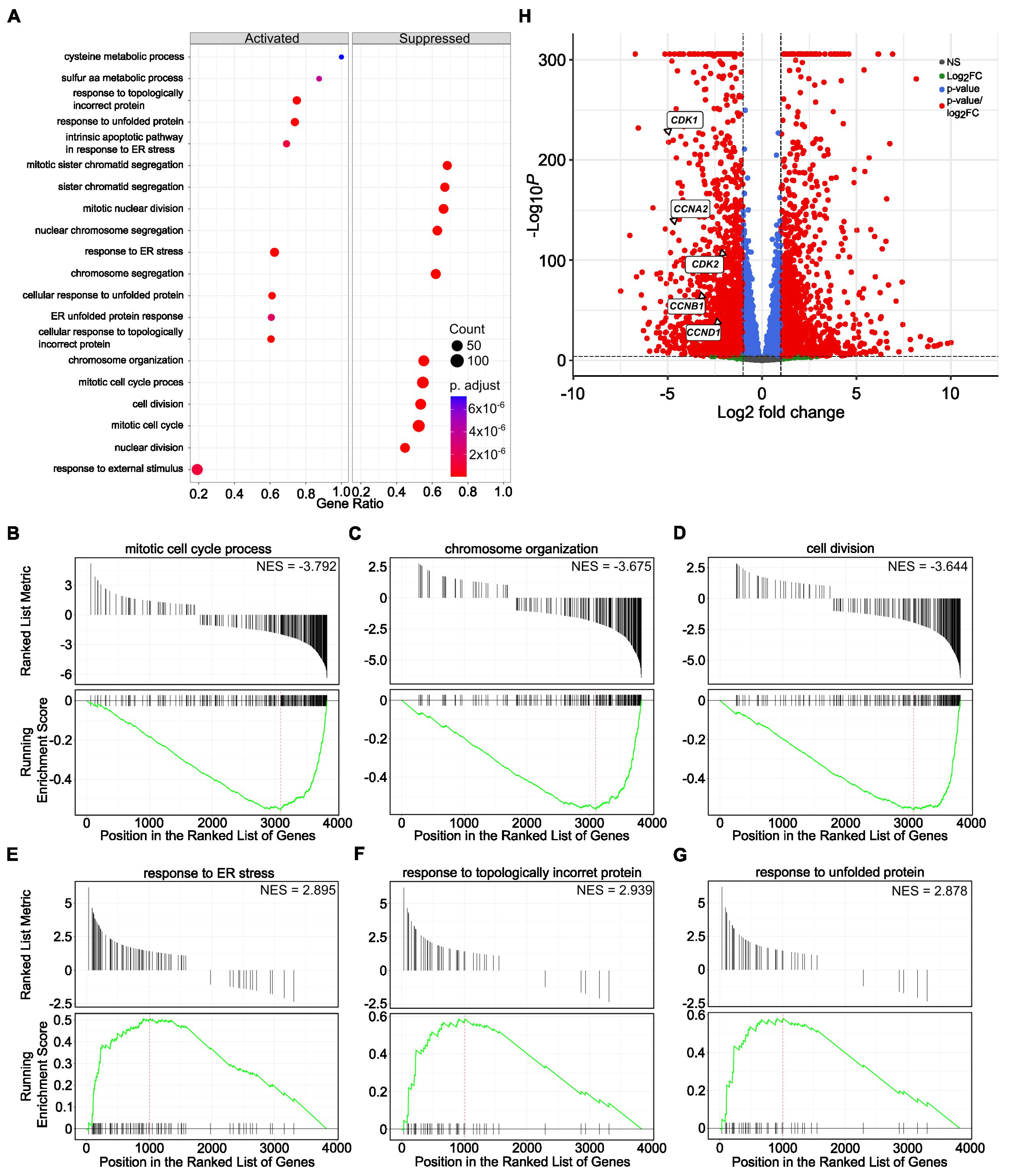
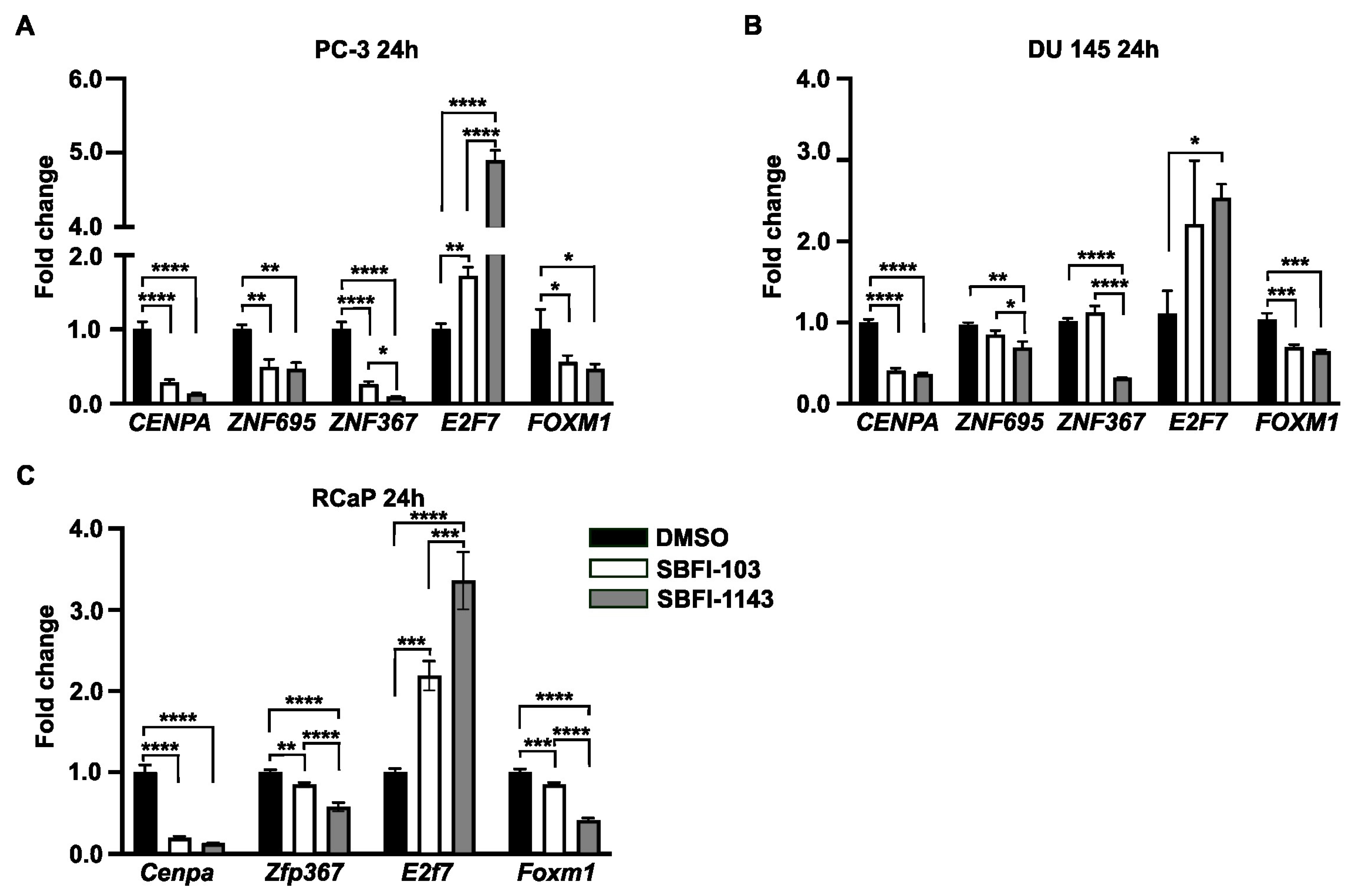
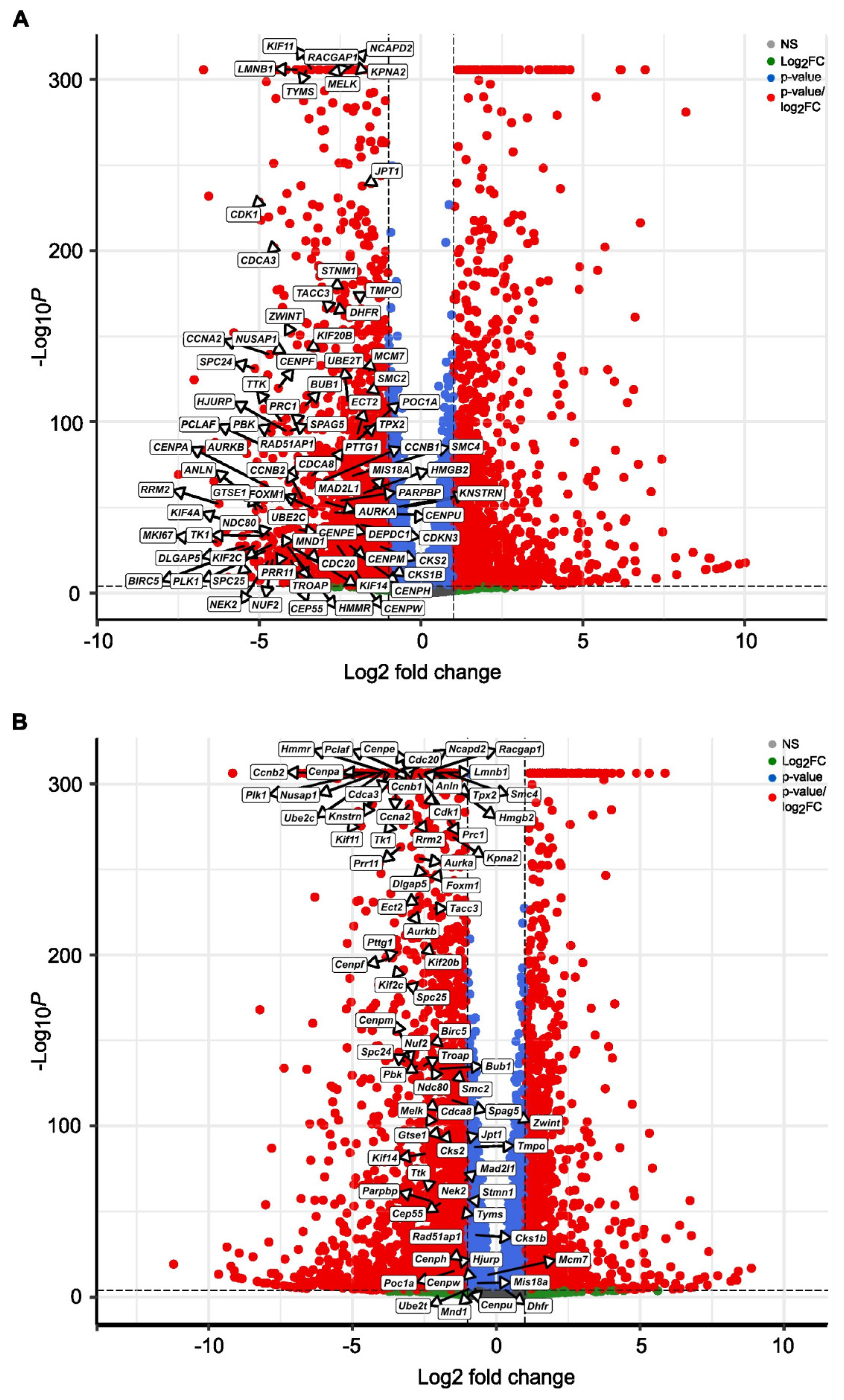
3.3. SBFI-103 and SBFI-1143 Modify the Transcriptome of PCa Cells
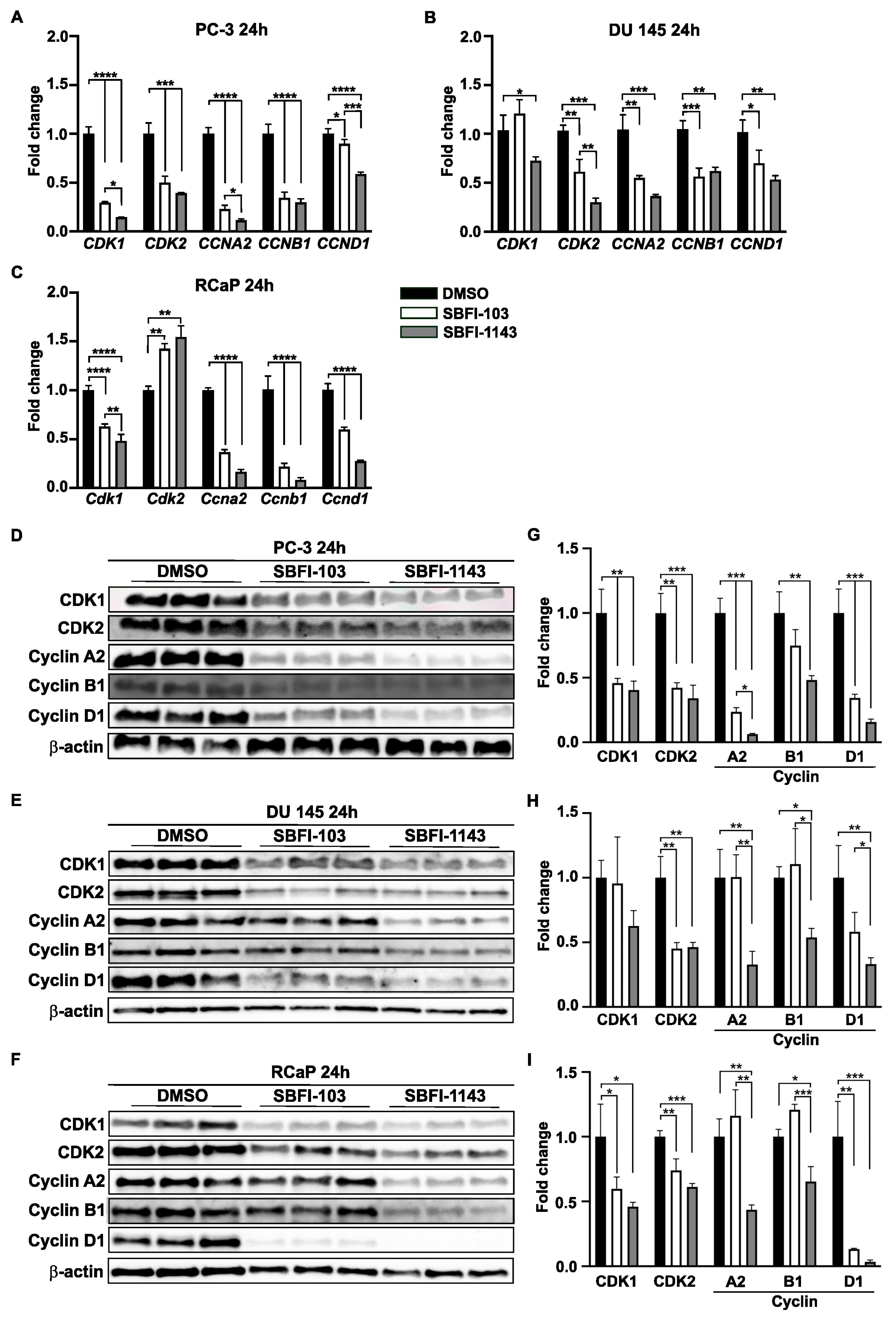
3.4. SBFI-1143 Alters Transcriptional Machinery and Impacts the Expression of Genes Involved in the Progression of mPC
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | Actin |
| ADGFR1 | Adhesion G protein-coupled receptor F |
| AGR2 | Anterior gradient 2 |
| AGTR2 | Angiotensin II Receptor Type 2 |
| ANLN | Anillin |
| AR | Androgen Receptor |
| ASPN | Asporin |
| ATF3 | Activating Transcription Factor 3 |
| ATF4 | Activating Transcription Factor 4 |
| BIRC5 | Baculoviral IAP repeat-containing protein 5 or Survivin |
| BRCA1 | Breast cancer susceptibility gene 1 |
| BRCA2 | Breast cancer susceptibility gene 2 |
| CCNA2 | Cyclin A2 |
| CCNB1 | Cyclin B1 |
| CCND1 | Cyclin D1 |
| CDC20 | Cell division cycle protein 20 homolog |
| CDK1 | Cyclin-dependent kinase 1 |
| CDK2 | Cyclin-dependent kinase 2 |
| CENPA | Centromere protein A |
| CD24 | CD24 antigen |
| CD248 | Endosialin |
| CHAC1 | ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1 |
| ChEA3 | ChIP-X Enrichment Analysis 3 |
| COL1A1 | Collagen type I alpha 1 chain |
| COL3A1 | Collagen type III alpha 1 chain |
| COX1 | Cyclooxygenase-1 |
| COX2 | Cyclooxygenase-2 |
| CRABP1 | Cellular retinoic acid binding protein 1 |
| CRPC | Castration-resistant prostate cancer |
| CSRNP1 | Cysteine and serine rich nuclear protein 1 |
| DDIT4 | DNA Damage-Inducible Transcript 4 |
| DMSO | Dimethyl sulfoxide |
| DNMT1 | DNA methyltransferase 1 |
| DNMT3A | DNA methyltransferase 3A |
| DNTM3B | DNA methyltransferase 3B |
| DPT | Dermatopontin |
| EED | Embryonic ectoderm development |
| EFEMP1 | Epidermal Growth Factor-Containing Fibulin-Like Extracellular Matrix Protein 1 |
| EZH2 | Enhancer of Zeste Homolog 2 |
| E2F7 | E2F Transcription Factor 7 |
| FABP5 | Fatty Acid-Binding Protein 5 |
| FAM111B | FAM111 Trypsin Like Peptidase B |
| FOXM1 | (Forkhead box protein M1) |
| FZD4 | Frizzled-4 |
| GAS1 | Growth arrest-specific protein 1 |
| GAS6 | Growth arrest-specific protein 6 |
| GATA2 | GATA binding protein 2 |
| GDF15 | Growth Differentiation Factor 15 |
| GSEA | Gene set enrichment analysis |
| HAS3 | Hyaluronan Synthase 3 |
| HMMR | Hyaluronan-mediated motility receptor |
| HMOX1 | Heme oxygenase 1 |
| HOXB13 | Homeobox B13 |
| HRP | Horseradish peroxidase |
| IGFBP5 | Insulin-like Growth Factor-Binding Protein 5 |
| IL1R1 | Interleukin 1 receptor, type I |
| JUN | Transcription factor Jun |
| LGR5 | Leucine-rich repeat containing G protein-coupled receptor 5 |
| LPC | A subpopulation of prostate cancer cells with a role in trans-differentiation, recurrence and poor prognosis |
| LPSig | Lineage plasticity-related signature |
| LRP4 | Low-density lipoprotein receptor-related protein 4 |
| LRP5 | Low-density lipoprotein receptor-related protein 5 |
| MAPK | Mitogen-activated protein kinase |
| MKI67 | Marker of Proliferation Ki-67 |
| μmol/L | Micromoles per liter |
| Mmol/L | Millimoles per liter |
| mPC | Metastatic prostate cancer |
| MYBL2 | Myeloblastosis oncogene-like 2 |
| MYC | C-MYC proto-oncogene |
| NFIL3 | Nuclear Factor Interleukin 3 Regulated |
| NIBAN1 | Niban Apoptosis Regulator 1 |
| NID2 | Nidogen-2 |
| PARP1 | Poly(ADP-Ribose) Polymerase 1 |
| PCa | Prostate cancer |
| PDGFB | Platelet derived growth factor subunit B |
| PHACTR3 | Phosphatase and actin regulator 3 |
| PLK1 | Polo-like kinase 1 |
| PTN | Pleiotrophin |
| RIN | RNA integrity number |
| RNA-seq | RNA-sequencing |
| RN7SK | 7SK small nuclear RNA |
| RPLP0P2 | Ribosomal Protein Lateral Stalk Subunit P0 Pseudogene 2 |
| RRM2 | Ribonucleotide Reductase Regulatory Subunit M2 |
| RSPO | R-spondin-1 |
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 |
| SBFI | Stony Brook FABP5 inhibitor |
| SESN2 | Sestrin-2 |
| SFRP2 | Secreted frizzled-related protein 2 |
| SLC7A11 | Solute carrier family 7 member 11 |
| SRPX | Sushi repeat containing protein X-linked |
| S100a4 | S100 calcium-binding protein A4 |
| TAME | Truxillic-acid monoester |
| TGFβ | Transforming growth factor beta |
| TMEM119 | Transmembrane protein 119 |
| TOP2A | DNA topoisomerase II alpha |
| TRIB3 | Tribbles Homolog 3 |
| Tris-HCl | Tris(hydroxymethyl)aminomethane hydrochloride |
| UBE2C | Ubiquitin-conjugating enzyme E2C |
| WNT4 | Wnt Family Member 4 |
| WNT7A | Wnt Family Member 7A |
| WNT7B | Wnt Family Member 7B |
| WNT10B | Wnt Family Member 10B |
| ZNF367 | Zinc Finger Protein 367 |
| ZNF695 | Zinc Finger Protein 695 |
References
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- Sayegh, N.; Swami, U.; Agarwal, N. Recent Advances in the Management of Metastatic Prostate Cancer. JCO Oncol. Pract. 2022, 18, 45–55. [Google Scholar] [CrossRef]
- Gebrael, G.; Sayegh, N.; Tripathi, N.; Goel, D.; McFarland, T.; Ebrahimi, H.; Nordblad, B.; Chigarira, B.; Mathew Thomas, V.; Sahu, K.K.; et al. Impact of Initial Timing of Metastatic Disease on Survival in Patients With Newly Diagnosed Metastatic Castration-Resistant Prostate Cancer Treated With Androgen Receptor Pathway Inhibitors. Urol. Pract. 2024, 11, 32–35. [Google Scholar] [CrossRef] [PubMed]
- LaTulippe, E.; Satagopan, J.; Smith, A.; Scher, H.; Scardino, P.; Reuter, V.; Gerald, W.L. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002, 62, 4499–4506. [Google Scholar]
- Marzec, J.; Ross-Adams, H.; Pirro, S.; Wang, J.; Zhu, Y.; Mao, X.; Gadaleta, E.; Ahmad, A.S.; North, B.V.; Kammerer-Jacquet, S.F.; et al. The Transcriptomic Landscape of Prostate Cancer Development and Progression: An Integrative Analysis. Cancers 2021, 13, 345. [Google Scholar] [CrossRef]
- Cajigas-Du Ross, C.K.; Martinez, S.R.; Woods-Burnham, L.; Duran, A.M.; Roy, S.; Basu, A.; Ramirez, J.A.; Ortiz-Hernandez, G.L.; Rios-Colon, L.; Chirshev, E.; et al. RNA sequencing reveals upregulation of a transcriptomic program associated with stemness in metastatic prostate cancer cells selected for taxane resistance. Oncotarget 2018, 9, 30363–30384. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, F.; Lin, Y.; Liu, M.; Zhou, H.; Cui, F.; Jin, Y.; Chen, L.; Sheng, X. Integrated analysis of single-cell and bulk transcriptomics develops a robust neuroendocrine cell-intrinsic signature to predict prostate cancer progression. Theranostics 2024, 14, 1065–1080. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, T.; Wei, J.; Chen, L.; Liu, Z.; Jin, Y.; Liu, M.; Zhou, H.; Hu, Y.; Sheng, X. Integrated single-cell transcriptomic analyses identify a novel lineage plasticity-related cancer cell type involved in prostate cancer progression. eBioMedicine 2024, 109, 105398. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; d’Oelsnitz, S.; Kaczocha, M. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 2019, 9, 18944. [Google Scholar] [CrossRef] [PubMed]
- Fidelito, G.; Todorovski, I.; Cluse, L.; Vervoort, S.J.; Taylor, R.A.; Watt, M.J. Lipid-metabolism-focused CRISPR screens identify enzymes of the mevalonate pathway as essential for prostate cancer growth. Cell Rep. 2025, 44, 115470. [Google Scholar] [CrossRef]
- Butler, L.M.; Mah, C.Y.; Machiels, J.; Vincent, A.D.; Irani, S.; Mutuku, S.M.; Spotbeen, X.; Bagadi, M.; Waltregny, D.; Moldovan, M.; et al. Lipidomic Profiling of Clinical Prostate Cancer Reveals Targetable Alterations in Membrane Lipid Composition. Cancer Res. 2021, 81, 4981–4993. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.W.; Kasai, M.; Yamamoto, S.; Fukuhara, H.; Karashima, T.; Kurabayashi, A.; Furihata, M.; Hanazaki, K.; Inoue, K.; Ogura, S.I. Metabolic shift towards oxidative phosphorylation reduces cell-density-induced cancer-stem-cell-like characteristics in prostate cancer in vitro. Biol. Open 2023, 12, bio059615. [Google Scholar] [CrossRef]
- Schopf, B.; Weissensteiner, H.; Schafer, G.; Fazzini, F.; Charoentong, P.; Naschberger, A.; Rupp, B.; Fendt, L.; Bukur, V.; Giese, I.; et al. OXPHOS remodeling in high-grade prostate cancer involves mtDNA mutations and increased succinate oxidation. Nat. Commun. 2020, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Gaffar, S.; Aathirah, A.S. Fatty-Acid-Binding Proteins: From Lipid Transporters to Disease Biomarkers. Biomolecules 2023, 13, 1753. [Google Scholar] [CrossRef]
- Storch, J.; Corsico, B. The Multifunctional Family of Mammalian Fatty Acid-Binding Proteins. Annu. Rev. Nutr. 2023, 43, 25–54. [Google Scholar] [CrossRef]
- Agellon, L.B. Importance of fatty acid binding proteins in cellular function and organismal metabolism. J. Cell. Mol. Med. 2024, 28, e17703. [Google Scholar] [CrossRef]
- Zhang, J.; He, G.; Jin, X.; Alenezi, B.T.; Naeem, A.A.; Abdulsamad, S.A.; Ke, Y. Molecular mechanisms on how FABP5 inhibitors promote apoptosis-induction sensitivity of prostate cancer cells. Cell Biol. Int. 2023, 47, 929–942. [Google Scholar] [CrossRef]
- Warren, W.G.; Osborn, M.; Yates, A.; Wright, K.; O’Sullivan, S.E. The emerging role of fatty acid binding protein 5 (FABP5) in cancers. Drug Discov. Today 2023, 28, 103628. [Google Scholar] [CrossRef]
- Naeem, A.A.; Abdulsamad, S.A.; Rudland, P.S.; Malki, M.I.; Ke, Y. Fatty acid-binding protein 5 (FABP5)-related signal transduction pathway in castration-resistant prostate cancer cells: A potential therapeutic target. Precis. Clin. Med. 2019, 2, 192–196. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Kaczocha, M. FABP5 as a novel molecular target in prostate cancer. Drug Discov. Today 2020, 25, 2056–2061. [Google Scholar] [CrossRef]
- Forootan, F.S.; Forootan, S.S.; Gou, X.; Yang, J.; Liu, B.; Chen, D.; Al Fayi, M.S.; Al-Jameel, W.; Rudland, P.S.; Hussain, S.A.; et al. Fatty acid activated PPARgamma promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget 2016, 7, 9322–9339. [Google Scholar] [CrossRef]
- Swamynathan, M.M.; Mathew, G.; Aziz, A.; Gordon, C.; Hillowe, A.; Wang, H.; Jhaveri, A.; Kendall, J.; Cox, H.; Giarrizzo, M.; et al. FABP5 Inhibition against PTEN-Mutant Therapy Resistant Prostate Cancer. Cancers 2023, 16, 60. [Google Scholar] [CrossRef]
- Naeem, A.A.; Abdulsamad, S.A.; Zeng, H.; He, G.; Jin, X.; Zhang, J.; Alenezi, B.T.; Ma, H.; Rudland, P.S.; Ke, Y. FABP5 can substitute for androgen receptor in malignant progression of prostate cancer cells. Int. J. Oncol. 2024, 64, 18. [Google Scholar] [CrossRef]
- Hillowe, A.; Gordon, C.; Wang, L.; Rizzo, R.C.; Trotman, L.C.; Ojima, I.; Bialkowska, A.; Kaczocha, M. Fatty acid binding protein 5 regulates docetaxel sensitivity in taxane-resistant prostate cancer cells. PLoS ONE 2023, 18, e0292483. [Google Scholar] [CrossRef] [PubMed]
- Al-Jameel, W.; Gou, X.; Jin, X.; Zhang, J.; Wei, Q.; Ai, J.; Li, H.; Al-Bayati, A.; Platt-Higgins, A.; Pettitt, A.; et al. Inactivated FABP5 suppresses malignant progression of prostate cancer cells by inhibiting the activation of nuclear fatty acid receptor PPARgamma. Genes. Cancer 2019, 10, 80–96. [Google Scholar] [CrossRef]
- Carbonetti, G.; Converso, C.; Clement, T.; Wang, C.; Trotman, L.C.; Ojima, I.; Kaczocha, M. Docetaxel/cabazitaxel and fatty acid binding protein 5 inhibitors produce synergistic inhibition of prostate cancer growth. Prostate 2020, 80, 88–98. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Swamynathan, M.M.; Rajput, S.; Jayanetti, K.; Rendina, D.; Takemura, K.; Bogdan, D.; Wang, L.; Rizzo, R.C.; et al. Fatty acid binding protein 5 inhibitors as novel anticancer agents against metastatic castration-resistant prostate cancer. Bioorganic Med. Chem. 2025, 122, 118136. [Google Scholar] [CrossRef] [PubMed]
- Al-Jameel, W.; Gou, X.; Forootan, S.S.; Al Fayi, M.S.; Rudland, P.S.; Forootan, F.S.; Zhang, J.; Cornford, P.A.; Hussain, S.A.; Ke, Y. Inhibitor SBFI26 suppresses the malignant progression of castration-resistant PC3-M cells by competitively binding to oncogenic FABP5. Oncotarget 2017, 8, 31041–31056. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Keenan, A.B.; Torre, D.; Lachmann, A.; Leong, A.K.; Wojciechowicz, M.L.; Utti, V.; Jagodnik, K.M.; Kropiwnicki, E.; Wang, Z.; Ma’ayan, A. ChEA3: Transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 2019, 47, W212–W224. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bolis, M.; Bossi, D.; Vallerga, A.; Ceserani, V.; Cavalli, M.; Impellizzieri, D.; Di Rito, L.; Zoni, E.; Mosole, S.; Elia, A.R.; et al. Dynamic prostate cancer transcriptome analysis delineates the trajectory to disease progression. Nat. Commun. 2021, 12, 7033. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sunkel, B.; Chen, Z.; Liu, X.; Ye, Z.; Li, Q.; Grenade, C.; Ke, J.; Zhang, C.; Chen, H.; et al. Three-tiered role of the pioneer factor GATA2 in promoting androgen-dependent gene expression in prostate cancer. Nucleic Acids Res. 2014, 42, 3607–3622. [Google Scholar] [CrossRef]
- He, B.; Lanz, R.B.; Fiskus, W.; Geng, C.; Yi, P.; Hartig, S.M.; Rajapakshe, K.; Shou, J.; Wei, L.; Shah, S.S.; et al. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc. Natl. Acad. Sci. USA 2014, 111, 18261–18266. [Google Scholar] [CrossRef]
- Rodriguez-Bravo, V.; Carceles-Cordon, M.; Hoshida, Y.; Cordon-Cardo, C.; Galsky, M.D.; Domingo-Domenech, J. The role of GATA2 in lethal prostate cancer aggressiveness. Nat. Rev. Urol. 2017, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Oh, K.J.; Park, R.Y.; Xuan, N.T.; Kang, T.W.; Kwon, D.D.; Choi, C.; Kim, M.S.; Nam, K.I.; Ahn, K.Y.; et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol. Cancer 2010, 9, 124. [Google Scholar] [CrossRef]
- Yeh, S.; Hu, Y.C.; Rahman, M.; Lin, H.K.; Hsu, C.L.; Ting, H.J.; Kang, H.Y.; Chang, C. Increase of androgen-induced cell death and androgen receptor transactivation by BRCA1 in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11256–11261. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kumaraswamy, E.; Harlan-Williams, L.M.; Jensen, R.A. The role of BRCA1 and BRCA2 in prostate cancer. Front. Biosci. 2013, 18, 1445–1459. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef]
- Deshmukh, D.; Qiu, Y. Role of PARP-1 in prostate cancer. Am. J. Clin. Exp. Urol. 2015, 3, 1–12. [Google Scholar] [PubMed]
- Saha, A.K.; Contreras-Galindo, R.; Niknafs, Y.S.; Iyer, M.; Qin, T.; Padmanabhan, K.; Siddiqui, J.; Palande, M.; Wang, C.; Qian, B.; et al. The role of the histone H3 variant CENPA in prostate cancer. J. Biol. Chem. 2020, 295, 8537–8549. [Google Scholar] [CrossRef]
- Lee, D.Y.; Chun, J.N.; So, I.; Jeon, J.H. Oncogenic role of FOXM1 in human prostate cancer (Review). Oncol. Rep. 2024, 51, 15. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, H.; Zhu, X.; Lin, D.; Chen, Z.; Dong, X.; Chen, J.; Shi, M.; Ni, Y.; Cao, J.; et al. Deciphering the Transcription Factor Landscape in Prostate Cancer Progression: A Novel Approach to Understand NE Transdifferentiation. Adv. Sci. 2025, 12, e2404938. [Google Scholar] [CrossRef] [PubMed]
- Machnik, M.; Cylwa, R.; Kielczewski, K.; Biecek, P.; Liloglou, T.; Mackiewicz, A.; Oleksiewicz, U. The expression signature of cancer-associated KRAB-ZNF factors identified in TCGA pan-cancer transcriptomic data. Mol. Oncol. 2019, 13, 701–724. [Google Scholar] [CrossRef]
- Westendorp, B.; Mokry, M.; Groot Koerkamp, M.J.; Holstege, F.C.; Cuppen, E.; de Bruin, A. E2F7 represses a network of oscillating cell cycle genes to control S-phase progression. Nucleic Acids Res. 2012, 40, 3511–3523. [Google Scholar] [CrossRef]
- Han, Z.; Mo, R.; Cai, S.; Feng, Y.; Tang, Z.; Ye, J.; Liu, R.; Cai, Z.; Zhu, X.; Deng, Y.; et al. Differential Expression of E2F Transcription Factors and Their Functional and Prognostic Roles in Human Prostate Cancer. Front. Cell Dev. Biol. 2022, 10, 831329. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, X.; Xu, P.; Tan, Z.; Zhu, Z.; Zhang, G.; Jiang, Z.; Deng, Z. E2F7, regulated by miR-30c, inhibits apoptosis and promotes cell cycle of prostate cancer cells. Oncol. Rep. 2020, 44, 849–862. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, Y.; Feng, Y.; Chen, T.; Liu, M.; Zeng, Y.; Yin, X.; Qu, S.; Huang, L.; Ke, Y.; et al. SBFI26 induces triple-negative breast cancer cells ferroptosis via lipid peroxidation. J. Cell. Mol. Med. 2024, 28, e18212. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Xu, L. FABP5 inhibitor SBFI-26 regulates FOXM1 expression and Wnt signaling pathway in ovarian granulosa cell of patients with polycystic ovary syndrome. Prev. Med. 2023, 174, 107634. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajput, S.; LaComb, J.F.; Gordon, C.; Wang, H.; Sarder, M.; Kaczocha, M.; Ojima, I.; Bialkowska, A.B. SBFI Inhibitors Reprogram Transcriptomic Landscape of Prostate Cancer Cells Leading to Cell Death. Cancers 2025, 17, 3723. https://doi.org/10.3390/cancers17233723
Rajput S, LaComb JF, Gordon C, Wang H, Sarder M, Kaczocha M, Ojima I, Bialkowska AB. SBFI Inhibitors Reprogram Transcriptomic Landscape of Prostate Cancer Cells Leading to Cell Death. Cancers. 2025; 17(23):3723. https://doi.org/10.3390/cancers17233723
Chicago/Turabian StyleRajput, Shubhra, Joseph F. LaComb, Chris Gordon, Hehe Wang, Manisha Sarder, Martin Kaczocha, Iwao Ojima, and Agnieszka B. Bialkowska. 2025. "SBFI Inhibitors Reprogram Transcriptomic Landscape of Prostate Cancer Cells Leading to Cell Death" Cancers 17, no. 23: 3723. https://doi.org/10.3390/cancers17233723
APA StyleRajput, S., LaComb, J. F., Gordon, C., Wang, H., Sarder, M., Kaczocha, M., Ojima, I., & Bialkowska, A. B. (2025). SBFI Inhibitors Reprogram Transcriptomic Landscape of Prostate Cancer Cells Leading to Cell Death. Cancers, 17(23), 3723. https://doi.org/10.3390/cancers17233723






