Serological Response Patterns to Assess Treatment Outcomes in Advanced Non-Small Cell Lung Cancer: A Real-World Exploratory Multi-Center Observational Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
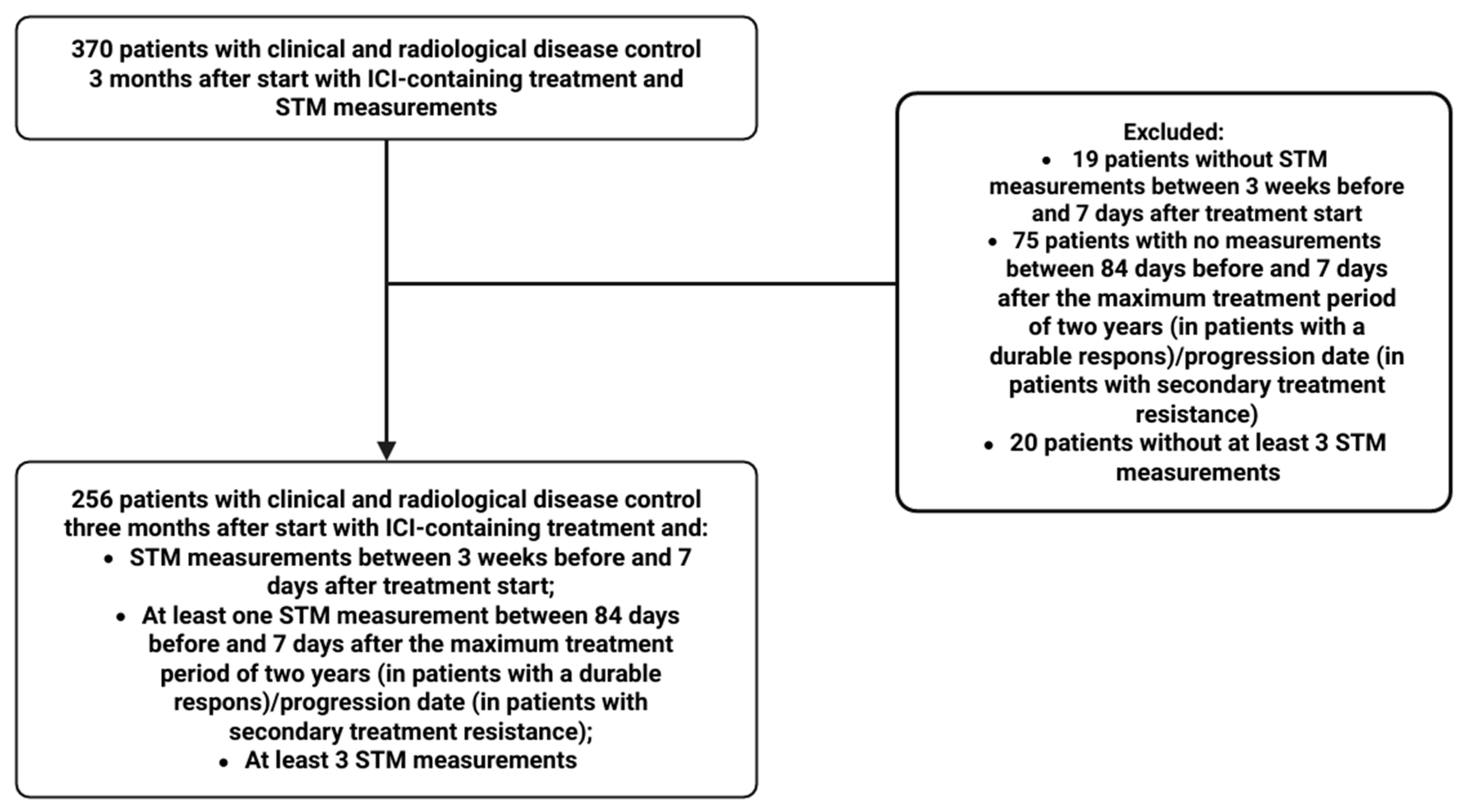
2.1. Study Design and Patient Population
2.2. Procedures
STM Analysis
2.3. Statistical Analysis
2.3.1. Patient Characteristics
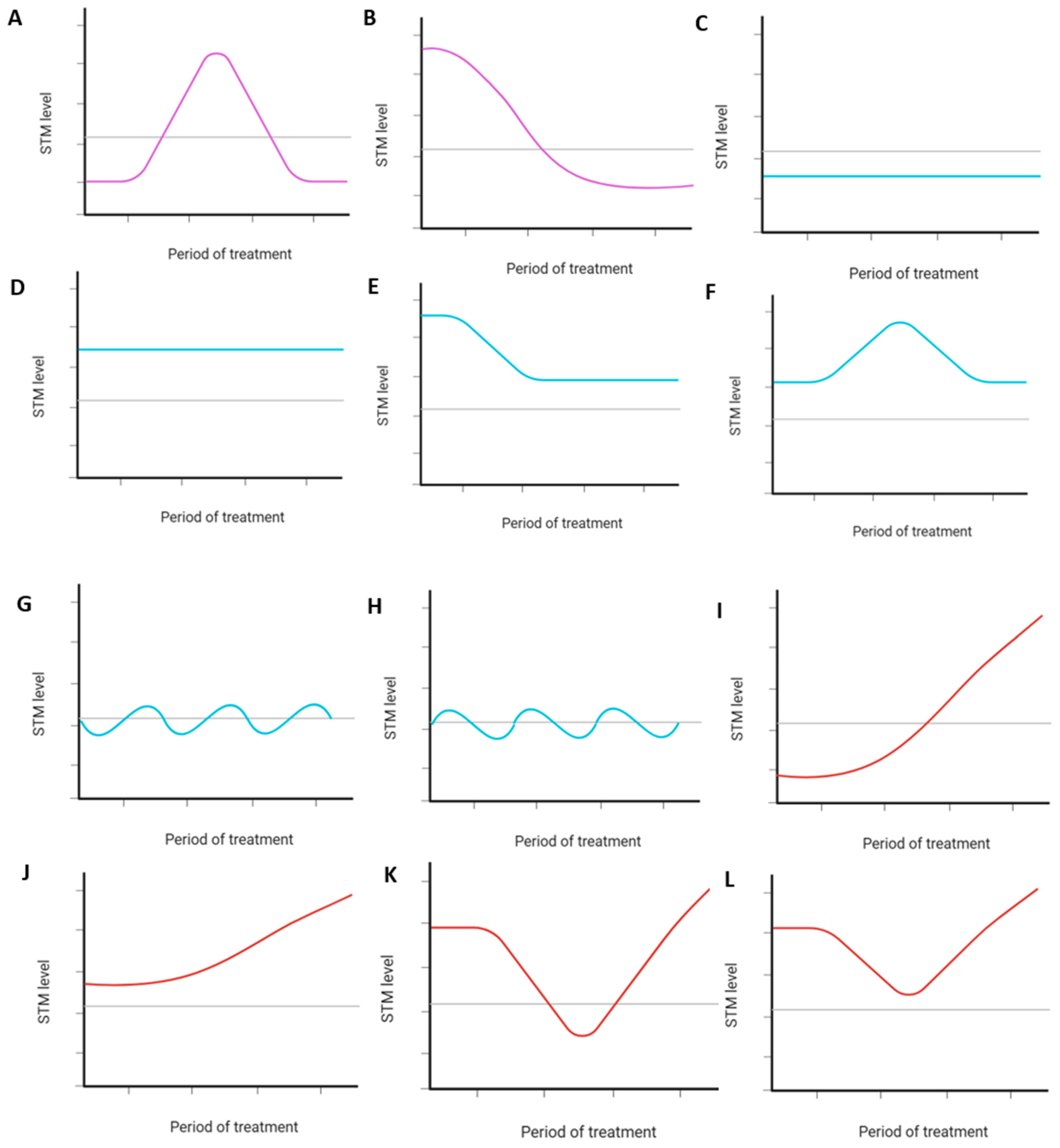
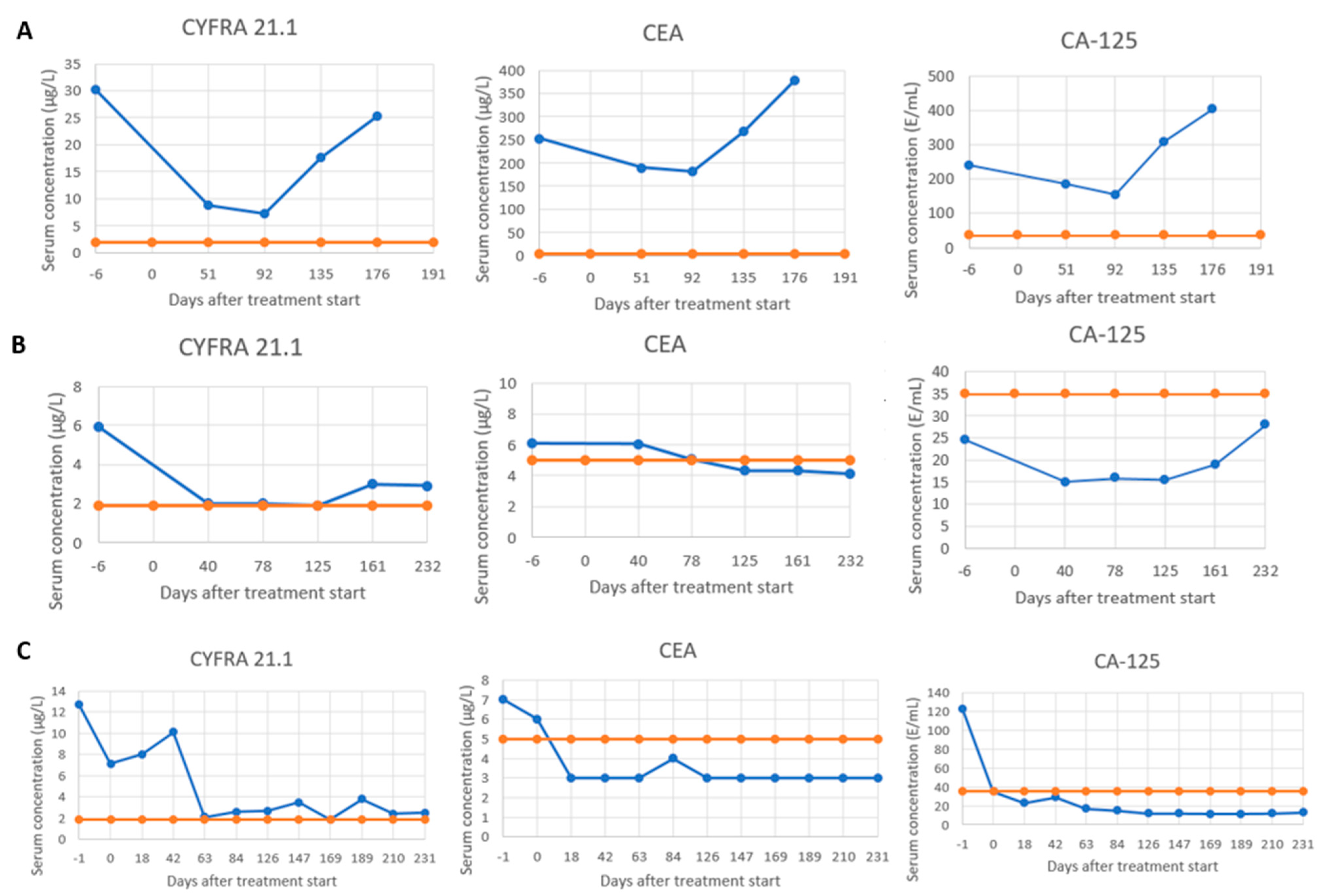
2.3.2. STM Dynamics During Treatment and Subclassification into Serological Response Patterns
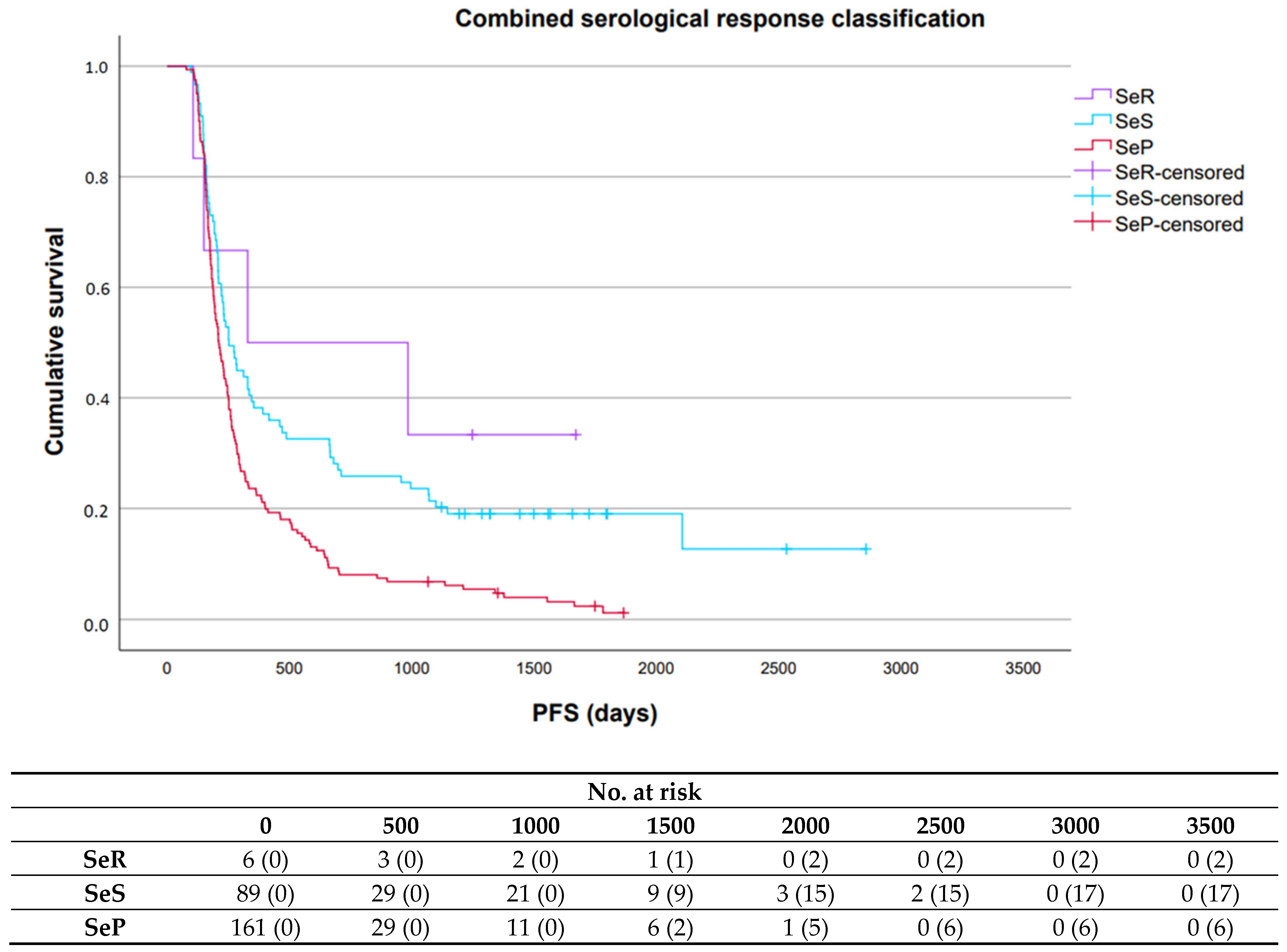
2.3.3. Prognostic Value Serological Response Patterns
3. Results
3.1. Patient Characteristics
3.2. STM Dynamics During Treatment and Subclassification into Serological Response Patterns
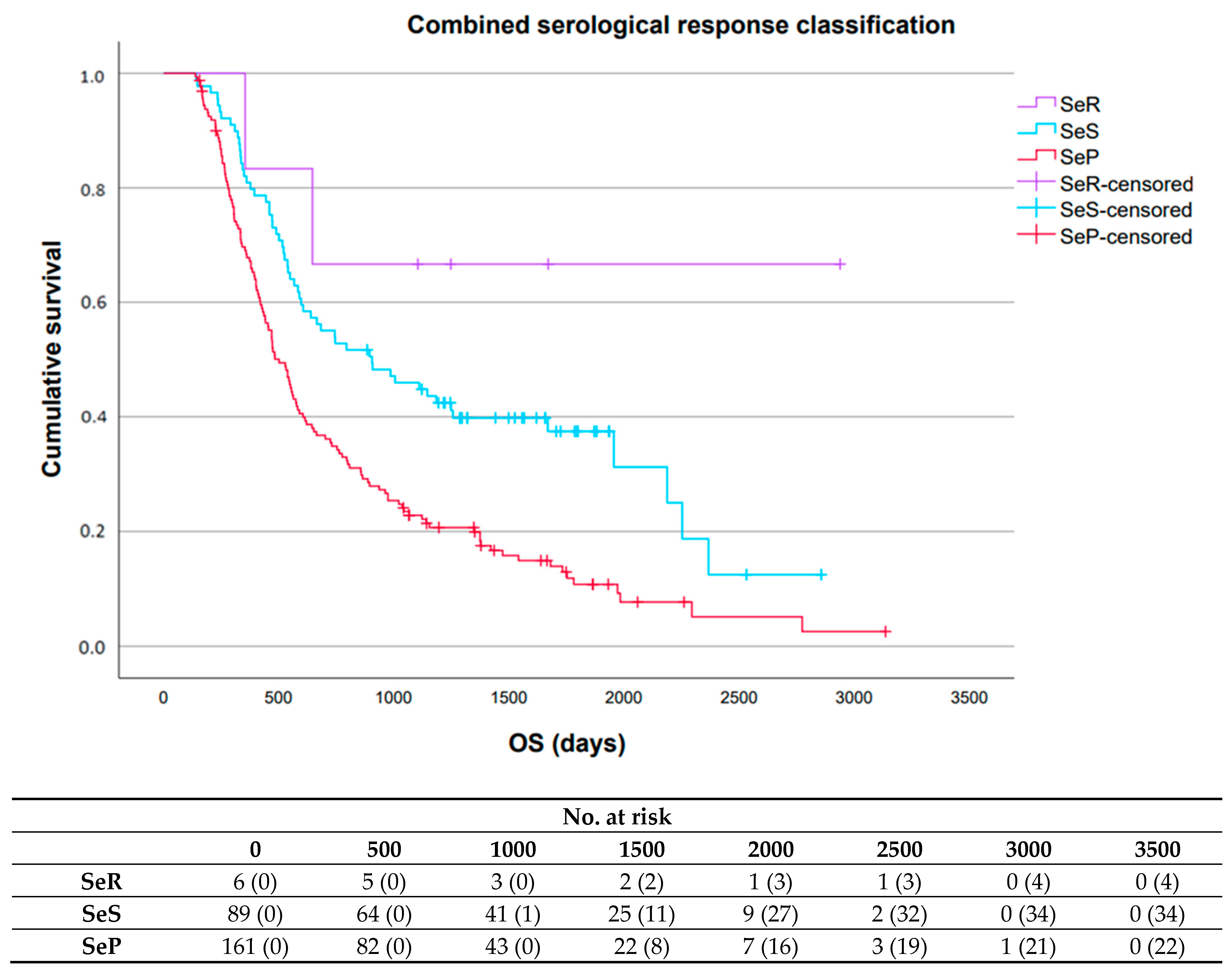
3.3. Prognostic Value Serological Response Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA-125 | Cancer Antigen-125 |
| CEA | CarcinoEmbryonic Antigen |
| CI | confidence interval |
| CR | complete remission |
| CT | Computed Tomography |
| ctDNA | circulating tumor DNA |
| Cyfra 21.1 | Cytokeratin 19 fragment antigen |
| ICI | immune checkpoint inhibitor |
| IQR | interquartile range |
| irAE | immune-related adverse event |
| NKI | Netherlands Cancer Institute |
| NR | not reached |
| NSCLC | non-small cell lung cancer |
| OS | overall survival |
| PFS | progression-free survival |
| PR | partial response |
| PD-L1 | programmed death-ligand 1 |
| Radboudumc | Radboud University Medical Centre |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | stable disease |
| StD | standard deviation |
| SeP | serological progression |
| SeR | serological remission |
| SeS | serological stable/unknown significance |
| STM | serum tumor marker |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Hernández, A.; del Portillo, E.G.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune checkpoint inhibitors in non-small cell lung cancer: From current perspectives to future treatments—A systematic review. Ann. Transl. Med. 2023, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Yoo, S.H.; Suh, K.J.; Kim, S.H.; Kim, Y.J.; Ock, C.-Y.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.-W.; et al. Clinical pattern of failure after a durable response to immune checkpoint inhibitors in non-small cell lung cancer patients. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Reinhorn, D.; Jacobi, O.; Icht, O.; Dudnik, E.; Rotem, O.; Zer, A.; A Goldstein, D. Treatment Beyond Progression with Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Immunotherapy 2020, 12, 235–243. [Google Scholar] [CrossRef]
- Vacher, L.; Bernadach, M.; Molnar, I.; Passildas-Jahanmohan, J.; Dubray-Longeras, P. The efficacy of immune checkpoint inhibitors following discontinuation for long-term response or toxicity in advanced or metastatic non-small-cell lung cancers: A retrospective study. Health Sci. Rep. 2024, 7, e1825. [Google Scholar] [CrossRef]
- Mahadevarao Premnath, S.; Zubair, M. Laboratory Evaluation of Tumor Biomarkers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK597378/ (accessed on 3 March 2025).
- Buma, A.I.; Schuurbiers, M.M.; van Rossum, H.H.; Heuvel, M.M.v.D. Clinical perspectives on serum tumor marker use in predicting prognosis and treatment response in advanced non-small cell lung cancer. Tumor. Biol. 2023, 46, S207–S217. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Yousef, M.; Zeineddine, M.A.; More, A.; Fanaeian, M.; Chowdhury, S.; Knafl, M.; Edelkamp, P.; Ito, I.; Gu, Y.; et al. Serum Tumor Markers and Outcomes in Patients with Appendiceal Adenocarcinoma. JAMA Netw. Open. 2024, 7, e240260. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, Y.; Li, L.-L.; Guan, Y.-P.; Feng, D.-F.; Yin, B.-B.; Liang, X.-F.; Yin, J.; Jiang, R.; Liang, J.; et al. Dynamics of Early Serum Tumour Markers and Neutrophil-to-Lymphocyte Ratio Predict Response to PD-1/PD-L1 Inhibitors in Advanced Non-Small-Cell Lung Cancer. Cancer Manag. Res. 2021, 13, 8241–8255. [Google Scholar] [CrossRef]
- Lang, D.; Haslinger, W.; Akbari, K.; Scala, M.; Hergan, B.; Asel, C.; Horner, A.; Wass, R.; Brehm, E.; Kaiser, B.; et al. Serum Tumor Marker Dynamics as Predictive Biomarkers in NSCLC Chemo-Immunotherapy and Mono-Immunotherapy Maintenance: A Registry-Based Descriptive Study. Lung Cancer 2020, 11, 113–121. [Google Scholar] [CrossRef]
- Muller, M.; Hoogendoorn, R.; Moritz, R.J.; van der Noort, V.; Lanfermeijer, M.; Korse, C.M.; Broek, D.v.D.; Hoeve, J.J.T.; Baas, P.; van Rossum, H.H.; et al. Validation of a clinical blood-based decision aid to guide immunotherapy treatment in patients with non-small cell lung cancer. Tumor. Biol. 2021, 43, 115–127. [Google Scholar] [CrossRef]
- Schuurbiers, M.M.F.; van Delft, F.A.; Koffijberg, H.; Ijzerman, M.J.; Monkhorst, K.; Ligtenberg, M.J.L.; Broek, D.v.D.; van Rossum, H.H.; Heuvel, M.M.v.D. External validation of a serum tumor marker algorithm for early prediction of no durable benefit to immunotherapy in metastastic non-small cell lung carcinoma. Tumor. Biol. 2025, 47. [Google Scholar] [CrossRef] [PubMed]
- van Delft, F.A.; Schuurbiers, M.; Muller, M.; Burgers, S.A.; van Rossum, H.H.; Ijzerman, M.J.; Koffijberg, H.; Heuvel, M.M.v.D. Modeling strategies to analyse longitudinal biomarker data: An illustration on predicting immunotherapy non-response in non-small cell lung cancer. Heliyon 2022, 8, e10932. [Google Scholar] [CrossRef] [PubMed]
- Dall’oLio, F.G.; Abbati, F.; Facchinetti, F.; Massucci, M.; Melotti, B.; Squadrilli, A.; Buti, S.; Formica, F.; Tiseo, M.; Ardizzoni, A. CEA and CYFRA 21-1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Ther. Adv. Med. Oncol. 2020, 12, 1758835920952994. [Google Scholar] [CrossRef]
- Noonan, S.A.; Patil, T.; Gao, D.; King, G.G.; Thibault, J.R.; Lu, X.; Bunn, P.A.; Doebele, R.C.; Purcell, W.T.; Barón, A.E.; et al. Baseline and On-Treatment Characteristics of Serum Tumor Markers in Stage IV Oncogene-Addicted Adenocarcinoma of the Lung. J. Thorac. Oncol. 2018, 13, 134–138. [Google Scholar] [CrossRef]
- Dal Bello, M.G.; Filiberti, R.A.; Alama, A.; Orengo, A.M.; Mussap, M.; Coco, S.; Vanni, I.; Boccardo, S.; Rijavec, E.; Genova, C.; et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J. Transl. Med. 2019, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiao, Y.; Zhou, Y.; Deng, H.; Yuan, Z.; Dong, L.; Lan, J.; Hu, H.; Huang, J.; Huang, S. Dynamic monitoring of serum tumor markers as prognostic factors in patients with advanced non-small-cell lung cancer treated with first-line immunotherapy: A multicenter retrospective study. Ther. Adv. Med. Oncol. 2023, 15, 17588359231206282. [Google Scholar] [CrossRef]
- Rosati, G.; Riccardi, F.; Tucci, A. Use of Tumor Markers in the Management of Head and Neck Cancer. Int. J. Biol. Markers 2000, 15, 179–183. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Cho, W.K.; Choi, D.H.; Park, H.C.; Park, W.; Yu, J.I.; Park, Y.S.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Kim, H.C.; et al. Elevated CEA is associated with worse survival in recurrent rectal cancer. Oncotarget 2017, 8, 105936–105941. [Google Scholar] [CrossRef]
- Desai, S.; Guddati, A.K. Carcinoembryonic Antigen, Carbohydrate Antigen 19-9, Cancer Antigen 125, Prostate-Specific Antigen and Other Cancer Markers: A Primer on Commonly Used Cancer Markers. World. J. Oncol. 2023, 14, 4–14. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Wang, H.; Zhao, W.-D.; Li, Y.-F.; Wang, J.-J.; Chen, X.-Y.; Huang, Y.-Q.; Wang, W.-J.; Wang, Y.; Sun, S.-C. Prognostic Value of Combined Lactate Dehydrogenase, C-Reactive Protein, Cancer Antigen 153 and Cancer Antigen 125 in Metastatic Breast Cancer. Cancer Control 2022, 29, 10732748211053150. [Google Scholar] [CrossRef]
- Oaknin, A.; Barretina, P.; Perez, X.; Jimenez, L.; Velasco, M.; Alsina, M.; Brunet, J.; Germa, J.R.; Beltran, M. CA-125 Response Patterns in Patients with Recurrent Ovarian Cancer Treated with Pegylated Liposomal Doxorubicin (PLD). Int. J. Gynecol. Cancer 2010, 20, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Ciccarese, C.; Caffo, O.; DE Giorgi, U.; Basso, U.; Tucci, M.; Mosillo, C.; Maruzzo, M.; Maines, F.; Casadei, C.; et al. The Role of Fast and Deep PSA Response in Castration-sensitive Prostate Cancer. Anticancer Res. 2021, 42, 165–172. [Google Scholar] [CrossRef]
- Reck, M.; Popat, S.; Reinmuth, N.; De Ruysscher, D.; Kerr, K.M.; Peters, S. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 25, iii27–iii39. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Song, Y.; Tang, J.; Zhang, B. What is the optimal duration of immune checkpoint inhibitors in malignant tumors? Front. Immunol. 2022, 13, 983581. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://cran.r-project.org/ (accessed on 19 May 2025).
- Nguyen, K.T.; Sakthivel, G.; Milano, M.T.; Qiu, H.; Singh, D.P. Oligoprogression in non-small cell lung cancer: A narrative review. J. Thorac. Dis. 2022, 14, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Trapé, J.; Fernández-Galán, E.; Auge, J.M.; Carbonell-Prat, M.; Filella, X.; Miró-Cañís, S.; González-Fernández, C. Factors influencing blood tumor marker concentrations in the absence of neo-plasia. Tumour. Biol. 2024, 46, S35–S63. [Google Scholar] [CrossRef]
- Petersen, P.H.; Sandberg, S.; Fraser, C.G.; Goldschmidt, H. Influence of index of individuality on false positives in repeated sam-pling from healthy individuals. Clin. Chem. Lab. Med. 2001, 39, 160–165. [Google Scholar] [CrossRef]
- Marques-Garcia, F.; Boned, B.; González-Lao, E.; Braga, F.; Carobene, A.; Coskun, A.; Díaz-Garzón, J.; Fernández-Calle, P.; Perich, M.C.; Simon, M.; et al. Critical review and meta-analysis of biological variation estimates for tumor markers. CCLM 2020, 60, 494–504. [Google Scholar] [CrossRef]
- Coşkun, A.; Aarsand, A.K.; Sandberg, S.; Guerra, E.; Locatelli, M.; Díaz-Garzón, J.; Fernandez-Calle, P.; Ceriotti, F.; Jonker, N.; Bartlett, W.A.; et al. Within- and between-subject biological variation data for tumor markers based on the European Biological Variation Study. CCLM 2020, 60, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Tumor Markers in Clinical Practice: A Review Focusing on Common Solid Cancers. Med. Princ. Pr. 2012, 22, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Xuzhang, W.; Huang, H.; Yu, Y.; Shen, L.; Li, Z.; Lu, S. Treatment strategies based on different oligoprogressive patterns after immunotherapy failure in metastatic NSCLC. Ther. Adv. Med. Oncol. 2023, 15, 17588359231156387. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recom-mendation. Lance. Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: Current status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Pando-Caciano, A.; Trivedi, R.; Pauwels, J.; Nowakowska, J.; Cavina, B.; Falkman, L.; Debattista, J.; Belényesi, S.-K.; Radhakrishnan, P.; Molina, M.A. Unlocking the promise of liquid biopsies in precision oncology. J. Liq. Biopsy 2024, 3, 100151. [Google Scholar] [CrossRef]
- Dixon, D.; Sattar, H.; Moros, N.; Kesireddy, S.R.; Ahsan, H.; Lakkimsetti, M.; Fatima, M.; Doshi, D.; Sadhu, K.; Hassan, M.J. Unveiling the Influence of AI Predictive Analytics on Patient Outcomes: A Comprehensive Narrative Review. Cureus 2024, 16, e59954. [Google Scholar] [CrossRef]
| Patients a, n = 256 (100%) | |
|---|---|
| General characteristics | |
| Age, median (IQR) | 64.0 (56.0–71.0) |
| Gender (male), No. (%) | 126 (49.2) |
| BMI, median (IQR) | 24.4 (22.1–27.5) |
| ECOG PS (≥2), No. (%) | 25 (9.8) |
| Ethnicity (Caucasian), No. (%) | 231 (93.1) |
| Albumin level < 35 g/L, No. (%) | 91 (37.0) |
| Kidney function ≤ 60 mL/min/1.73 m2, No. (%) | 40 (15.9) |
| Smoking | |
| Smoking status, No. (%) | |
| Never smoker | 24 (9.8) |
| Ex-smoker | 168 (68.9) |
| Current smoker | 52 (21.3) |
| Pack years, median (IQR) | 30.0 (16.0–40.0) |
| Tumor characteristics | |
| Histology, No. (%) | |
| Adenocarcinoma | 190 (75.4) |
| Squamous cell carcinoma | 40 (15.9) |
| Other | 22 (8.7) |
| PD-L1 expression, No. (%) | |
| Negative (<1%) | 91 (42.7) |
| Weak positive (1–49%) | 48 (22.5) |
| Strong positive (≥50%) | 74 (34.7) |
| Mutation status, No. (%) | |
| KRAS positive | 84 (36.4) |
| EGFR positive | 29 (12.4) |
| BRAF positive | 17 (7.0) |
| ALK positive | 2 (0.9) |
| Lung cancer stage, No. (%) | |
| Stage ≤ III | 18 (7.2) |
| Localization of distant metastases, No. (%) | |
| Brain | 48 (18.8) |
| Bone | 70 (27.3) |
| Liver | 24 (9.4) |
| Adrenal gland(s) | 50 (19.5) |
| Serum tumor markers | |
| Elevated baseline levels (yes), No. (%) | |
| Cyfra 21.1 | 167 (84.8%) |
| CEA | 119 (62.0%) |
| CA-125 | 103 (59.5%) |
| Treatment | |
| Current, No. (%) | |
| ICI monotherapy | 114 (44.5) |
| Nivolumab | 59 (51.8) |
| Pembrolizumab | 48 (42.1) |
| Atezolizumab | 5 (4.4) |
| Other | 2 (1.8) |
| Dual ICI therapy | 1 (0.0) |
| Nivolumab + ipilimumab | 1 (100.0) |
| ICI and chemotherapy | 141 (55.1) |
| Pembrolizumab + cis-/carboplatin + paclitaxel | 22 (15.6) |
| Pembrolizumab + cis-/carboplatin + pemetrexed | 88 (62.4) |
| Atezolizumab + bevacizumab + carboplatin + paclitaxel | 30 (21.3) |
| Other | 1 (0.0) |
| Line of treatment, No. (%) | |
| ≥Second-line | 120 (46.9) |
| Site of inclusion | |
| Radboudumc (yes), No. (%) | 151 (59.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buma, A.I.G.; Laarakker, F.; van Delft, F.A.; Schuurbiers, M.M.F.; Smit, J.; van Herwaarden, A.E.; van Rossum, H.H.; van den Heuvel, M.M. Serological Response Patterns to Assess Treatment Outcomes in Advanced Non-Small Cell Lung Cancer: A Real-World Exploratory Multi-Center Observational Cohort Study. Cancers 2025, 17, 3647. https://doi.org/10.3390/cancers17223647
Buma AIG, Laarakker F, van Delft FA, Schuurbiers MMF, Smit J, van Herwaarden AE, van Rossum HH, van den Heuvel MM. Serological Response Patterns to Assess Treatment Outcomes in Advanced Non-Small Cell Lung Cancer: A Real-World Exploratory Multi-Center Observational Cohort Study. Cancers. 2025; 17(22):3647. https://doi.org/10.3390/cancers17223647
Chicago/Turabian StyleBuma, Alessandra I. G., Femke Laarakker, Frederik A. van Delft, Milou M. F. Schuurbiers, Jasper Smit, Antonius E. van Herwaarden, Huub H. van Rossum, and Michel M. van den Heuvel. 2025. "Serological Response Patterns to Assess Treatment Outcomes in Advanced Non-Small Cell Lung Cancer: A Real-World Exploratory Multi-Center Observational Cohort Study" Cancers 17, no. 22: 3647. https://doi.org/10.3390/cancers17223647
APA StyleBuma, A. I. G., Laarakker, F., van Delft, F. A., Schuurbiers, M. M. F., Smit, J., van Herwaarden, A. E., van Rossum, H. H., & van den Heuvel, M. M. (2025). Serological Response Patterns to Assess Treatment Outcomes in Advanced Non-Small Cell Lung Cancer: A Real-World Exploratory Multi-Center Observational Cohort Study. Cancers, 17(22), 3647. https://doi.org/10.3390/cancers17223647







