Simple Summary
Primary central nervous system lymphoma is a form of aggressive brain tumor that requires rapid diagnosis to enable timely treatment. Although accurate, the results of conventional histopathology often take several days. In this study, we evaluated the usefulness of flow cytometry, which can deliver diagnostic information within hours. We retrospectively analyzed 67 patients with suspected primary central nervous system lymphoma and compared the flow cytometry findings with the final pathology; most cases confirmed by pathology were correctly identified by flow cytometry, which showed its high sensitivity and perfect specificity, although some cases were discordant due to reactive T-cell infiltration. Importantly, this is the first study to provide detailed subset analyses of lymphocytes in primary central nervous system lymphoma using flow cytometry. These results demonstrate that flow cytometry is a valuable complement to standard pathology, offering rapid and reliable diagnostic guidance that can accelerate treatment decisions and improve patient care.
Abstract
Background/Objectives: Primary central nervous system lymphoma (PCNSL) has a markedly high proliferation rate, making early diagnosis and prompt therapeutic intervention essential. To accelerate diagnosis, our institution adopted flow cytometry (FCM) in conjunction with conventional histopathology, and this study therefore evaluated the diagnostic performance of FCM for PCNSL. Methods: We retrospectively analyzed 67 consecutive patients with suspected PCNSL who underwent intraoperative FCM between 2010 and 2023 based on preoperative imaging. B-cell clonality was defined as ≥20% CD19/CD20-positive cells with a κ/λ ratio of >3.0 or <0.5. Results: Using histopathology, we confirmed the presence of PCNSL in 42 patients, all diagnosed as having diffuse large B-cell lymphoma. Six cases (14.3%) were discordant (FCM-D). The sensitivity, specificity, and positive predictive value of FCM were 85.7%, 100%, and 100%, respectively. T-cell markers were significantly elevated in FCM-D cases (p < 0.01), although these were pathologically diagnosed as diffuse large B-cell lymphoma based on histology and immunohistochemistry. Conclusions: FCM yields reliable diagnostic information within hours of tissue collection and supports early therapeutic decisions in PCNSL. Discordant results may reflect reactive T-cell infiltration. This is the first study to present detailed subset analyses in PCNSL using FCM in correlation with pathology, underscoring its utility as a rapid diagnostic tool.
1. Introduction
Primary central nervous system lymphoma (PCNSL) is a rare extranodal non-Hodgkin lymphoma, accounting for approximately 4% of primary brain tumors and 1% of all non-Hodgkin lymphomas. Most PCNSL cases are diffuse large B-cell lymphomas (DLBCLs), characterized by rapid proliferation and an aggressive clinical course [1,2,3,4,5]. PCNSL incidence has risen, particularly among immunocompetent older adults, reflecting demographic shifts and advances in diagnostic imaging [1,6].
Clinically, PCNSL manifests with diverse, nonspecific neurological symptoms—such as cognitive decline, hemiparesis, seizures, and visual disturbances—that may resemble glioblastoma, demyelinating disease, or metastases. Early and accurate diagnosis is crucial as PCNSL responds to chemotherapy and radiotherapy, especially high-dose methotrexate (HD-MTX)-based regimens [1,2,6].
Patients who receive prompt therapy demonstrate improved progression-free and overall survival, whereas diagnostic delays correlate with poorer outcomes [1].
There are various reports addressing state-of-the-art imaging-based approaches; however, diagnosis remains challenging [7,8,9,10], as there are no disease-specific signs. MRI typically reveals homogeneously enhanced deep-seated lesions, but lacks specificity [1,6,11]. PET/CT can exclude systemic lymphoma but is insufficient for intracranial diagnosis [12]. CSF cytology offers limited sensitivity and molecular approaches such as MYD88 mutation detection or interleukin-10 and C-X-C motif chemokine ligand 13 (CXCL13) quantification are promising but not widely adopted in clinical practice [13,14,15,16]. Consequently, stereotactic biopsy remains the diagnostic gold standard. However, histopathology requires fixation, embedding, staining, and immunohistochemistry, delaying treatment initiation by several days [17].
Established in hematology for diagnosing systemic lymphomas, flow cytometry (FCM) provides rapid immunophenotyping. By assessing surface markers and κ/λ ratios, it can determine B-cell clonality within hours of tissue collection [18]. Several reports have explored intraoperative FCM for CNS lymphomas, indicating that it can differentiate PCNSLs from other tumors and reduce diagnostic delays [19,20,21,22].
Nonetheless, discordant cases occur, often due to reactive T-cell infiltration, including reactive perivascular T-cell infiltration (RPVI)—a histological hallmark of PCNSLs—that may obscure B-cell clonality and yield false negatives [19,23,24].
To address these limitations, we retrospectively analyzed patients who underwent intraoperative FCM for suspected PCNSL at our institution. We aimed to evaluate diagnostic accuracy, characterize discordant cases, and integrate immunophenotypic subset analyses with histopathology. To our knowledge, this is the first study to correlate FCM with pathology in PCNSL at the subset level, thereby clarifying the immunological basis of diagnostic discordance.
2. Materials and Methods
2.1. Study Design and Patients
For this retrospective cohort study, we included consecutive patients who underwent surgical biopsy or resection for suspected PCNSL at Nagasaki University Hospital between January 2010 and December 2023. Inclusion criteria were (1) preoperative neuroimaging (MRI or CT) suggestive of PCNSL, characterized by homogeneous gadolinium enhancement, deep-seated lesion distribution, or other typical radiological features, and (2) the availability of intraoperative specimens to process for FCM in addition to routine histopathology. Patients with systemic lymphoma, secondary CNS involvement, or insufficient tumor tissue were excluded.
2.2. Surgical Procedures and Tissue Handling
All patients underwent either stereotactic biopsy or open resection, depending on lesion characteristics and clinical condition. Tumor specimens were divided: one portion was processed for histopathology and the other was immediately prepared for FCM. For the latter, tissue was mechanically dissociated in RPMI-1640 medium supplemented with 10% fetal bovine serum, filtered through a 40 μm mesh, and processed within one hour after collection to preserve antigenicity.
2.3. Flow Cytometry Protocol
Cell suspensions were stained with a panel of monoclonal antibodies targeting B- and T-cell surface markers, including CD3 (clone SK7, number 347347), CD4 (RPA-T4, 3338054), CD5 (UCHT2, 340696), CD8 (Leu-2a, 340046), CD10 (HI10a, 340921), CD19 (4G7, 347543), and CD20 (L27, 346595) (BD Biosciences, San Jose, CA, USA). Light chain restriction was assessed using antibodies against κ and λ immunoglobulin light chains (F0434 and F0435) (Agilent Technologies, Inc., Santa Clara, CA, USA). Data acquisition was performed using an FACSLyric Flow Cytometer (BD Biosciences), and analysis was carried out with FACSuit software (Version 1.2.1) (BD Biosciences).
Consistent with established criteria, B-cell clonality was defined as ≥20% of lymphocytes expressing CD19 or CD20, combined with an abnormal κ/λ ratio (>3.0 or <0.5) [19,21]. T-cell markers were analyzed to evaluate reactive perivascular infiltration. Discordance (FCM-D) was defined as when FCM failed to demonstrate B-cell clonality despite histopathological confirmation of PCNSL.
2.4. Histopathological Examination
Specimens were fixed in 10% neutral buffered formalin, paraffin-embedded, and stained with hematoxylin and eosin (HE). For immunohistochemistry (IHC), we employed antibodies against CD3, CD10, CD20, BCL-6, MUM1, and Ki-67, following standard protocols. Diagnoses were established by board-certified neuropathologists in accordance with the World Health Organization (WHO) classification of hematopoietic and lymphoid tumors [25]. Cases showing reactive perivascular T-cell infiltration (RPVI) were specifically recorded.
2.5. Clinical and Radiological Data
Baseline demographic and clinical data included age, sex, Karnofsky Performance Status (KPS), biopsy type, and tumor number (single vs. multiple). MRI findings, including contrast enhancement patterns, hemorrhage, and calcification, were independently reviewed by two neuroradiologists.
2.6. Statistical Analysis
Continuous variables were tested for normality using the Shapiro–Wilk test. As most variables were not normally distributed, they were expressed as the median and compared using the Mann–Whitney U test. Categorical variables were evaluated using Fisher’s exact or Chi-square tests, as appropriate.
The diagnostic accuracy of FCM was calculated using histopathology as the reference standard, with sensitivity, specificity, positive predictive value, and negative predictive value reported. Subgroup analyses compared FCM-concordant (FCM-C) and FCM-discordant (FCM-D) patients regarding their immunophenotypic and clinical features. Statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a modified version of R Commander for biostatistics [26], with statistical significance set at p < 0.05.
2.7. Ethical Considerations
This study was approved by the Institutional Review Board of Nagasaki University Hospital (Approval No. 2106117). Owing to its retrospective design and use of anonymized data, informed consent was waived. All procedures adhered to the principles of the Declaration of Helsinki.
3. Results
3.1. Patient Characteristics
Between January 2010 and December 2023, 67 patients with suspected PCNSL underwent biopsy or tumor removal with intraoperative FCM. Histopathological evaluation confirmed PCNSL in 42 patients, while 25 were diagnosed with other conditions: glioblastoma (n = 12), other gliomas (n = 5), metastases (n = 2), and miscellaneous tumors, including meningioma or inflammatory lesions (n = 6) (Figure 1). All confirmed PCNSL cases were classified as DLBCL.
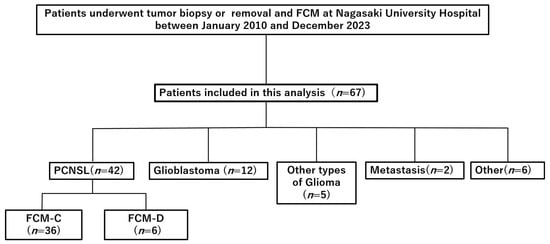
Figure 1.
Flow diagram of patient selection. Retrospective cohort of patients who underwent tumor biopsy or removal with intraoperative flow cytometry (FCM) at Nagasaki University Hospital between January 2010 and December 2023. Abbreviations: FCM, flow cytometry; PCNSL, primary central nervous system lymphoma; FCM-C, FCM concordant; FCM-D, FCM discordant.
Among the 42 patients with PCNSL, FCM and histopathology were concordant in 36 (85.7%, FCM-C) and discordant in 6 (14.3%, FCM-D). Baseline demographic and clinical data are presented in Table 1. The median age of the PCNSL cohort was 71.5 years, with no significant difference between FCM-C (72.0 years) and FCM-D (66.5 years) groups (p = 0.35). Male patients accounted for 58.3% of FCM-C and 50.0% of FCM-D cases (p = 1.0). Most diagnoses were obtained via stereotactic needle biopsy (92.9%), and the median of preoperative Karnofsky Performance Status (KPS) was 50 overall. Radiological findings included homogeneous gadolinium enhancement in 69.0% of PCNSL cases, hemorrhage in 40.5%, and no calcifications. None of these features differed significantly between FCM-C and FCM-D groups.

Table 1.
Baseline patient characteristics.
3.2. Diagnostic Accuracy of Flow Cytometry
We evaluated the diagnostic performance of FCM using histopathology as the reference standard. Of the 42 PCNSL cases, 36 were FCM-C, while all 25 non-PCNSL tumors were FCM-D. Sensitivity was 85.7% (36/42), specificity 100% (25/25), positive predictive value 100% (36/36), and negative predictive value 80.6% (25/31). These results underscore the high specificity of FCM, as no false positives occurred; however, false negatives were observed in 14.3% of patients with PCNSL, highlighting the need to interpret discordant results cautiously.
3.3. Lymphocyte Subset Analysis
Flow cytometric analysis revealed clear differences between FCM-C and FCM-D (Figure 2). In FCM-C, B-cell markers were consistently elevated: CD10 positivity averaged 23.8 ± 35.3% versus 4.8 ± 7.7% in FCM-D (p < 0.01); CD19 was 81.1 ± 19.6% in FCM-C compared with 19.4 ± 36.1% in FCM-D (p < 0.01); and CD20 was 76.8 ± 24.2% in FCM-C versus 32.0 ± 43.8% in FCM-D (p < 0.01). These findings demonstrate strong B-cell clonality, combined with abnormalities in the k/λ ratio in FCM-C.
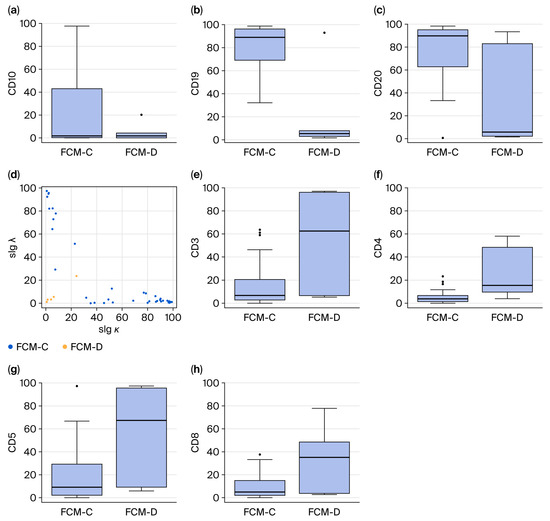
Figure 2.
Lymphocyte subset analysis (a–c,e–h) and scatter plots (d) determined by FCM. Percentages of B-cell surface markers (a–c). Scatter plot of slgκ and slgλ (d). Percentages of T-cell surface markers (e–h). Abbreviations: FCM-C, flow cytometry concordant; FCM-D, flow cytometry discordant; slgκ, surface immunoglobulin κ; slgλ, surface immunoglobulin λ.
Conversely, T-cell markers predominated in FCM-D: CD3 expression averaged 55.1 ± 41.0% in FCM-D versus 15.4 ± 18.2% in FCM-C (p < 0.01). CD4 also increased (25.3 ± 22.5% vs. 5.3 ± 5.4%, p < 0.01), as did CD5 (57.1 ± 40.6% vs. 17.3 ± 22.4%, p < 0.01) and CD8 (33.9 ± 28.6% vs. 8.7 ± 9.7%, p < 0.01). These results suggest that T-cell predominance in discordant cases is obscured B-cell clonality in FCM.
3.4. Histopathological Correlates of Discordant Cases
Histopathology provided additional insights into the six FCM-D cases. HE staining demonstrated RPVI in four patients (66.7%) (Figure 3), while in the other two cases, tumor tissue showed diffuse lymphomatous infiltration without marked RPVI, indicating that sampling error or low tumor cell content may also explain discordance.
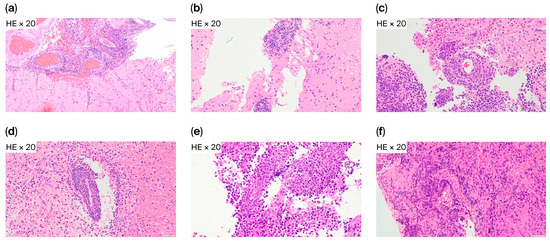
Figure 3.
Reactive perivascular T-cell infiltration in FCM-D cases. Representative HE staining from six FCM-D cases. Reactive perivascular T-cell infiltration (RPVI) was present in four cases (a–d) and absent in two (e,f). Abbreviations: FCM-D, FCM discordant; HE, hematoxylin and eosin; RPVI, reactive perivascular T-cell infiltration.
Despite the lack of FCM-detected clonality, all six FCM-D cases were confirmed as B-cell lymphoma by IHC. CD20 immunostaining revealed high intratumoral B-cell proportions, with positivity ranging from 72% to 98% (median 85%) (Figure 4). CD3 immunostaining revealed low T-cell proportions, with positivity ranging from 2.6% to 53.4% (median 6.4%), although one case could not be evaluated. These findings confirm that FCM-D cases represented DLBCL.
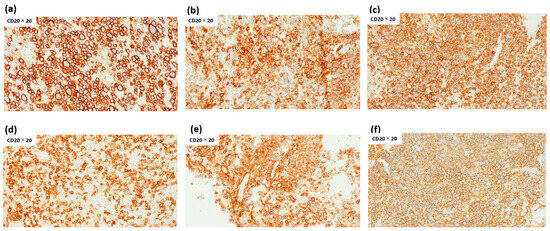
Figure 4.
Percentage of intratumoral CD20-positive lymphocytes in FCM-D cases. Percentages of intratumoral CD20-positive lymphocytes were 79% (a), 83% (b), 93% (c), 72% in (d), 87% (e), and 98% (f), with a median of 85% (range 72–98%). Abbreviations: FCM-D, FCM discordant.
4. Discussion
The results of our study demonstrate that intraoperative FCM is a rapid and highly specific diagnostic tool for PCNSL, with sensitivity of 85.7% and specificity of 100%. These results are consistent with previous investigations of FCM in stereotactic brain biopsies [19,20,21,22,27] but extend prior work by incorporating detailed subset analyses and correlating discordant findings with histopathological features. We identify an important biological factor—immune microenvironmental influence—that may confound cytometric evaluation in PCNSL.
4.1. Diagnostic Value of FCM
FCM’s high specificity and positive predictive value underscore it as a strong diagnostic adjunct. The absence of false positives indicates that once B-cell clonality is detected, clinicians can confidently initiate lymphoma-directed therapy. This is particularly valuable in neurosurgical practice, where delays in chemotherapy can worsen outcomes [1]. Although histopathology remains definitive, it requires several days for tissue processing and immunohistochemistry to be conducted. By contrast, FCM yields results within hours, enabling earlier treatment planning [17,19,20,21].
4.2. Discordant Cases and the Role of the Immune Microenvironment
A unique contribution of this study is the characterization of discordant cases. Although histopathology confirmed B-cell lymphomas, FCM-D cases demonstrated the predominance of T-cell markers. Histological review revealed RPVI in most discordant cases, supporting the interpretation that immune microenvironmental factors can obscure B-cell clonality. RPVI has been described as characteristic of PCNSL and may carry prognostic relevance [2,23,24,28,29]. Our findings emphasize that reactive T-cell infiltrates can alter cytometric profiles, masking underlying lymphomas.
The immunological implications of RPVI warrant attention. Several reports suggest that perivascular T-cell infiltrates not only confound diagnosis but may also represent an active host immune response with potential survival benefits [2,28,29].
This dual role illustrates the complexity of the PCNSL microenvironment. Clinically, a T-cell-dominant FCM profile should not exclude PCNSL, particularly when radiological and clinical evidence is compelling. Biologically, RPVI may provide insight into immune surveillance mechanisms that could be harnessed therapeutically [2,23,24,28,29].
4.3. Comparison with the Previous Literature
The sensitivity and specificity observed in our study are comparable to those reported by Takeuchi et al. [19] and Inoue et al. [21]. Our results align with those of Cordone et al., who demonstrated the feasibility of stereotactic biopsy FCM and showed that it has a high degree of agreement (89%) with immunohistochemistry in brain lymphoma identification [22]. For CNS lymphomas, Koriyama et al. further showed that intraoperative FCM differentiates PCNSL from glioblastoma—a distinction often difficult on imaging [30]. However, these studies did not explore discordance in detail. By integrating subset analysis with histopathological correlation, our study identifies reactive T-cell infiltration as a major contributor to discordance and emphasizes cautious interpretation of FCM-negative results.
4.4. Clinical Implications
Incorporating FCM into intraoperative workflows offers several advantages. First, it enables rapid confirmation of B-cell clonality, facilitating early chemotherapy initiation. Second, combining FCM with intraoperative IHC and cytology may enhance accuracy in cases with marked T-cell infiltration [21,22].
Recognizing that discordant FCM results may reflect an immune-rich tumor microenvironment also carries prognostic and counseling-related implications. As the field of oncology moves toward precision strategies, incorporating immunological features such as RPVI into diagnostic and prognostic models may become increasingly relevant [2,23,24,28,29].
The authors of future studies should validate FCM protocols in multicenter prospective cohorts, standardize gating strategies, and establish universally accepted cut-off values. Ultimately, integrating FCM with histopathology, molecular diagnostics, and advanced imaging may provide a multimodal framework for the rapid, reliable diagnosis of PCNSL.
4.5. Limitations
This study has limitations inherent to its retrospective, single-center design. The small number of discordant cases (n = 6) restricted statistical power for subgroup analyses. Technical variability, including tissue handling and sample size, may also have affected our results. Moreover, our clonality thresholds (≥20% CD19/CD20 with κ/λ > 3.0 or <0.5) were adapted from prior reports and require multicenter validation.
5. Conclusions
Flow cytometry is a rapid, accurate, and highly specific adjunct to histopathology for diagnosing PCNSL. Although discordance may arise from reactive T-cell infiltration and there remains room for improvement in this area, incorporating FCM into intraoperative workflows can expedite therapy, improve outcomes, and support multimodal diagnostic strategies.
Author Contributions
Conceptualization, H.N., T.H. and T.K.; methodology, H.N.; software, H.N.; validation, H.N. and T.H.; formal analysis, H.N.; investigation, H.N.; resources, H.N.; data curation, H.N. and T.H.; writing—original draft preparation, H.N. and T.H.; writing—review and editing, H.N., T.H., T.K., N.U., A.M., M.Y., S.B., K.U., K.Y. (Koichi Yoshida), H.K., Y.H., H.H., K.A., K.Y. (Katsunori Yanagihara), M.N., Y.M. and T.M.; visualization, H.N.; supervision, K.Y. (Katsunori Yanagihara), M.N., Y.M. and T.M.; project administration, H.N. and T.H.; funding acquisition, H.N. and T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Nagasaki University Hospital (Approval No. 2106117, approved on 20 February 2025). All procedures adhered to the principles of the Declaration of Helsinki.
Informed Consent Statement
Owing to this study’s retrospective design and use of anonymized data, the need for informed consent was waived.
Data Availability Statement
All relevant data are included within the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PCNSL | Primary Central Nervous System Lymphoma |
| DLBCL | Diffuse Large B-Cell Lymphoma |
| HD-MTX | High-Dose Methotrexate |
| MRI | Magnetic Resonance Imaging |
| PET/CT | Positron Emission Tomograph/Computed Tomography |
| CSF | Cerebrospinal Fluid |
| CXCL13 | C-X-C Motif Chemokine Ligand 13 |
| FCM | Flow Cytometry |
| RPVI | Reactive Perivascular T-Cell Infiltration |
| FCM-D | Flow Cytometry Discordant |
| HE | Hematoxylin and Eosin |
| IHC | Immunohistochemistry |
| WHO | World Health Organization |
| KPS | Karnofsky Performance Status |
| SD | Standard Deviation |
References
- Ferreri, A.J.M.; Calimeri, T.; Cwynarski, K.; Dietrich, J.; Grommes, C.; Hoang-Xuan, K.; Illerhaus, G.; Makino, K.; Marturano, E.; Rubenstein, J.L.; et al. Primary central nervous system lymphoma. Nat. Rev. Dis. Primers 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Lauw, M.I.S.; Lucas, C.-H.G.; Ohgami, R.S.; Wen, K.W. Primary central nervous system lymphomas: A diagnostic overview of key histomorphologic, immunophenotypic, and genetic features. Diagnostics 2020, 10, 1076. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, T.; Hiu, T.; Imaizumi, Y.; Morimoto, S.; Niino, D.; Yamaguchi, S.; Baba, S.; Ujifuku, K.; Yoshida, K.; et al. Treatment of new-onset primary central nervous system lymphoma in elderly patients using RMPV chemotherapy: A single-institution experience. Int. J. Hematol. 2023, 118, 333–339. [Google Scholar] [CrossRef]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef]
- Deckert, M.; Brunn, A.; Montesinos-Rongen, M.; Terreni, M.R.; Ponzoni, M. Primary lymphoma of the central nervous system—A diagnostic challenge. Hematol. Oncol. 2014, 32, 57–67. [Google Scholar] [CrossRef]
- Grommes, C.; DeAngelis, L.M. Primary CNS lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Z.J.; Li, W.H.; Yang, Y.; Zhang, J.; Yang, X.B.; Zuo, L.; Xiao, G.; Wang, S.Z.; Yan, L.F.; et al. Differentiation between primary central nervous system lymphoma and atypical glioblastoma based on MRI morphological feature and signal intensity ratio: A retrospective multicenter study. Front. Oncol. 2022, 12, 811197. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.D.; Anh, N.N.; Minh, N.D.; Huyen, D.K.; Duc, N.M. Differentiation of glioblastoma and primary central nervous system lymphomas using multiparametric diffusion and perfusion magnetic resonance imaging. Biomed. Rep. 2023, 19, 82. [Google Scholar] [CrossRef]
- Neska-Matuszewska, M.; Bladowska, J.; Sąsiadek, M.; Zimny, A. Differentiation of glioblastoma multiforme, metastases and primary central nervous system lymphomas using multiparametric perfusion and diffusion MR imaging of a tumor core and a peritumoral zone—Searching for a practical approach. PLoS ONE 2018, 13, e0191341. [Google Scholar] [CrossRef]
- Malikova, H.; Koubska, E.; Weichet, J.; Klener, J.; Rulseh, A.; Liscak, R.; Vojtech, Z. Can morphological MRI differentiate between primary central nervous system lymphoma and glioblastoma? Cancer Imaging 2016, 16, 40. [Google Scholar] [CrossRef]
- Pons-Escoda, A.; Souza, L.; Vargas, M.I.; Thurnher, M.M.; Smirniotopoulos, J.G.; Castillo, M. Imaging of lymphomas involving the CNS: An update. AJNR Am. J. Neuroradiol. 2023, 44, 421–431. [Google Scholar] [CrossRef]
- Chiavazza, C.; Pellerino, A.; Ferrio, F.; Cistaro, A.; Soffietti, R.; Rudà, R. Primary CNS lymphomas: Challenges in diagnosis and treatment. Biomed. Res. Int. 2018, 2018, 3606970. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Sasaki, N.; Nakano, Y.; Matsushita, Y.; Omura, T.; Shimizu, S.; Saito, K.; Kobayashi, K.; Narita, Y.; Kondo, A.; et al. Liquid biopsy of cerebrospinal fluid for MYD88 L265P mutation is useful for diagnosis of central nervous system lymphoma. Cancer Sci. 2021, 112, 4702–4710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, D.; Yin, J.; Zhang, L.; Zhang, X.; Wang, W.; Zhang, M.; Zhou, D.; Zhang, W. Changes in cerebrospinal fluid interleukin-10 levels display better performance in predicting disease relapse than conventional magnetic resonance imaging in primary central nervous system lymphoma. BMC Cancer 2021, 21, 183. [Google Scholar] [CrossRef]
- Zorofchian, S.; Lu, G.; Zhu, J.J.; Duose, D.Y.; Windham, J.; Esquenazi, Y.; Ballester, L.Y. Detection of the MYD88 p.L265P mutation in the CSF of a patient with CNS lymphoma. Front. Oncol. 2018, 8, 382. [Google Scholar] [CrossRef]
- Fischer, L.; Korfel, A.; Pfeiffer, S.; Kiewe, P.; Volk, H.-D.; Cakiroglu, H.; Widmann, T.; Thiel, E. CXCL13 and CXCL12 in Central Nervous System Lymphoma Patients. Clin. Cancer Res. 2009, 15, 5968–5973. [Google Scholar] [CrossRef]
- Debliquis, A.; Voirin, J.; Harzallah, I.; Maurer, M.; Lerintiu, F.; Drénou, B.; Ahle, G. Cytomorphology and flow cytometry of brain biopsy rinse fluid enables faster and multidisciplinary diagnosis of large B-cell lymphoma of the central nervous system. Cytom. B Clin. Cytom. 2018, 94, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Craig, F.E.; Foon, K.A. Flow cytometric immunophenotyping for hematologic neoplasms. Blood 2008, 111, 3941–3967. [Google Scholar] [CrossRef]
- Takeuchi, H.; Inaba, T.; Shishido-Hara, Y.; Tsukamoto, T.; Mizutani, S.; Okamoto, T.; Tanigawa, S.; Yamanaka, T.; Takahashi, Y.; Konishi, E.; et al. Analysis of false-negative findings of the incomparable accuracy and swiftness of flow cytometric diagnosis of primary central nervous system lymphoma. Neurol. Med. Chir. 2023, 63, 495–502. [Google Scholar] [CrossRef]
- Romeo, E.; Markopoulos, G.; Voulgaris, S.; Vartholomatos, G.; Alexiou, G.A. The role of intraoperative flow cytometry on intracranial tumor surgery: A scoping review. Neurosurg. Rev. 2025, 48, 631. [Google Scholar] [CrossRef]
- Inoue, A.; Miyazaki, Y.; Watanabe, H.; Nishikawa, M.; Kusakabe, K.; Ohnishi, T.; Taniwaki, M.; Honda, T.; Kondo, T.; Kinnami, S.; et al. Reliable intraoperative diagnostic methods for PCNSL: Utility of combining intraoperative immunohistochemistry, cytology, and flow cytometry in achieving optimal treatment. Acta Neurol. Belg. 2025, 125, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Cordone, I.; Masai, S.; Carosi, M.; Vidiei, A.; Marchwsi, M.; Marino, M.; Tekera, S.; Pasquale, A.; Mengarelli, A.; Conti, L.; et al. Brain stereotactic biopsy flow cytometry for central nervous system lymphoma characterization: Advantages and pitfalls. J. Exp. Clin. Cancer Res. 2016, 35, 128. [Google Scholar] [CrossRef]
- Bashir, R.; Chamberlain, M.; Ruby, E.; Hochberg, F.H. T-cell infiltration of primary CNS lymphoma. Neurology 1996, 46, 440–444. [Google Scholar] [CrossRef]
- Riemersma, S.A.; Oudejans, J.J.; Vonk, M.J.; Dreef, E.J.; Prins, F.A.; Jansen, P.M.; Vermeer, M.H.; Blok, P.; Kibbelaar, R.E.; Muris, J.J.F.; et al. High numbers of tumour-infiltrating activated cytotoxic T lymphocytes, and frequent loss of HLA class I and II expression, are features of aggressive B-cell lymphomas of the brain and testis. J. Pathol. 2005, 206, 328–336. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; ISBN 978-92-832-4506-3. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- van der Meulen, M.; Bromberg, J.E.C.; Lam, K.H.; Dammers, R.; Langerak, A.W.; Doorduijn, J.K.; Kros, J.M.; van den Bent, M.J.; van der Velden, V.H.J. Flow cytometry shows added value in diagnosing lymphoma in brain biopsies. Cytom. Part B Clin. Cytom. 2018, 94, 928–934. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zuo, C.; Wang, J.; Liu, J.; Jiao, B.; Zheng, J.; Cai, Z. Prognostic significance of the aggregative perivascular growth pattern of tumor cells in primary central nervous system diffuse large B-cell lymphoma. Neuro Oncol. 2013, 15, 727–734. [Google Scholar] [CrossRef]
- Ponzoni, M.; Berger, F.; Chassagne-Clément, C.; Tinguely, M.; Jouvet, A.; Ferreri, A.J.M.; Dell’Oro, S.; Terreni, M.R.; Doglioni, C.; Weis, J.; et al. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br. J. Haematol. 2007, 138, 316–323. [Google Scholar] [CrossRef]
- Koriyama, S.; Nitta, M.; Saito, N.; Hirano, M.; Tominaga, T. Intraoperative flow cytometry enables differentiation between PCNSL and glioblastoma. World Neurosurg. 2018, 112, e261–e268. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).