Impact of Quitting Smoking at Diagnosis on Overall Survival in Lung Cancer Patients: A Comprehensive Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Selection Process
2.4. Data Collection Process
2.5. Assessment of Risk of Bias
2.6. Synthesis Methods
2.7. Assessment of Publication Bias
3. Results
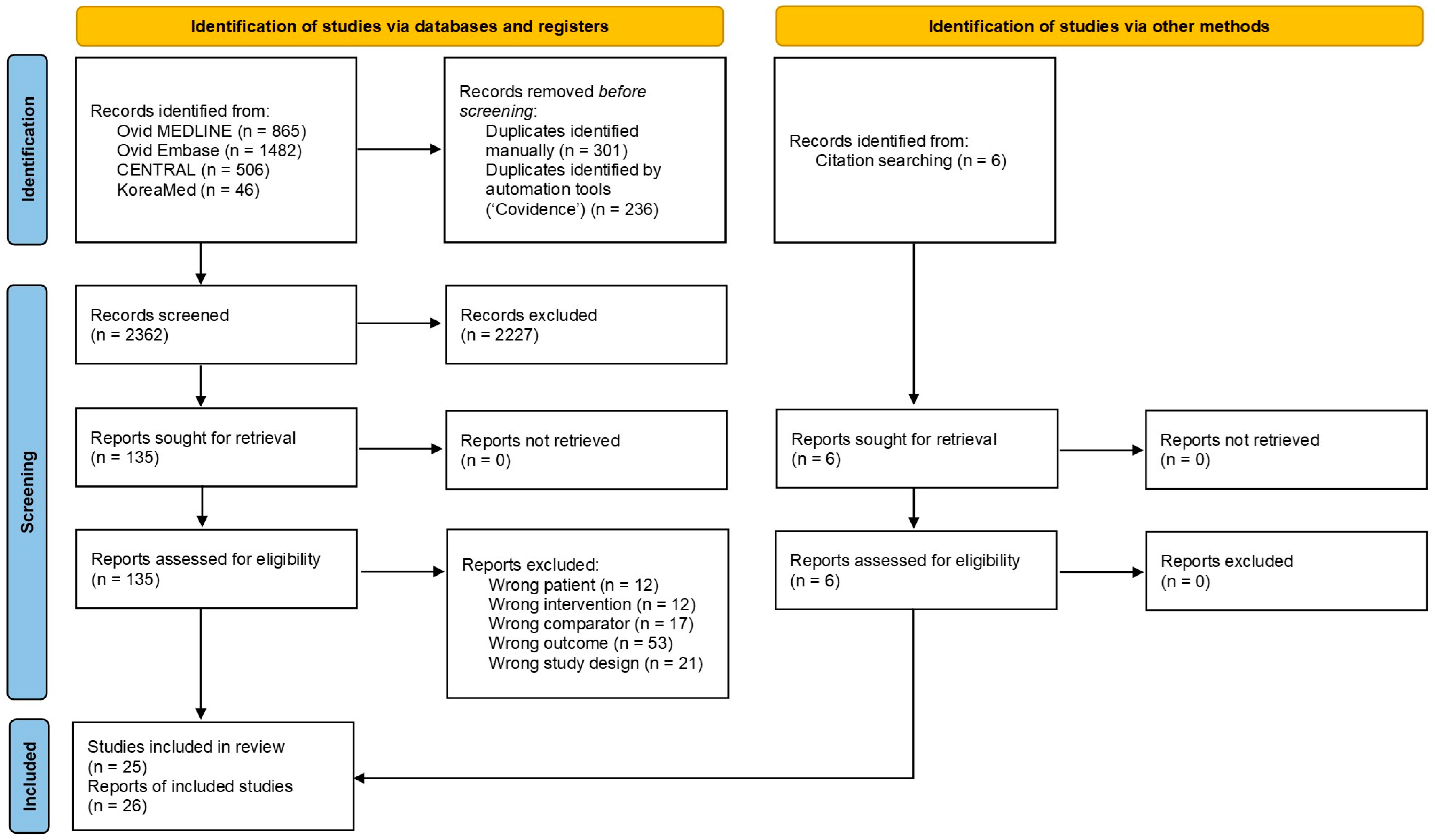
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Included Studies
3.4. Impact of Quitting Smoking at Diagnosis on Overall Survival
3.4.1. Qualitative Analysis of Evidence
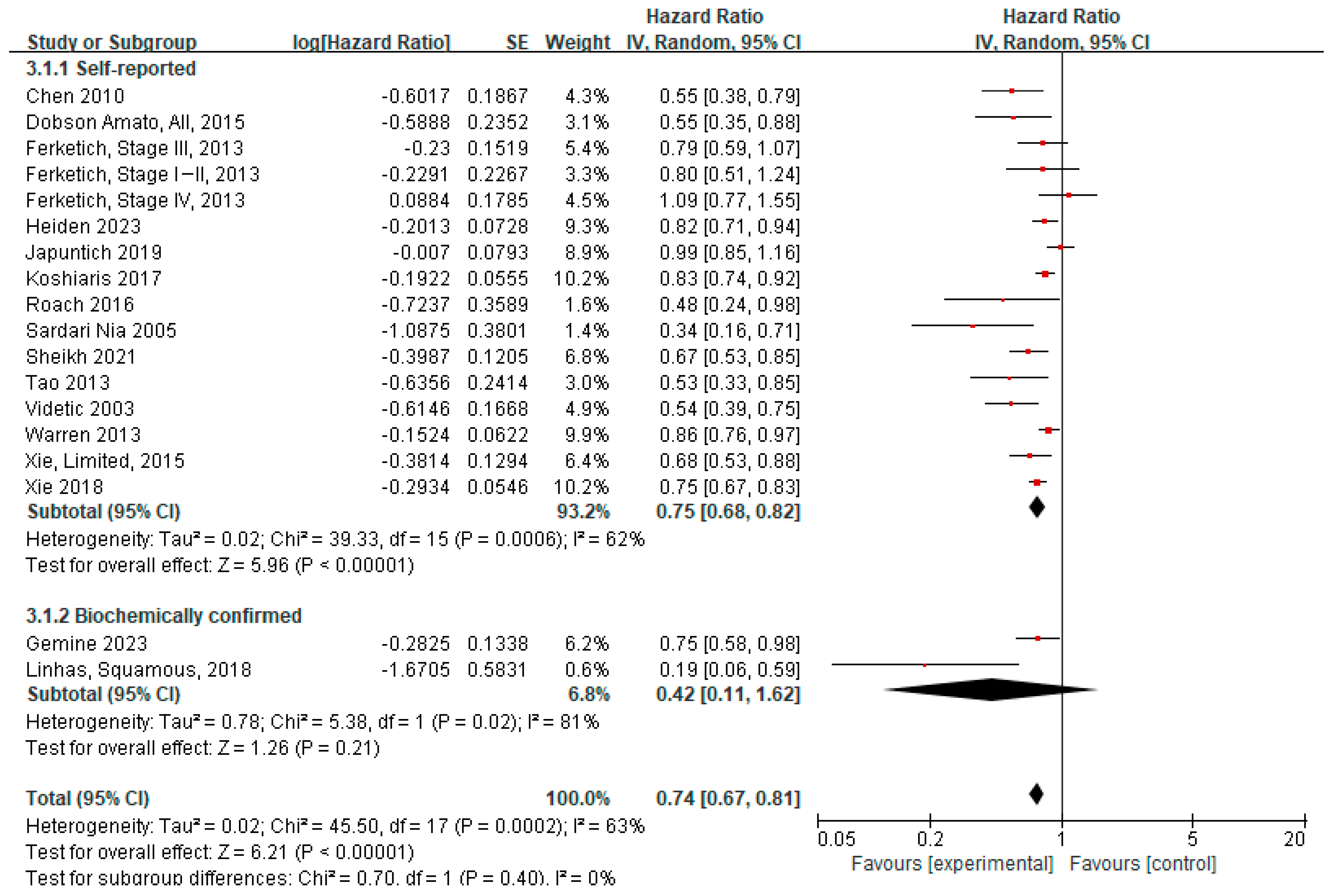
3.4.2. Meta-Analysis
3.4.3. Subgroup Analyses
3.4.4. Sensitivity Analysis
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CO | Carbon monoxide |
| HR | Hazard ratio |
| KM | Kaplan–Meier |
| MST | Median survival time |
| NSCLC | Non-small cell lung cancer |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| RCT | Randomized controlled trials |
| RevMan | Review Manager |
| RoB 2 | Risk of bias tool 2 |
| RoBANS 2 | Risk of Bias for Nonrandomized Studies 2 |
| SCLC | Small cell lung cancer |
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Oh, C.M.; Shin, A.; Lee, J.S. Survival of korean adult cancer patients by stage at diagnosis, 2006–2010: National cancer registry study. Cancer Res. Treat. 2013, 45, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Brawley, O.W.; Glynn, T.J.; Khuri, F.R.; Wender, R.C.; Seffrin, J.R. The first Surgeon General’s report on smoking and health: The 50th anniversary. CA Cancer J. Clin. 2014, 64, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Videtic, G.M.; Stitt, L.W.; Dar, A.R.; Kocha, W.I.; Tomiak, A.T.; Truong, P.T.; Vincent, M.D.; Yu, E.W. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J. Clin. Oncol. 2003, 21, 1544–1549. [Google Scholar] [CrossRef]
- Daniel, M.; Keefe, F.J.; Lyna, P.; Peterson, B.; Garst, J.; Kelley, M.; Bepler, G.; Bastian, L.A. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J. Pain 2009, 10, 323–328. [Google Scholar] [CrossRef]
- Duffy, S.A.; Ronis, D.L.; Valenstein, M.; Fowler, K.E.; Lambert, M.T.; Bishop, C.; Terrell, J.E. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics 2007, 48, 142–148. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Rosner, B.A.; Colditz, G.A. Smoking and smoking cessation in relation to mortality in women. JAMA 2008, 299, 2037–2047. [Google Scholar] [CrossRef]
- Fares, A.F.; Li, Y.; Jiang, M.; Brown, M.C.; Lam, A.C.L.; Aggarwal, R.; Schmid, S.; Leighl, N.B.; Shepherd, F.A.; Wang, Z.; et al. Association between duration of smoking abstinence before non-small-cell lung cancer diagnosis and survival: A retrospective, pooled analysis of cohort studies. Lancet Public Health 2023, 8, e691–e700. [Google Scholar] [CrossRef]
- Gemine, R.E.; Ghosal, R.; Collier, G.; Parry, D.; Campbell, I.; Davies, G.; Davies, K.; Lewis, K.E. Longitudinal study to assess impact of smoking at diagnosis and quitting on 1-year survival for people with non-small cell lung cancer. Lung Cancer 2019, 129, 1–7. [Google Scholar] [CrossRef]
- Kovacs, G.; Barsai, A.; Szilasi, M. Smoking: A prognostic factor of lung cancer survival. Magy. Onkol. 2012, 56, 187–191. [Google Scholar]
- Warren, G.W.; Kasza, K.A.; Reid, M.E.; Cummings, K.M.; Marshall, J.R. Smoking at diagnosis and survival in cancer patients. Int. J. Cancer 2013, 132, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Allen, M.S.; Marks, R.; Jiang, G.; Sun, Z.; Nichols, F.; Zhang, M.; Chen, C.; Aubry, M.C.; Jatoi, A.; et al. Nomogram prediction of overall survival for patients with non-small-cell lung cancer incorporating pretreatment peripheral blood markers. Eur. J. Cardiothorac. Surg. 2018, 53, 1214–1222. [Google Scholar] [CrossRef]
- Caini, S.; Del Riccio, M.; Vettori, V.; Scotti, V.; Martinoli, C.; Raimondi, S.; Cammarata, G.; Palli, D.; Banini, M.; Masala, G.; et al. Quitting Smoking At or Around Diagnosis Improves the Overall Survival of Lung Cancer Patients: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2022, 17, 623–636. [Google Scholar] [CrossRef]
- Westmaas, J.L.; Newton, C.C.; Stevens, V.L.; Flanders, W.D.; Gapstur, S.M.; Jacobs, E.J. Does a Recent Cancer Diagnosis Predict Smoking Cessation? An Analysis From a Large Prospective US Cohort. J. Clin. Oncol. 2015, 33, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Baser, S.; Shannon, V.R.; Eapen, G.A.; Jimenez, C.A.; Onn, A.; Lin, E.; Morice, R.C. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest 2006, 130, 1784–1790. [Google Scholar] [CrossRef]
- Bergman, B.; Sörenson, S. Smoking and effect of chemotherapy in small cell lung cancer. Eur. Respir. J. 1988, 1, 932–937. [Google Scholar] [CrossRef]
- Johnston-Early, A.; Cohen, M.H.; Minna, J.D.; Paxton, L.M.; Fossieck, B.E., Jr.; Ihde, D.C.; Bunn, P.A., Jr.; Matthews, M.J.; Makuch, R. Smoking abstinence and small cell lung cancer survival. An association. JAMA 1980, 244, 2175–2179. [Google Scholar] [CrossRef]
- Linhas, A.R.D.; Dias, M.C.P.; Barroso, A.M.P. Smoking cessation before initiation of chemotherapy in metastatic non-small cell lung cancer: Influence on prognosis. J. Bras. Pneumol. 2018, 44, 436–438. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Seo, H.-J.; Kim, S.Y.; Lee, Y.J.; Park, J.-E. RoBANS 2: A revised risk of bias assessment tool for nonrandomized studies of interventions. Korean J. Fam. Med. 2023, 44, 249. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H.; Murad, M.H.; Hong, C.; Qu, Z.; Cole, S.R.; Chen, Y. Empirical comparison of publication bias tests in meta-analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef]
- Gemine, R.E.; Davies, G.R.; Lanyon, K.; Rees, S.E.; Campbell, I.; Lewis, K.E. Quitting smoking improves two-year survival after a diagnosis of non-small cell lung cancer. Lung Cancer 2023, 186, 107388. [Google Scholar] [CrossRef]
- Koshiaris, C.; Aveyard, P.; Oke, J.; Ryan, R.; Szatkowski, L.; Stevens, R.; Farley, A. Smoking cessation and survival in lung, upper aero-digestive tract and bladder cancer: Cohort study. Br. J. Cancer 2017, 117, 1224–1232. [Google Scholar] [CrossRef]
- Tao, L.; Wang, R.; Gao, Y.T.; Yuan, J.M. Impact of postdiagnosis smoking on long-term survival of cancer patients: The Shanghai cohort study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Choe, Y.R.; Oh, I.J.; Kim, M.S.; Kho, B.G.; Shin, H.J.; Park, C.K.; Kim, Y.I.; Kim, Y.C.; Ahn, H.R.; et al. Efficacy of an inpatient smoking cessation program at a single regional cancer center: A prospective observational study. Medicine 2021, 100, e24745. [Google Scholar] [CrossRef]
- Dobson Amato, K.A.; Hyland, A.; Reed, R.; Mahoney, M.C.; Marshall, J.; Giovino, G.; Bansal-Travers, M.; Ochs-Balcom, H.M.; Zevon, M.A.; Cummings, K.M.; et al. Tobacco Cessation May Improve Lung Cancer Patient Survival. J. Thorac. Oncol. 2015, 10, 1014–1019. [Google Scholar] [CrossRef]
- Heiden, B.T.; Eaton, D.B., Jr.; Chang, S.H.; Yan, Y.; Schoen, M.W.; Chen, L.S.; Smock, N.; Patel, M.R.; Kreisel, D.; Nava, R.G.; et al. Assessment of Duration of Smoking Cessation Prior to Surgical Treatment of Non-small Cell Lung Cancer. Ann. Surg. 2023, 277, e933–e940. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.C.; Rehman, S.; DeWees, T.A.; Abraham, C.D.; Bradley, J.D.; Robinson, C.G. It’s never too late: Smoking cessation after stereotactic body radiation therapy for non-small cell lung carcinoma improves overall survival. Pract. Radiat. Oncol. 2016, 6, 12–18. [Google Scholar] [CrossRef]
- Doerr, F.; Leschczyk, T.; Grapatsas, K.; Menghesha, H.; Baldes, N.; Schlachtenberger, G.; Heldwein, M.B.; Michel, M.; Quaas, A.; Hagmeyer, L.; et al. Postoperative Tobacco Cessation Improves Quality of Life, Lung Function and Long-Term Survival in Non-Small-Cell Lung Cancer Patients. Cancers 2024, 16, 465. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, R.; Garces, Y.I.; Jatoi, A.; Stoddard, S.M.; Sun, Z.; Marks, R.S.; Liu, Y.; Yang, P. Prognostic factors for limited-stage small cell lung cancer: A study of 284 patients. Lung Cancer 2010, 67, 221–226. [Google Scholar] [CrossRef]
- Ferketich, A.K.; Niland, J.C.; Mamet, R.; Zornosa, C.; D’Amico, T.A.; Ettinger, D.S.; Kalemkerian, G.P.; Pisters, K.M.; Reid, M.E.; Otterson, G.A. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer 2013, 119, 847–853. [Google Scholar] [CrossRef]
- Japuntich, S.J.; Kumar, P.; Pendergast, J.F.; Juarez Caballero, G.Y.; Malin, J.L.; Wallace, R.B.; Chrischilles, E.A.; Keating, N.L.; Park, E.R. Smoking Status and Survival Among a National Cohort of Lung and Colorectal Cancer Patients. Nicotine Tob. Res. 2019, 21, 497–504. [Google Scholar] [CrossRef]
- Sardari Nia, P.; Weyler, J.; Colpaert, C.; Vermeulen, P.; Van Marck, E.; Van Schil, P. Prognostic value of smoking status in operated non-small cell lung cancer. Lung Cancer 2005, 47, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Mukeriya, A.; Shangina, O.; Brennan, P.; Zaridze, D. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 1232–1239. [Google Scholar] [CrossRef]
- Xie, D.; Marks, R.; Zhang, M.; Jiang, G.; Jatoi, A.; Garces, Y.I.; Mansfield, A.; Molina, J.; Yang, P. Nomograms Predict Overall Survival for Patients with Small-Cell Lung Cancer Incorporating Pretreatment Peripheral Blood Markers. J. Thorac. Oncol. 2015, 10, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Setter, C.; Schild, S.E.; Dunst, J. Effect of smoking during radiotherapy, respiratory insufficiency, and hemoglobin levels on outcome in patients irradiated for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Lugg, S.T.; Tikka, T.; Agostini, P.J.; Kerr, A.; Adams, K.; Kalkat, M.S.; Steyn, R.S.; Rajesh, P.B.; Bishay, E.; Thickett, D.R.; et al. Smoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgery. J. Cardiothorac. Surg. 2017, 12, 52. [Google Scholar] [CrossRef]
- Saito-Nakaya, K.; Nakaya, N.; Fujimori, M.; Akizuki, N.; Yoshikawa, E.; Kobayakawa, M.; Nagai, K.; Nishiwaki, Y.; Tsubono, Y.; Uchitomi, Y. Marital status, social support and survival after curative resection in non-small-cell lung cancer. Cancer Sci. 2006, 97, 206–213. [Google Scholar] [CrossRef]
- Parsons, A.; Daley, A.; Begh, R.; Aveyard, P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 2010, 340, b5569. [Google Scholar] [CrossRef]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef]
- Jassem, J. Tobacco smoking after diagnosis of cancer: Clinical aspects. Transl. Lung Cancer Res. 2019, 8 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Do, K.A.; Johnson, M.M.; Lee, J.J.; Wu, X.F.; Dong, Q.; Hong, W.K.; Khuri, F.R.; Spitz, M.R. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer 2004, 101, 2837–2842. [Google Scholar] [CrossRef]
- Sitas, F.; Weber, M.F.; Egger, S.; Yap, S.; Chiew, M.; O’Connell, D. Smoking cessation after cancer. J. Clin. Oncol. 2014, 32, 3593–3595. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004, 328, 1519. [Google Scholar] [CrossRef]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Grønkjær, M.; Eliasen, M.; Skov-Ettrup, L.S.; Tolstrup, J.S.; Christiansen, A.H.; Mikkelsen, S.S.; Becker, U.; Flensborg-Madsen, T. Preoperative smoking status and postoperative complications: A systematic review and meta-analysis. Ann. Surg. 2014, 259, 52–71. [Google Scholar] [CrossRef]
- Cai, L.; Bai, H.; Duan, J.; Wang, Z.; Gao, S.; Wang, D.; Wang, S.; Jiang, J.; Han, J.; Tian, Y.; et al. Epigenetic alterations are associated with tumor mutation burden in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 198. [Google Scholar] [CrossRef]
- Kim, Y.-A.; Hodzic, E.; Amgalan, B.; Saslafsky, A.; Wojtowicz, D.; Przytycka, T.M. Mutational Signatures as Sensors of Environmental Exposures: Analysis of Smoking-Induced Lung Tissue Remodeling. Biomolecules 2022, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Bossé, Y.; Postma, D.S.; Sin, D.D.; Lamontagne, M.; Couture, C.; Gaudreault, N.; Joubert, P.; Wong, V.; Elliott, M.; van den Berge, M.; et al. Molecular signature of smoking in human lung tissues. Cancer Res. 2012, 72, 3753–3763. [Google Scholar] [CrossRef]
- Park, B.; Kong, S.Y.; Kim, J.; Kim, Y.; Park, I.H.; Jung, S.Y.; Lee, E.S. Health Behaviors of Cancer Survivors in Nationwide Cross-Sectional Survey in Korea: Higher Alcohol Drinking, Lower Smoking, and Physical Inactivity Pattern in Survivors with Higher Household Income. Medicine 2015, 94, e1214. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Boyes, A.W.; Hall, A.; Girgis, A.; D’Este, C.; Sitas, F. Prevalence and factors related to smoking and smoking cessation 6 months following a cancer diagnosis: A population-based study. J. Cancer Surviv. 2016, 10, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Marshall, J.R.; Cummings, K.M.; Toll, B.; Gritz, E.R.; Hutson, A.; Dibaj, S.; Herbst, R.; Dresler, C. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J. Thorac. Oncol. 2013, 8, 543–548. [Google Scholar] [CrossRef]


| Lung Cancer Subtype | Subgroups/Total | Unadjusted HR | 95% CI | p Value for Difference | Adjusted HR | 95% CI | p Value for Difference |
|---|---|---|---|---|---|---|---|
| NSCLC | Stage I–III, only | 0.42 | 0.25–0.72 | 0.002 | 0.67 | 0.54–0.83 | 0.0003 |
| Stage IV, only | 1.17 | 0.77–1.77 | 0.47 | 0.49 | 0.09–2.75 | 0.42 | |
| Any stage, unspecified * | 0.75 | 0.55–1.01 | 0.06 | 0.76 | 0.68–0.84 | <0.00001 | |
| Overall pooled estimate | 0.72 | 0.53–0.99 | 0.04 | 0.73 | 0.64–0.83 | <0.00001 | |
| SCLC | Limited stage, only | 0.53 | 0.37–0.77 | 0.0008 | 0.61 | 0.51–0.72 | <0.00001 |
| Extensive stage, only | 0.89 | 0.74–1.06 | 0.19 | - | – | - | |
| Any stage, unspecified * | 0.82 | 0.66–1.01 | 0.07 | - | – | - | |
| Overall pooled estimate | 0.78 | 0.63–0.95 | 0.01 | 0.61 | 0.51–0.72 | <0.00001 | |
| Both or unspecified subtypes # | Overall pooled estimate | 0.71 | 0.64–0.78 | <0.00001 | 0.85 | 0.75–0.97 | 0.02 |
| All studies † | Stage I–III or limited stage | 0.50 | 0.37–0.67 | <0.0001 | 0.64 | 0.56–0.74 | <0.00001 |
| Stage IV or extensive stage | 0.95 | 0.75–1.21 | 0.68 | 0.49 | 0.09–2.75 | 0.42 | |
| Any stage, unspecified * | 0.73 | 0.67–0.79 | <0.00001 | 0.81 | 0.74–0.88 | <0.00001 | |
| Overall pooled estimate | 0.75 | 0.66–0.84 | <0.00001 | 0.74 | 0.67–0.81 | <0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M.; Suh, H.-W.; Lee, H.-J.; Choi, M.; Kim, J.S.; Lee, K.; Kim, S.-H.; Sohn, J.W.; Yoon, H.J.; Paek, Y.-J.; et al. Impact of Quitting Smoking at Diagnosis on Overall Survival in Lung Cancer Patients: A Comprehensive Meta-Analysis. Cancers 2025, 17, 3623. https://doi.org/10.3390/cancers17223623
Lee JM, Suh H-W, Lee H-J, Choi M, Kim JS, Lee K, Kim S-H, Sohn JW, Yoon HJ, Paek Y-J, et al. Impact of Quitting Smoking at Diagnosis on Overall Survival in Lung Cancer Patients: A Comprehensive Meta-Analysis. Cancers. 2025; 17(22):3623. https://doi.org/10.3390/cancers17223623
Chicago/Turabian StyleLee, Jong Min, Hyo-Weon Suh, Hyeon-Jeong Lee, Miyoung Choi, Ji Soo Kim, Kiheon Lee, Sang-Heon Kim, Jang Won Sohn, Ho Joo Yoon, Yu-Jin Paek, and et al. 2025. "Impact of Quitting Smoking at Diagnosis on Overall Survival in Lung Cancer Patients: A Comprehensive Meta-Analysis" Cancers 17, no. 22: 3623. https://doi.org/10.3390/cancers17223623
APA StyleLee, J. M., Suh, H.-W., Lee, H.-J., Choi, M., Kim, J. S., Lee, K., Kim, S.-H., Sohn, J. W., Yoon, H. J., Paek, Y.-J., Lee, C. M., & Park, D. W. (2025). Impact of Quitting Smoking at Diagnosis on Overall Survival in Lung Cancer Patients: A Comprehensive Meta-Analysis. Cancers, 17(22), 3623. https://doi.org/10.3390/cancers17223623






