Loss of Argininosuccinate Synthetase-1 (ASS1) Occurs in Esophageal Adenocarcinoma and Represents a Promising Biomarker for Therapy with Pegargiminase
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
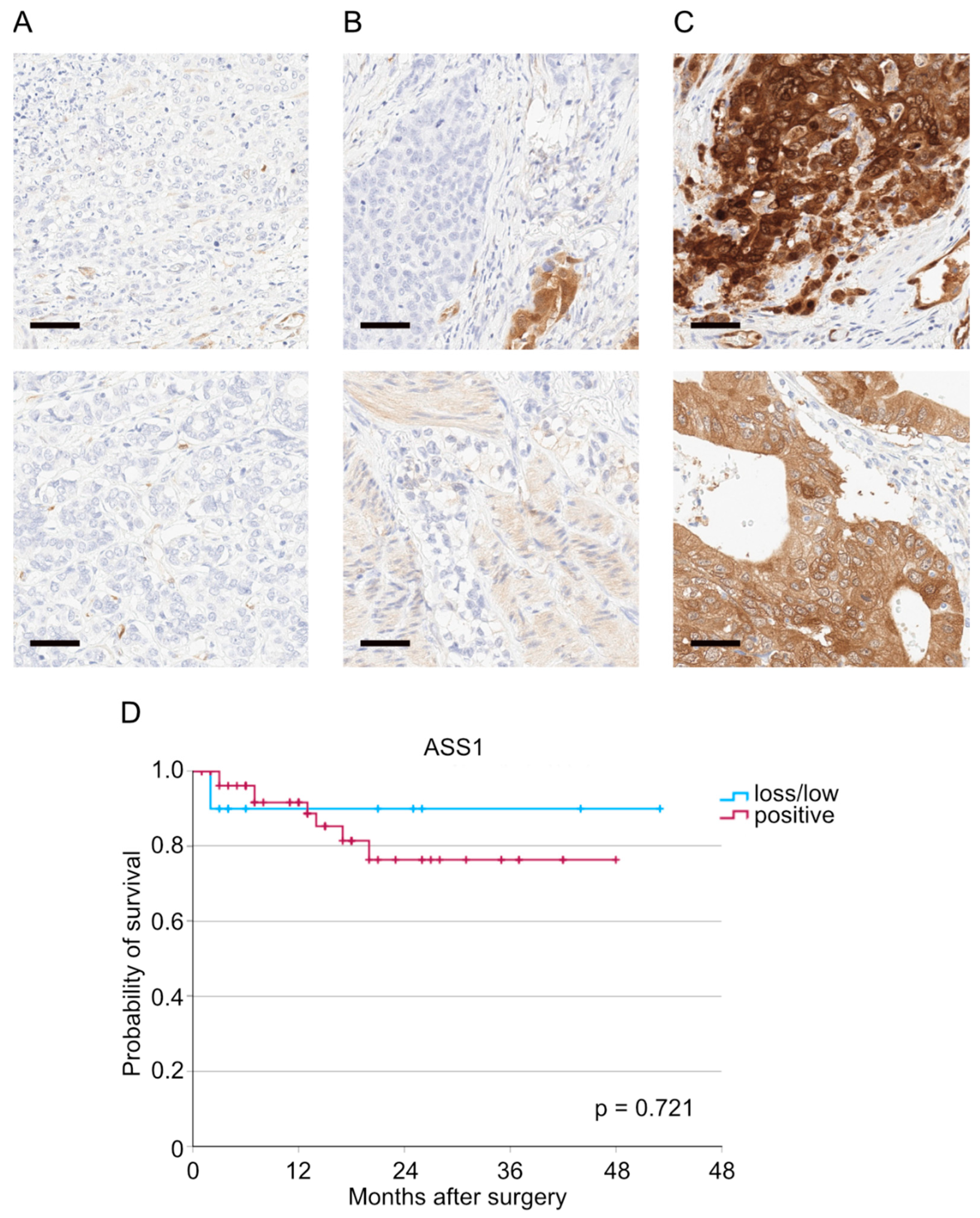
2.2. Immunohistochemistry
2.3. RNAScopeTM
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AEG | Siewert classification of the adenocarcinoma of the esophagogastric junction |
| ASS1 | Argininosuccinate Synthetase-1 |
| CROSS | Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study |
| et al. | et alia (and others) |
| FDA | Food and Drug Administration |
| FLOT | fluorouracil, leucovorin, oxaliplatin and docetaxel |
| L | lymph vessel infiltration |
| µm | micrometer |
| RNA | Ribonucleic acid |
| V | blood vessel infiltration |
| (y)pN | pathological tumor status (after neoadjuvant therapy) |
| (y)pT | pathological lymph node status (after neoadjuvant therapy) |
References
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lievre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. New Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Husson, A.; Brasse-Lagnel, C.; Fairand, A.; Renouf, S.; Lavoinne, A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 2003, 270, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhao, X. Argininosuccinate synthase 1, arginine deprivation therapy and cancer management. Front. Pharmacol. 2022, 13, 935553. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zuo, Q.; Ruan, H.; Wang, H.; Jin, H.; Cheng, Z.; Ly, Y.; Qin, W.; Wang, C. Argininosuccinate synthase 1 suppresses cancer cell invasion by inhibiting STAT3 pathway in hepatocellular carcinoma. Acta Biochim. Biophys Sin 2019, 51, 263–276. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wu, W.R.; Wang, Y.H.; Wang, J.W.; Fang, F.M.; Tsai, J.W.; Li, S.H.; Hung, H.C.; Yu, S.C.; Lan, J.; et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: Aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin. Cancer Res. 2013, 19, 2861–2872. [Google Scholar] [CrossRef]
- Luo, W.; Zou, Z.; Nie, Y.; Luo, J.; Ming, Z.; Hu, X.; Luo, T.; Ouyang, M.; Liu, M.; Tang, H.; et al. ASS1 inhibits triple-negative breast cancer by regulating PHGDH stability and de novo serine synthesis. Cell. Death Dis. 2024, 15, 319. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Harris, A.L.; Koukourakis, M.I. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 2021, 9, 28. [Google Scholar] [CrossRef]
- Lan, J.; Tai, H.C.; Lee, S.W.; Chen, T.J.; Huang, H.Y.; Li, C.F. Deficiency in expression and epigenetic DNA Methylation of ASS1 gene in nasopharyngeal carcinoma: Negative prognostic impact and therapeutic relevance. Tumour Biol. 2014, 35, 161–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Chung, S.F.; Tam, S.Y.; Leung, Y.C.; Guan, X. Arginine deprivation as a strategy for cancer therapy: An insight into drug design and drug combination. Cancer Lett. 2021, 502, 58–70. [Google Scholar] [CrossRef]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar]
- Szlosarek, P.W.; Steele, J.P.; Nolan, L.; Gilligan, D.; Taylor, P.; Spicer, J.; Lind, M.; Mitra, S.; Shamash, J.; Phillips, M.M.; et al. Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.E. TNM Atlas, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar]
- Schneider, P.M.; Baldus, S.E.; Metzger, R.; Kocher, M.; Bongartz, R.; Bollschweiler, E.; Schaefer, H.; Thiele, J.; Dienes, H.P.; Mueller, R.P.; et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: Implications for response classification. Ann. Surg. 2005, 242, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.I.; Simon, A.G.; Jung, J.O.; Fretter, C.; Schröder, W.; Bruns, C.J.; Schmidt, T.; Quaas, A.; Knipper, K. Hexokinase 2 as an independent risk factor for worse patient survival in esophageal adenocarcinoma and as a potential therapeutic target protein: A retrospective, single--center cohort study. Oncol. Lett. 2024, 28, 495. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Creelan, B.C.; Sarkodie, T.; Nolan, L.; Taylor, P.; Olevsky, O.; Grosso, F.; Cortinovis, D.; Chitnis, M.; Roy, A.; et al. Pegargiminase Plus First-Line Chemotherapy in Patients With Nonepithelioid Pleural Mesothelioma: The ATOMIC-Meso Randomized Clinical Trial. JAMA Oncol. 2024, 10, 475–483. [Google Scholar] [CrossRef]
- Chang, K.Y.; Chiang, N.J.; Wu, S.Y.; Yen, C.J.; Chen, S.H.; Yeh, Y.M.; Li, C.F.; Feng, X.; Qu, K.; Johnston, A.; et al. Phase 1b study of pegylated arginine deiminase (ADI-PEG 20) plus Pembrolizumab in advanced solid cancers. Oncoimmunology 2021, 10, 1943253. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Wimalasingham, A.G.; Phillips, M.M.; Hall, P.E.; Chan, P.Y.; Conibear, J.; Lim, L.; Rashid, S.; Steele, J.; Wells, P.; et al. Phase 1, pharmacogenomic, dose-expansion study of pegargiminase plus pemetrexed and cisplatin in patients with ASS1-deficient non-squamous non-small cell lung cancer. Cancer Med. 2021, 10, 6642–6652. [Google Scholar] [CrossRef]
- Feng, X.; Ji, Z.; Yang, G. ASS1 regulates immune microenvironment via CXCL8 signaling in ovarian cancer. Biochem. Biophys. Res. Commun. 2022, 631, 86–92. [Google Scholar] [CrossRef]
- Hall, P.E.; Ready, N.; Johnston, A.; Bomalaski, J.S.; Venhaus, R.R.; Sheaff, M.; Krug, L.; Szlosarek, P.W. Phase II Study of Arginine Deprivation Therapy With Pegargiminase in Patients With Relapsed Sensitive or Refractory Small-cell Lung Cancer. Clin. Lung Cancer 2020, 21, 527–533. [Google Scholar] [CrossRef]
- Beddowes, E.; Spicer, J.; Chan, P.Y.; Khadeir, R.; Corbacho, J.G.; Repana, D.; Steele, J.P.; Schmid, P.; Szyszko, T.; Cook, G.; et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers. J. Clin. Oncol. 2017, 35, 1778–1785. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Klabatsa, A.; Pallaska, A.; Sheaff, M.; Smith, P.; Crook, T.; Grimshaw, M.J.; Steele, J.P.; Rudd, R.M.; Balkwill, F.R.; et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin. Cancer Res. 2006, 12, 7126–7131. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.; Song, Y.; Lee, S.Y.; Choi, I.; Park, I.S.; Kim, J.; Kim, J.S.; Kim, K.M.; Seo, H.R. Argininosuccinate synthase 1 suppresses tumor progression through activation of PERK/eIF2α/ATF4/CHOP axis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 127. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wang, Y.Y.; Jing, Y.; Xu, M.H.; Zhu, Z.T.; Wang, Q.J. Abnormal arginine metabolism is associated with prognosis in patients of gastric cancer. Transl. Cancer Res. 2021, 10, 2451–2469. [Google Scholar] [CrossRef]
- Ding, Q.; Li, R.; Wang, Q.; Yu, L.; Zi, F. A pan-cancer analysis of the role of argininosuccinate synthase 1 in human tumors. Front. Oncol. 2023, 13, 1049147. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, H.; Jia, C.; Ma, X.; Guo, W.; Li, H. Clinicopathological features and prognosis of patients <45 years old with esophageal adenocarcinoma comparing to other age groups. J. Thorac. Dis. 2016, 8, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Meng, Q.; Cao, S.; Jiang, Q.; Chen, Y.; Wu, B.; Li, X.; Bao, S. The role of ASS1 in mouse embryonic stem cell differentiation into mesendoderm lineages. Stem. Cell. Res. Ther. 2025, 16, 479. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef]
- Lim, L.Q.J.; Adler, L.; Hajaj, E.; Soria, L.R.; Perry, R.B.; Darzi, N.; Brody, R.; Furth, N.; Lichtenstein, M.; Bab-Dinitz, E.; et al. ASS1 metabolically contributes to the nuclear and cytosolic p53-mediated DNA damage response. Nat. Metab. 2024, 6, 1294–1309. [Google Scholar] [CrossRef]
- Knipper, K.; Damanakis, A.I.; Zhao, Y.; Bruns, C.J.; Schmidt, T.; Popp, F.C.; Quaas, A.; Lyu, S.I.; Pancalyze Study Group. Specific Subtypes of Carcinoma-Associated Fibroblasts Are Correlated with Worse Survival in Resectable Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 2049. [Google Scholar] [CrossRef]
| Characteristic | ASS1 | ||||
|---|---|---|---|---|---|
| Total | Loss | Low | Positive | p-Value | |
| n (%) | n (%) | n (%) | n (%) | ||
| No. of patients | 97 (100) | 6 (100) | 6 (100) | 85 (100) | |
| Sex | 0.600 | ||||
| Male | 84 (86.6) | 5 (83.3) | 6 (100.0) | 73 (85.9) | |
| Female | 13 (13.4) | 1 (16.7) | 0 (0.0) | 12 (14.1 | |
| Age | 0.007 | ||||
| ≤65 | 38 (39.2) | 2 (33.3) | 6 (100.0) | 30 (35.3) | |
| >65 | 59 (60.8) | 4 (66.7) | 0 (0.0) | 55 (64.7) | |
| AEG | 0.211 | ||||
| I | 44 (45.4) | 1 (16.7) | 5 (83.3) | 38 (44.7) | |
| II | 51 (52.6) | 5 (83.3) | 1 (16.7) | 45 (52.9) | |
| III | 2 (2.1) | 0 (0.0) | 0 (0.0) | 2 (2.4) | |
| Perioperative/ neoadjuvant therapy | 0.063 | ||||
| No | 47 (48.5) | 1 (16.7) | 1 (16.7) | 55 (64.7) | |
| Yes | 50 (51.5) | 5 (83.3) | 5 (83.3) | 45 (52.9) | |
| Type of perioperative/ neoadjuvant therapy | 0.564 | ||||
| FLOT | 35 (70.0) | 5 (100.0) | 3 (60.0) | 27 (67.5) | |
| CROSS | 13 (26.0) | 0 (0.0) | 2 (40.0) | 11 (27.5) | |
| Other | 2 (4.0) | 0 (0.0) | 0 (0.0) | 2 (5.0) | |
| Surgical approach | 0.581 | ||||
| thoracoabdominal esophagectomy | 83 (85.6) | 5 (83.3) | 6 (100.0) | 72 (84.7) | |
| transhiatal extended gastrectomy | 14 (14.4) | 1 (16.7) | 0 (0.0) | 13 (15.3) | |
| Surgical technique | 0.687 | ||||
| Open | 24 (24.7) | 2 (33.3) | 1 (16.7) | 21 (24.7) | |
| Hybrid | 54 (55.7) | 4 (66.7) | 3 (50.0) | 47 (55.3) | |
| Total minimally invasive | 19 (19.6) | 0 (0.0) | 2 (33.3) | 17 (20.0) | |
| Clavien-Dindo classification | 0.018 | ||||
| 0 | 36 (37.1) | 2 (33.3) | 1 (16.7) | 33 (38.3) | |
| I | 3 (3.1) | 0 (0.0) | 2 (33.3) | 1 (1.2) | |
| II | 8 (8.2) | 1 (16.7) | 0 (0.0) | 7 (8.2) | |
| IIIa | 23 (23.7) | 1 (16.7) | 2 (33.3) | 20 (23.5) | |
| IIIb | 13 (13.4) | 1 (16.7) | 0 (0.0) | 12 (14.1) | |
| IVa | 8 (8.2) | 1 (16.7) | 0 (0.0) | 7 (8.2) | |
| IVb | 3 (3.1) | 0 (0.0) | 0 (0.0) | 3 (3.5) | |
| V | 3 (3.1) | 0 (0.0) | 1 (16.7) | 2 (2.4) | |
| Anastomotic leakage | 0.497 | ||||
| No | 88 (90.7) | 6 (100.0) | 6 (100.0) | 76 (89.4) | |
| Yes | 9 (9.3) | 0 (0.0) | 0 (0.0) | 9 (10.6) | |
| (y)pT | 0.667 | ||||
| 0 | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | |
| 1 | 19 (19.6) | 0 (0.0) | 0 (0.0) | 19 (22.4) | |
| 2 | 9 (9.3) | 0 (0.0) | 1 (16.7) | 8 (9.4) | |
| 3 | 61 (62.9) | 5 (83.3) | 5 (83.3) | 51 (60.0) | |
| 4 | 7 (7.2) | 1 (16.7) | 0 (0.0) | 6 (7.1) | |
| (y)pN | 0.348 | ||||
| 0 | 31 (32.0) | 0 (0.0) | 2 (33.3) | 29 (34.1) | |
| 1 | 21 (21.6) | 1 (16.7) | 1 (16.7) | 19 (22.4) | |
| 2 | 20 (20.6) | 1 (16.7) | 1 (16.7) | 18 (21.2) | |
| 3 | 25 (25.8) | 4 (66.6) | 2 (33.3) | 23 (22.4) | |
| L | 0.297 | ||||
| 0 | 24 (24.7) | 0 (0.0) | 1 (16.7) | 23 (27.1) | |
| 1 | 73 (75.3) | 6 (100.0) | 5 (83.3) | 62 (72.9) | |
| V | 0.057 | ||||
| 0 | 50 (52.1) | 1 (16.7) | 1 (16.7) | 48 (56.5) | |
| 1 | 46 (46.9) | 5 (83.3) | 4 (66.6)) | 37 (43.5) | |
| Unknown | 1 (1.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | |
| Grading | 0.893 | ||||
| 1 | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 1.2) | |
| 2 | 21 (21.6) | 0 (0.0) | 0 (0.0) | 21 (24.7) | |
| 3 | 23 (23.7) | 1 (16.7) | 1 (16.7) | 21 (24.7) | |
| Unknown/not. applicable | 53 (54.6) | 5 (83.3) | 5 (83.3) | 42 (49.4) | |
| Characteristic | Borders | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Sex | male vs. female | 0.615 | 0.134–2.814 | 0.531 |
| Age | ≥65 vs. <65 | 0.455 | 0.144–1.438 | 0.180 |
| Clavien-Dindo classification | ≥IIIb vs. <IIIb | 2.426 | 0.781–7.537 | 0.126 |
| (y)pT | ≥2 vs. <2 | 3.334 | 1.165–9.543 | 0.025 |
| (y)pN | ≥1 vs. 0 | 1.583 | 0.944–2.654 | 0.081 |
| L | 1 vs. 0 | 2.952 | 0.380–22.901 | 0.300 |
| V | ≥1 vs. 0 | 2.351 | 0.704–7.852 | 0.165 |
| ASS1 | positive vs. loss/low | 0.882 | 0.337–2.313 | 0.799 |
| Characteristic | Borders | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Age | ≥65 vs. <65 | 0.331 | 0.094–1.167 | 0.085 |
| Clavien-Dindo classification | ≥IIIb vs. <IIIb | 4.766 | 1.450–15.667 | 0.010 |
| (y)pT | ≥2 vs. <2 | 4.542 | 1.363–15.132 | 0.014 |
| (y)pN | ≥1 vs. 0 | 1.113 | 0.607–2.041 | 0.730 |
| V | ≥1 vs. 0 | 1.052 | 0.260–4.261 | 0.944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knipper, K.; Lyu, S.I.; Tzitzili, E.; Spielmann, S.-M.; Bruns, C.J.; Schmidt, T.; Popp, F.C.; Quaas, A. Loss of Argininosuccinate Synthetase-1 (ASS1) Occurs in Esophageal Adenocarcinoma and Represents a Promising Biomarker for Therapy with Pegargiminase. Cancers 2025, 17, 3624. https://doi.org/10.3390/cancers17223624
Knipper K, Lyu SI, Tzitzili E, Spielmann S-M, Bruns CJ, Schmidt T, Popp FC, Quaas A. Loss of Argininosuccinate Synthetase-1 (ASS1) Occurs in Esophageal Adenocarcinoma and Represents a Promising Biomarker for Therapy with Pegargiminase. Cancers. 2025; 17(22):3624. https://doi.org/10.3390/cancers17223624
Chicago/Turabian StyleKnipper, Karl, Su Ir Lyu, Eleni Tzitzili, Sarah-Michele Spielmann, Christiane J. Bruns, Thomas Schmidt, Felix C. Popp, and Alexander Quaas. 2025. "Loss of Argininosuccinate Synthetase-1 (ASS1) Occurs in Esophageal Adenocarcinoma and Represents a Promising Biomarker for Therapy with Pegargiminase" Cancers 17, no. 22: 3624. https://doi.org/10.3390/cancers17223624
APA StyleKnipper, K., Lyu, S. I., Tzitzili, E., Spielmann, S.-M., Bruns, C. J., Schmidt, T., Popp, F. C., & Quaas, A. (2025). Loss of Argininosuccinate Synthetase-1 (ASS1) Occurs in Esophageal Adenocarcinoma and Represents a Promising Biomarker for Therapy with Pegargiminase. Cancers, 17(22), 3624. https://doi.org/10.3390/cancers17223624







