Suspicion for Sarcoma: Clinical Presentation, Multi-Modality Imaging Evaluation, and Ultrasound Artificial Intelligence-Based Decision Support
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Acquisition and Patients
2.2. Imaging
2.3. Imaging Analysis
2.4. AI Decision Support
2.5. Statistical Analysis
3. Results
3.1. Diagnosis and Clinical Presentation
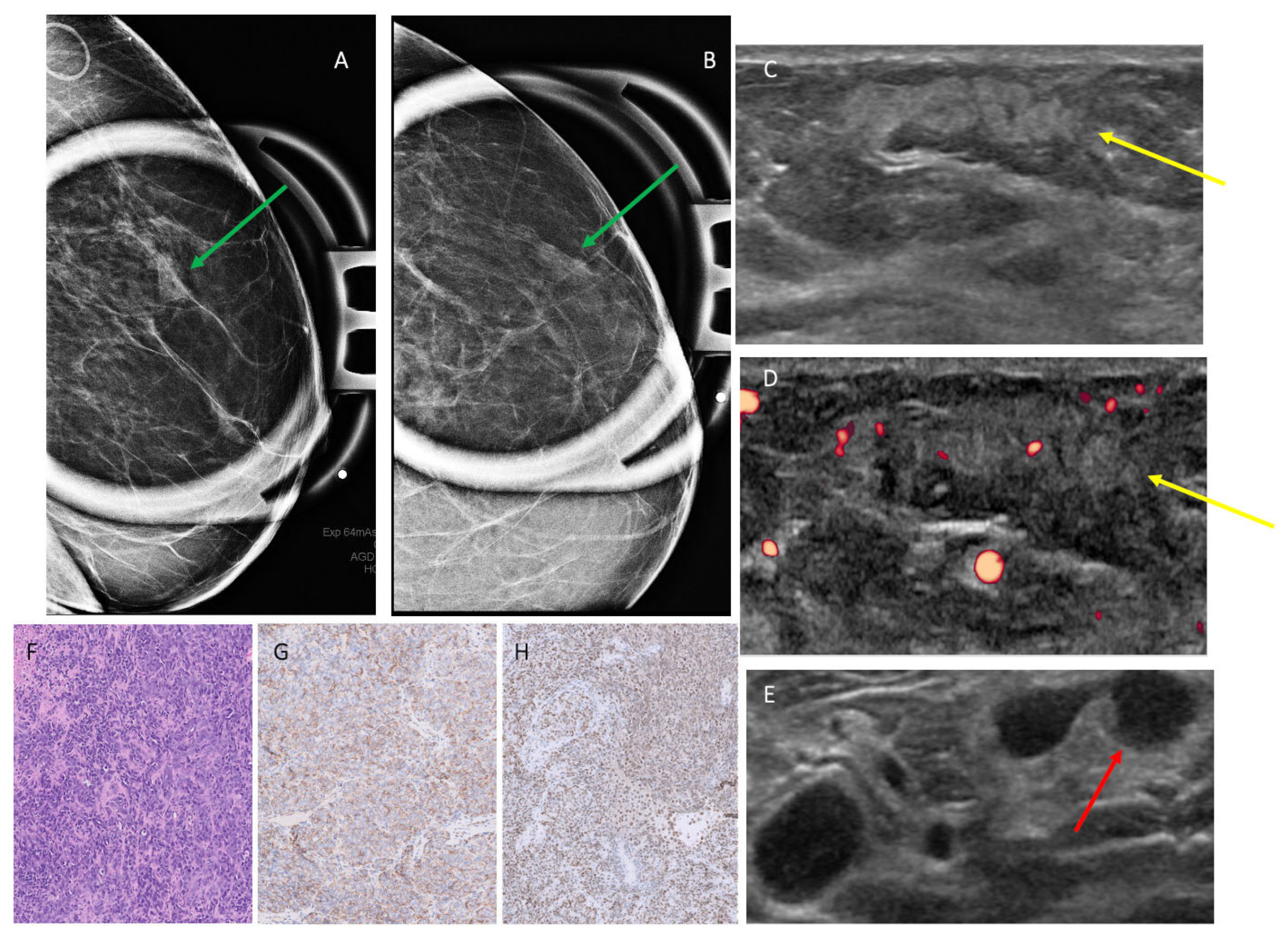
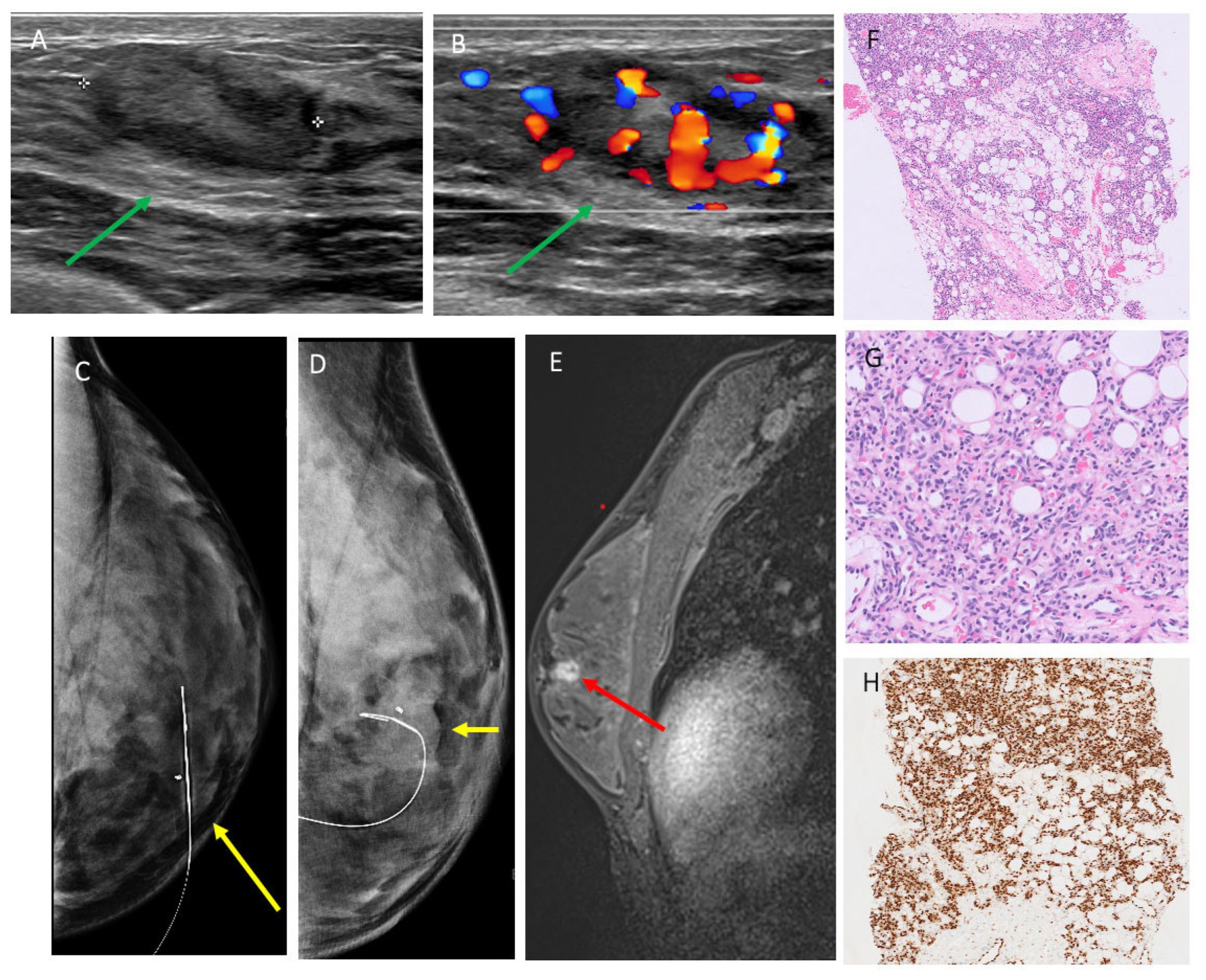
3.2. Mammographic Findings
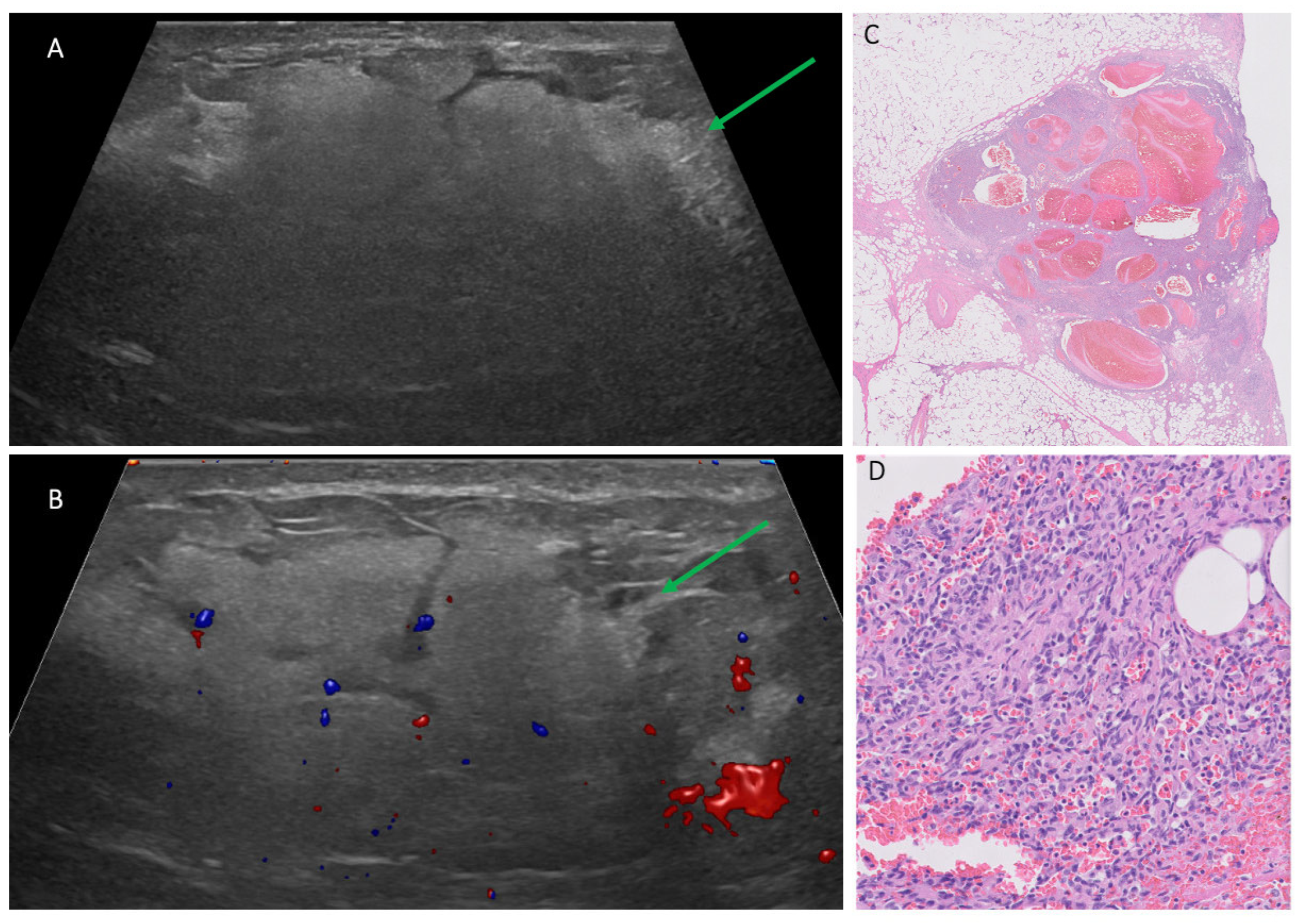
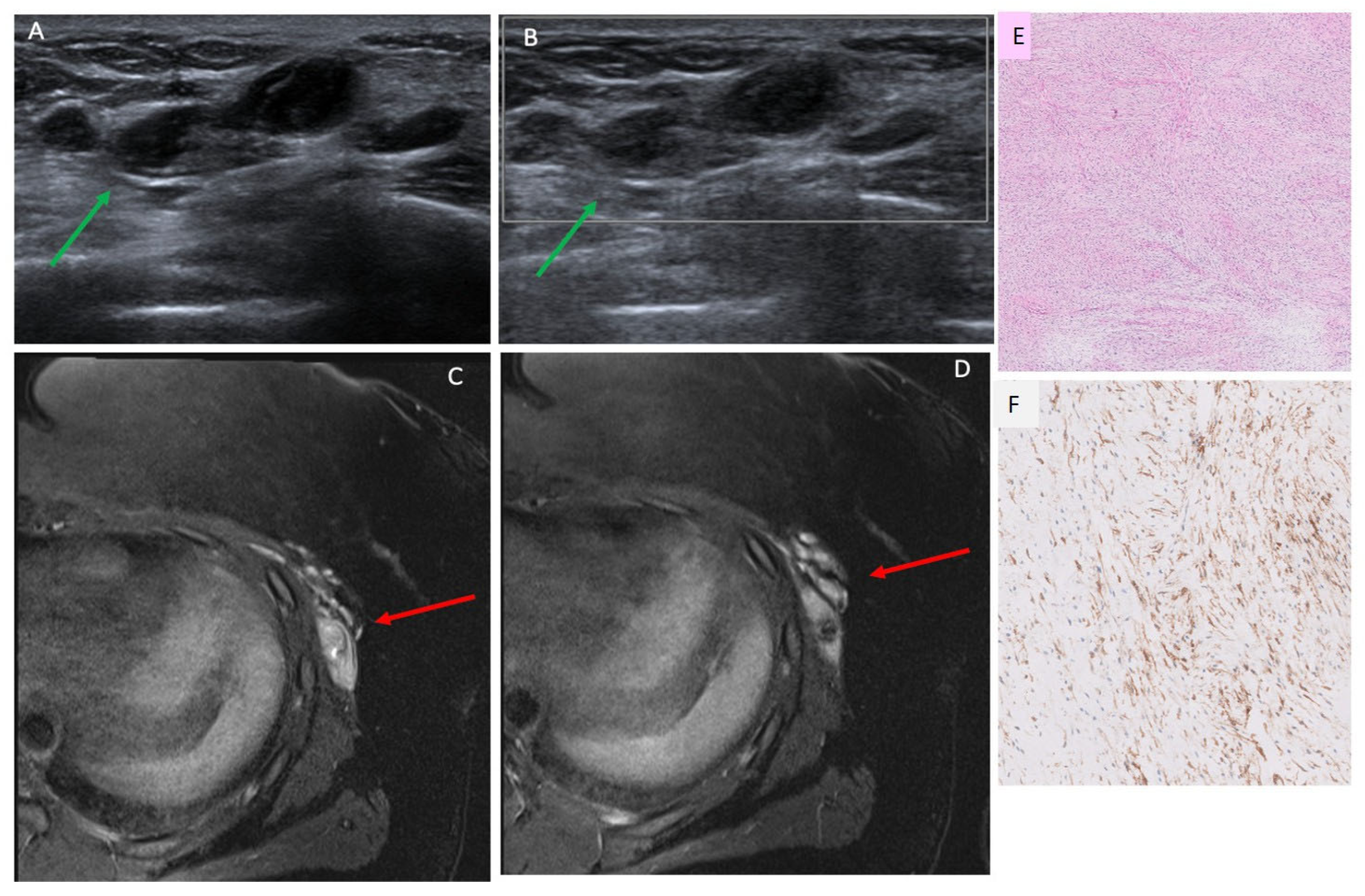
3.3. Ultrasound Findings
3.4. MRI Findings
3.5. AI Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGowan, T.S.; Cummings, B.J.; O’Sullivan, B.; Catton, C.N.; Miller, N.; Panzarella, T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Pollard, S.G.; Marks, P.V.; Temple, L.N.; Thompson, H.H. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer 1990, 66, 941–944. [Google Scholar] [CrossRef]
- Terrier, P.; Terrier-Lacombe, M.J.; Mouriesse, H.; Friedman, S.; Spielmann, M.; Contesso, G. Primary breast sarcoma: A review of 33 cases with immunohistochemistry and prognostic factors. Breast Cancer Res. Treat. 1989, 13, 39–48. [Google Scholar] [CrossRef]
- Elson, B.C.; Ikeda, D.M.; Andersson, I.; Wattsgård, C. Fibrosarcoma of the breast: Mammographic findings in five cases. AJR Am. J. Roentgenol. 1992, 158, 993–995. [Google Scholar] [CrossRef]
- Liberman, L.; Dershaw, D.D.; Kaufman, R.J.; Rosen, P.P. Angiosarcoma of the breast. Radiology 1992, 183, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Gilcrease, M.Z.; Santiago, L.; Hunt, K.K.; Yang, W.T. Imaging features of primary breast sarcoma. AJR Am. J. Roentgenol. 2012, 198, W386–W393. [Google Scholar] [CrossRef]
- Surov, A.; Holzhausen, H.J.; Ruschke, K.; Spielmann, R.P. Primary breast sarcoma: Prevalence, clinical signs, and radiological features. Acta Radiol. 2011, 52, 597–601. [Google Scholar] [CrossRef]
- Al-Benna, S.; Poggemann, K.; Steinau, H.U.; Steinstraesser, L. Diagnosis and management of primary breast sarcoma. Breast Cancer Res. Treat. 2010, 122, 619–626. [Google Scholar] [CrossRef]
- Feder, J.M.; de Paredes, E.S.; Hogge, J.P.; Wilken, J.J. Unusual breast lesions: Radiologic-pathologic correlation. Radiographics 1999, 19, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yoon, K.; Onyshchenko, M. Sarcoma of the Breast: Clinical Characteristics and Outcomes of 991 Patients from the National Cancer Database. Sarcoma 2021, 2021, 8828158. [Google Scholar] [CrossRef]
- Yin, M.; Mackley, H.B.; Drabick, J.J.; Harvey, H.A. Primary female breast sarcoma: Clinicopathological features, treatment and prognosis. Sci. Rep. 2016, 6, 31497. [Google Scholar] [CrossRef]
- Radu, I.; Scripcariu, V.; Panuța, A.; Rusu, A.; Afrăsânie, V.A.; Cojocaru, E.; Aniței, M.G.; Alexa-Stratulat, T.; Terinte, C.; Șerban, C.F.; et al. Breast Sarcomas-How Different Are They from Breast Carcinomas? Clinical, Pathological, Imaging and Treatment Insights. Diagnostics 2023, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Khaoula, M.; Sirine, B.; Sana, M.; Eya, A.; Ghada, S.; Karima, M. Primary breast sarcoma: Case report and literature review. Int. J. Surg. Case Rep. 2024, 119, 109587. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.K.; Terracina, K.P.; Soong, J.; Idowu, M.O.; Takabe, K. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014, 3, 28–34. [Google Scholar] [CrossRef]
- Farzaliyev, F.; Hamacher, R.; Steinau, H.U.; Bertram, S.; Podleska, L.E. Secondary angiosarcoma: A fatal complication of chronic lymphedema. J. Surg. Oncol. 2020, 121, 85–90. [Google Scholar] [CrossRef]
- Mesli, S.N.; Ghouali, A.K.; Benamara, F.; Taleb, F.A.; Tahraoui, H.; Abi-Ayad, C. Stewart-Treves Syndrome Involving Chronic Lymphedema after Mastectomy of Breast Cancer. Case Rep. Surg. 2017, 2017, 4056459. [Google Scholar] [CrossRef]
- Matsumoto, R.; Hsieh, S.J.K.; Chala, L.F.; de Mello, G.G.N.; de Barros, N. Sarcomas of the breast: Findings on mammography, ultrasound, and magnetic resonance imaging. Radiol. Bras. 2018, 51, 401–406. [Google Scholar] [CrossRef]
- Yoon, I.N.; Cha, E.S.; Kim, J.H.; Lee, J.E.; Chung, J. Breast Cancer after Radiation Therapy in a Patient with Li-Fraumeni Syndrome: A Case Report. Taehan Yongsang Uihakhoe Chi 2022, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cusidó, M.T.; Navarro, B.; Tresserra, F.; Baulies, S.; Ara, C.; Fabregas, R. Breast sarcoma. A case report and review of literature. Int. J. Surg. Case Rep. 2016, 24, 203–205. [Google Scholar] [CrossRef][Green Version]
- Osman, M.H.; Rabie, N.A.; Elmehrath, A.O.; Bedair, H.M.; Fala, S.Y.; Ghaith, H.S.; Refaat, M.A. Primary and Secondary Breast Sarcoma: Clinical and Pathological Characteristics, Prognostic Factors, and Nomograms for Predicting Survival. Clin. Breast Cancer 2022, 22, e753–e763. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, X.; Shi, K.; Rominger, A.; Caobelli, F. AI in Breast Cancer Imaging: An Update and Future Trends. Semin. Nucl. Med. 2025, 55, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.Ε.; Papageorgiou, D.-P.; Petmezas, G.; Dogoulis, P.; Maglaveras, N.; Tsaklidis, G. Transfer Learning-Boosted CNN for Computationally Efficient Multi-Cancer Detection; Springer: Singapore, 2025; pp. 1–12. [Google Scholar]
- Papageorgiou, V.E.; Dogoulis, P.; Papageorgiou, D.-P. A Convolutional Neural Network of Low Complexity for Tumor Anomaly Detection; Springer: Singapore, 2024; pp. 973–983. [Google Scholar]
- Papageorgiou, V. Brain Tumor Detection Based on Features Extracted and Classified Using a Low-Complexity Neural Network. Trait. Du Signal 2021, 38, 547–554. [Google Scholar] [CrossRef]
- Bahl, M.; Chang, J.M.; Mullen, L.A.; Berg, W.A. Artificial Intelligence for Breast Ultrasound: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2024, 223, e2330645. [Google Scholar] [CrossRef]
- Wanderley, M.C.; Soares, C.M.A.; Morais, M.M.M.; Cruz, R.M.; Lima, I.R.M.; Chojniak, R.; Bitencourt, A.G.V. Application of artificial intelligence in predicting malignancy risk in breast masses on ultrasound. Radiol. Bras. 2023, 56, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Fruchtman Brot, H.; Mango, V.L. Artificial intelligence in breast ultrasound: Application in clinical practice. Ultrasonography 2024, 43, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.; Yang, A.; Welch, C.; Jadav, V.; Posch, L.; Thoreson, N.; Morris, D.; Chouhdry, F.; Szabo, J.; Mendelson, D.; et al. Validating racial and ethnic non-bias of artificial intelligence decision support for diagnostic breast ultrasound evaluation. J. Med. Imaging 2023, 10, 061108. [Google Scholar] [CrossRef]
- Mango, V.L.; Sun, M.; Wynn, R.T.; Ha, R. Should We Ignore, Follow, or Biopsy? Impact of Artificial Intelligence Decision Support on Breast Ultrasound Lesion Assessment. AJR Am. J. Roentgenol. 2020, 214, 1445–1452. [Google Scholar] [CrossRef]
- Amir, T.; Coffey, K.; Reiner, J.S.; Sevilimedu, V.; Mango, V.L. Utilization of artificial intelligence to triage patients with delayed follow-up of probably benign breast ultrasound findings. Ultrasonography 2025, 44, 145–152. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A.; Bassett, L.W.; Berg, W.A.; Burnside, E.S.; Feig, S.A.; Lehman, C.D.; Newell, M.S. Breast Imaging Reporting and Data System (BI-RADS) Atlas, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Koios DSTM Breast—Koios Medical. Available online: https://koiosmedical.com/products/koios-ds-breast/ (accessed on 12 May 2025).
- Wienbeck, S.; Meyer, H.J.; Herzog, A.; Nemat, S.; Teifke, A.; Heindel, W.; Schäfer, F.; Kinner, S.; Müller-Schimpfle, M.; Surov, A. Imaging findings of primary breast sarcoma: Results of a first multicenter study. Eur. J. Radiol. 2017, 88, 1–7. [Google Scholar] [CrossRef]
- Pramanik, R.; Gogia, A.; Malik, P.S.; Gogi, R. Metastatic Primary Angiosarcoma of the Breast: Can We Tame It the Metronomic Way. Indian J. Med. Paediatr. Oncol. 2017, 38, 228–231. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, M.H.; Heo, S.H.; Kim, J.W.; Chang, N.K.; Song, S.G.; Kang, H.K. Myeloid sarcoma of the breast mimicking hamartoma on sonography. J. Ultrasound Med. 2008, 27, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- Hristov, B.; Biswas, S. A Rare Case of Spindle Cell Sarcoma Presenting as a Recurrent Breast Cyst. Clin. Oncol. 2017, 2, 1292. [Google Scholar]
- Jairaj, A.; (Koios Medical, New York, NY, USA). E-mail communication of unpublished work, 2025.
- Barinov, L.; Jairaj, A.; Becker, M.; Seymour, S.; Lee, E.; Schram, A.; Lane, E.; Goldszal, A.; Quigley, D.; Paster, L. Impact of Data Presentation on Physician Performance Utilizing Artificial Intelligence-Based Computer-Aided Diagnosis and Decision Support Systems. J. Digit. Imaging 2019, 32, 408–416. [Google Scholar] [CrossRef] [PubMed]
| PT | Age | Gender | PBS vs. SBS | Prior RT? | SBS Latency from RT (Months) | Clinical Manifestation | Histopathologic Subtype/Grade | IHC Markers | Recurrence? |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | F | SBS | Y | 13 | Palpable lump | Spindle Cell/High | P63+, ER−, PR−, Her2− | N |
| 2 | 22 | F | PBS | N | N/a | Asymmetric breast enlargement | Granulocytic/N/a | CD68+, CD34+, MPO+, lysozyme+, CD43+, CD117+ | N |
| 3 | 72 | M | PBS | N | N/a | Increased nipple sensitivity | Pleomorphic/High | Desmin+, scattered stromal cells ER+ and PR+, CD163+, SMA−, myoD−, Myf4 | N |
| 4 | 57 | F | PBS | N | N/a | Palpable lump | Spindle Cell/High | ER−, PR-Her2−, | N |
| 5 | 20 | F | PBS | N | N/a | Palpable lump | Fibromyxoid/Low | FXIII+, BCL-2+ | N |
| 6 | 65 | F | SBS | Y | 5 | Skin changes | Angiosarcoma/High | CD31+, CD34+, ERG+, C-MYC+, ER−, PR−, Her-2− | N |
| 7 | 74 | F | SBS | Y | 6 | Skin changes | Angiosarcoma/N/a | Ki67+, MYC+, | N |
| 8 | 80 | F | SBS | Y | 5 | Palpable lump | Angiosarcoma/High | N/a | N |
| 9 | 25 | F | PBS | N | N/a | Palpable lump | Angiosarcoma/Low–intermediate | N/a | N |
| 10 | 69 | F | PBS | N | N/a | Palpable lump | Angiosarcoma/N/a | N/a | N |
| 11 | 53 | F | SBS | Y | 8 | Skin changes | Angiosarcoma/N/a | ERG+, CD34+, C-MYC− | N |
| 12 | 79 | F | SBS | Y | 18 | Skin changes | Angiosarcoma/High | CD31+, C-MYC+, FLI1+ | N |
| 13 | 72 | F | SBS | Y | 7 | Palpable lump | Angiosarcoma/Low | N/a | N |
| 14 | 22 | F | PBS | N | N/a | Palpable lump | Angiosarcoma/High | ERG+, CD31+, Ki67+ (50–60%) | N |
| 15a | 40 | F | PBS | N | N/a | Palpable lump with overlying skin changes | Angiosarcoma/Intermediate–High | N/a | N |
| 15b | 41 | F | PBS | N | N/a | Palpable lump | Angiosarcoma/Intermediate–High | N/a | Y |
| 16 | 29 | F | PBS | N | N/a | Palpable lump | Angiosarcoma/High | Ki67+ (60%) | N |
| 17a | 61 | F | SBS | Y | 4 | Palpable lump with overlying skin changes | Angiosarcoma/High | N/a | N |
| 17b | 62 | F | SBS | Y | 5 | Palpable lump | Angiosarcoma/High | ERG+, CD31+, FVIII+ | Y |
| 18 | 68 | F | SBS | Y | 8 | Skin changes | Angiosarcoma/High | MYC+, ERG+. Ki67+ (75%), CD34+, F8+ | N |
| Patient | Size (cm) | Biopsy Method | MG Features | US Features | AI DS | MRI Features |
|---|---|---|---|---|---|---|
| 1 | 1.8 | Ultrasound core needle biopsy | Irregular high-density mass with indistinct margins | Irregular, non-parallel hypoechoic mass with microlobulated margins, posterior acoustic enhancement and no Doppler flow | Suspicious, 4A–4B | Irregular mass with irregular margins and heterogeneous enhancement |
| 2 | 1.4 | Ultrasound core needle biopsy | Global asymmetry | Irregular, non-parallel, heterogeneous mass with indistinct margins, posterior acoustic shadowing and Doppler flow | Suspicious, 4A–4B | N/a |
| 3 | 0.9 | Ultrasound core needle biopsy | Gynecomastia, otherwise no suspicious findings | Oval, parallel, hypoechoic mass with circumscribed margins, posterior acoustic enhancement and no Doppler flow | Suspicious, 4A–4B | N/a |
| 4 | 3.5 | Ultrasound core needle biopsy | Focal asymmetry associated with amorphous calcifications | Irregular, parallel, hypoechoic mass with indistinct margins, posterior acoustic shadowing and Doppler flow | Probably Malignant, 4C | N/a |
| 5 | 3.4 | Ultrasound core needle biopsy | N/a | Irregular, parallel, hypoechoic masses with indistinct/angular margins, posterior acoustic enhancement and no Doppler flow | Suspicious, 4A–4B | Lobulated mass with irregular margins and heterogeneous enhancement |
| 6 | N/a | Skin punch biopsy | Skin thickening only | N/a | N/a | N/a |
| 7 | 2.7 | Skin punch biopsy | N/a | Irregular, parallel, hypoechoic mass with microlobulated margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | N/a |
| 8 | N/a | Skin punch biopsy | Architectural distortion | Irregular skin thickening only | N/a | Diffuse irregular skin thickening of almost entire right breast with discontinuous areas of linear and nodular enhancement within the thickened skin |
| 9 | 2.5 | Ultrasound core needle biopsy | N/a | Oval, parallel, heterogeneous mass with indistinct margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | Irregular mass with irregular margins and heterogeneous enhancement |
| 10 | 2.5 | Ultrasound core needle biopsy | Irregular high-density mass with indistinct margins | Irregular, parallel, heterogeneous mass with indistinct margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | Irregular mass with irregular margins and heterogeneous enhancement |
| 11 | N/a | Skin punch biopsy | No suspicious findings | No suspicious findings | N/a | Skin thickening and enhancement |
| 12 | 2.9 | Ultrasound core needle biopsy | Focal asymmetry | Irregular, parallel, hyperechoic mass with obscured margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | N/a |
| 13 | 0.8 | Skin punch biopsy | N/a | Irregular, parallel, heterogeneous mass with obscured margins, posterior acoustic shadowing and no Doppler flow | Suspicious, 4A–4B | N/a |
| 14 | 7.1 | Ultrasound core needle biopsy | N/a | Irregular, parallel, heterogeneous mass with obscured margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | N/a |
| 15a | 3.7 | Ultrasound core needle biopsy | N/a | Irregular, parallel, heterogeneous mass with obscured margins, posterior acoustic shadowing and Doppler flow | Suspicious, 4A–4B | N/a |
| 15b | 0.5 | Ultrasound core needle biopsy | Focal asymmetry | Irregular, parallel, hypoechoic mass with obscured margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | Irregular mass with irregular margins and skin enhancement |
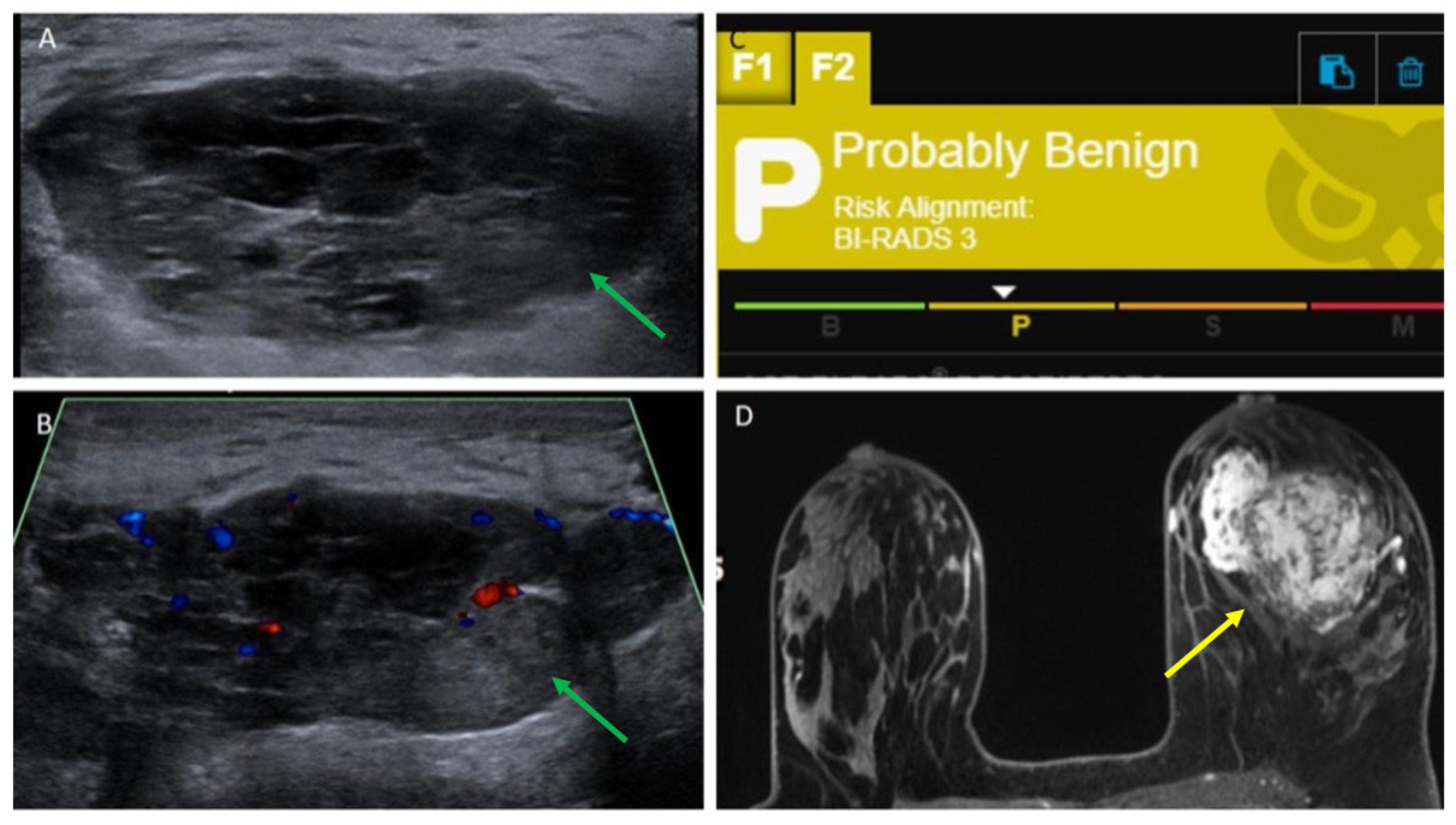
| 16 | 5.5 | Ultrasound core needle biopsy | Irregular, equal-density mass with indistinct margins | Oval, parallel, hypoechoic mass with obscured margins, posterior acoustic enhancement and Doppler flow | Probably Benign, 3 | Irregular mass with irregular margins and heterogeneous enhancement |
| 17a | 1.2 | Ultrasound core needle biopsy | Skin thickening only | Irregular, parallel, hypoechoic mass with obscured margins, posterior acoustic enhancement and Doppler flow | Suspicious, 4A–4B | N/a |
| 17b | 1.7 | Ultrasound core needle biopsy | N/a | Irregular, parallel, hypoechoic mass with indistinct margins, posterior acoustic enhancement and no Doppler flow | Suspicious, 4A–4B | N/a |
| 18 | N/a | Skin punch biopsy | Skin thickening only | No suspicious finding | N/a | Irregular skin thickening of the left breast with heterogeneously enhancing skin lesion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehran, N.A.; Rooney, E.; Shah, H.; Gomolin, T.; Zeizafoun, N.; Williams, D.; Margolies, L.R.; Chen, C. Suspicion for Sarcoma: Clinical Presentation, Multi-Modality Imaging Evaluation, and Ultrasound Artificial Intelligence-Based Decision Support. Cancers 2025, 17, 3626. https://doi.org/10.3390/cancers17223626
Mehran NA, Rooney E, Shah H, Gomolin T, Zeizafoun N, Williams D, Margolies LR, Chen C. Suspicion for Sarcoma: Clinical Presentation, Multi-Modality Imaging Evaluation, and Ultrasound Artificial Intelligence-Based Decision Support. Cancers. 2025; 17(22):3626. https://doi.org/10.3390/cancers17223626
Chicago/Turabian StyleMehran, Nikki A., Emily Rooney, Harsh Shah, Tamar Gomolin, Nebras Zeizafoun, Dayna Williams, Laurie R. Margolies, and Christine Chen. 2025. "Suspicion for Sarcoma: Clinical Presentation, Multi-Modality Imaging Evaluation, and Ultrasound Artificial Intelligence-Based Decision Support" Cancers 17, no. 22: 3626. https://doi.org/10.3390/cancers17223626
APA StyleMehran, N. A., Rooney, E., Shah, H., Gomolin, T., Zeizafoun, N., Williams, D., Margolies, L. R., & Chen, C. (2025). Suspicion for Sarcoma: Clinical Presentation, Multi-Modality Imaging Evaluation, and Ultrasound Artificial Intelligence-Based Decision Support. Cancers, 17(22), 3626. https://doi.org/10.3390/cancers17223626






