Patient Perception of the Relevance of Quality-of-Life Questionnaires in Waldenström Macroglobulinaemia: An International Survey
Simple Summary
Abstract
1. Introduction
2. Methods
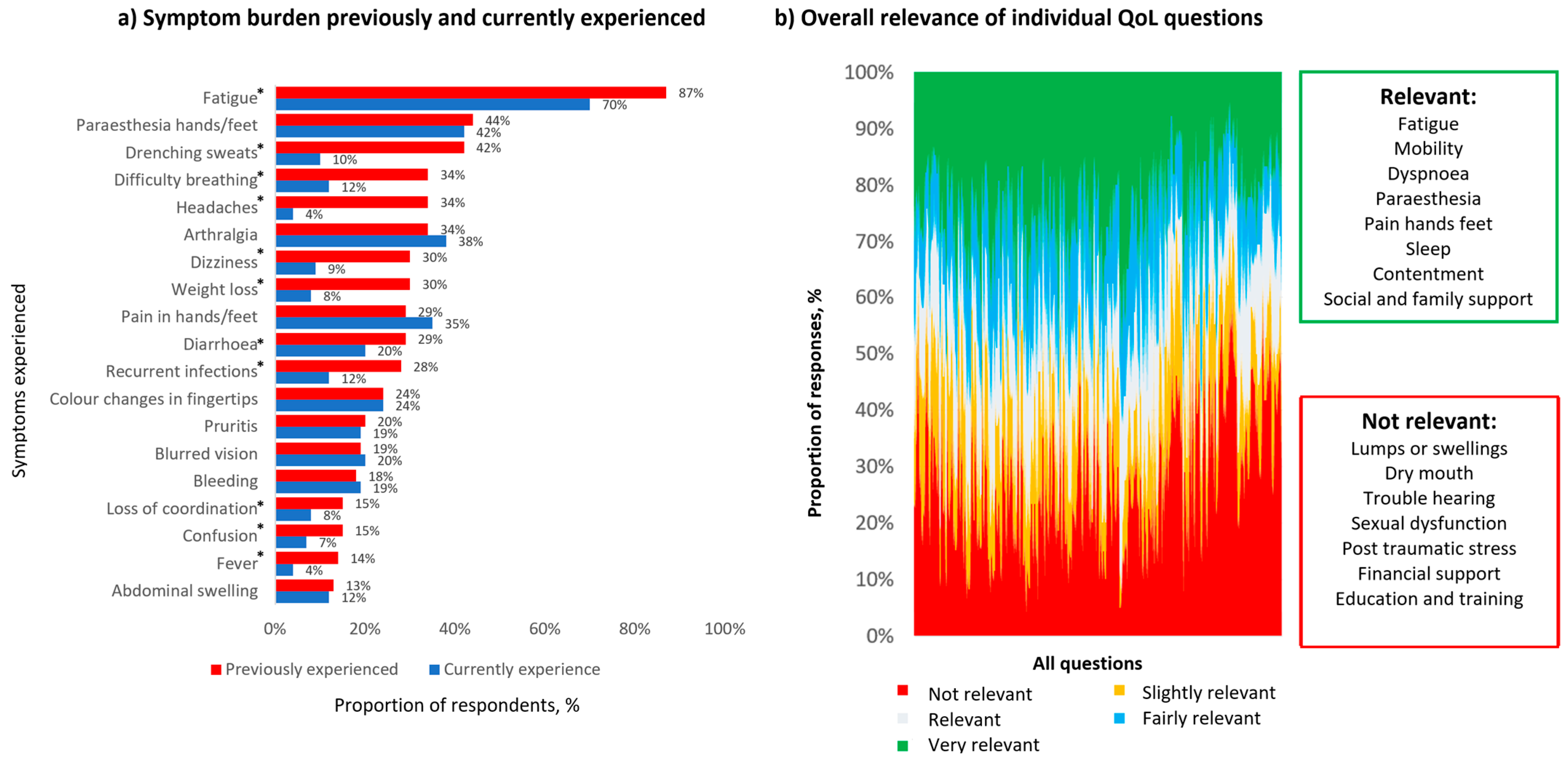
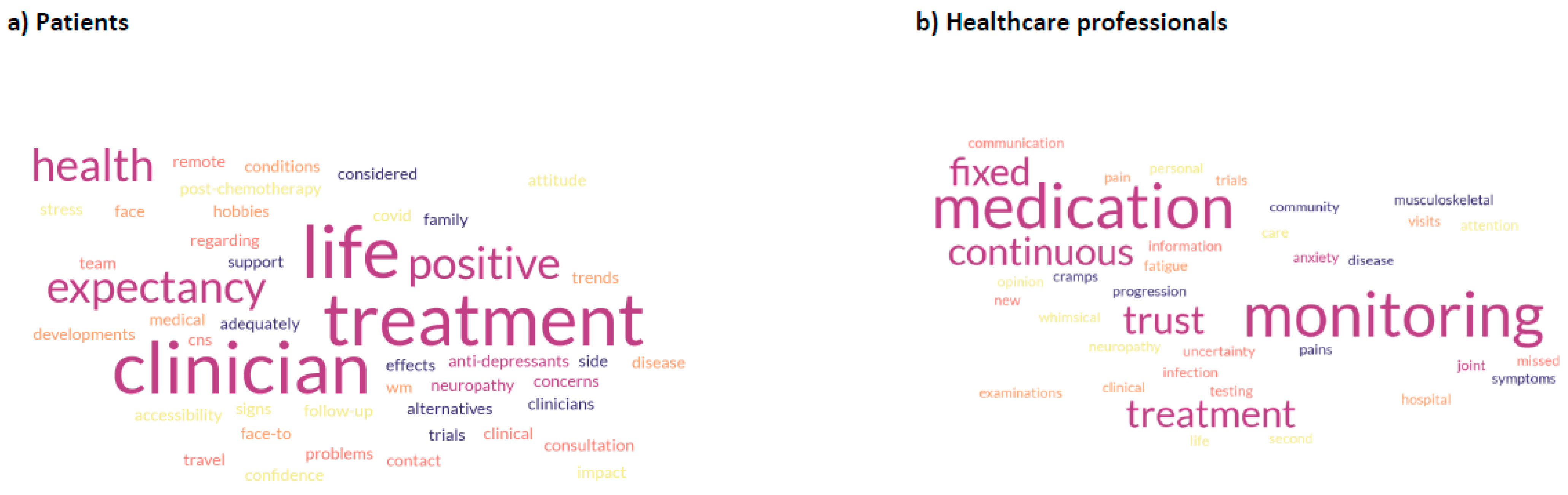
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveira Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. Correction: “The 5th edition of The World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms” Leukemia. 2022 Jul;36(7):1720-1748. Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Amaador, K.; Kersten, M.J.; Visser, O.; Brink, M.; Posthuma, E.F.M.; Minnema, M.C.; Vos, J.M.I.; Dinmohamed, A.G. Primary therapy and relative survival in patients with lymphoplasmacytic lymphoma/Waldenström macroglobulinaemia: A population-based study in the Netherlands, 1989–2018. Br. J. Haematol. 2021, 196, 660–669. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. Available online: https://hqlo.biomedcentral.com/articles/10.1186/1477-7525-4-79 (accessed on 1 August 2025). [CrossRef]
- Groenvold, M.; Klee, M.C.; Sprangers, M.A.; Aaronson, N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997, 50, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Bristogiannis, S.; Khwaja, J.; Lwin, Y.; Uppal, E.; D’Sa, S.; Kyriakou, C. Systematic literature review of quality-of-life questionnaires in Waldenström macroglobulinaemia-need for a disease-specific tool. EJHaem 2023, 4, 555–558. [Google Scholar] [CrossRef]
- van der Velden, N.C.A.; Han, P.K.J.; van Laarhoven, H.W.M.; de Vos, F.Y.F.L.; Hendriks, L.E.L.; Burgers, S.A.; Dingemans, A.C.; van Haarst, J.M.W.; Dits, J.; Smets, E.M.A.; et al. Prevalence and Predictors of Physician-Patient Discordance in Prognostic Percep-tions in Advanced Cancer. Oncologist 2023, 28, e653–e668. [Google Scholar] [CrossRef]
- Basch, E.; Abernethy, A.P.; Mullins, C.D.; Reeve, B.B.; Smith, M.L.; Coons, S.J.; Sloan, J.; Wenzel, K.; Chauhan, C.; Eppard, W.; et al. Recommendations for Incorporating Patient-Reported Outcomes Into Clinical Comparative Effectiveness Research in Adult Oncology. J. Clin. Oncol. 2012, 30, 4249–4255. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Baccarani, M.; Breccia, M.; Saussele, S.; Abel, G.; Caocci, G.; Guilhot, F.; Cocks, K.; Naeem, A.; Sprangers, M.; et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: The EORTC QLQ-CML24. Qual. Life Res. 2013, 23, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Tam, C.S.; Owen, R.G.; Buske, C.; Leblond, V.; Dimopoulos, M.; Garcia-Sanz, R.; Castillo, J.J.; Trotman, J.; Treon, S.P.; et al. Health-related quality of life in patients with Waldenström macroglobulinemia: Results from the ASPEN trial. Futur. Oncol. 2024, 20, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Amaador, K.; Nieuwkerk, P.T.; Minnema, M.C.; Kersten, M.J.; Vos, J.M.I. Patient preferences regarding treatment options for Wal-denström’s macroglobulinemia: A discrete choice experiment. Cancer Med. 2023, 12, 3376–3386. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V. Subcutaneous bortezomib: A step towards optimised drug use. Lancet Oncol. 2011, 12, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Kapoor, P.; Castillo, J.; Buske, C.; Ansell, S.; Branagan, A.; Kimby, E.; Li, Y.; Palomba, M.; Qiu, L.; et al. Report of consensus panel 7 from the 11th international workshop on Waldenström macroglobulinemia on priorities for novel clinical trials. Semin. Hematol. 2023, 60, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Morel, P.; Duhamel, A.; Gavriatopoulou, M.; Kyrtsonis, M.C.; Durot, E.; Symeonidis, A.; Laribi, K.; Hatjiharissi, E.; Ysebaert, L.; et al. A revised international prognostic score system for Waldenström’s macroglobulinemia. Leukemia 2019, 33, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Ferrari, A.; Frustaci, A.M.; Ferretti, V.V.; Rizzi, R.; Motta, M.; Piazza, F.; Merli, M.; Benevolo, G.; Visco, C.; et al. Younger patients with Waldenström Macroglobulinemia exhibit low risk profile and excellent outcomes in the era of immunotherapy and targeted therapies. Am. J. Hematol. 2020, 95, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, J.; Uppal, E.; Bristogiannis, S.; McCarthy, H.; Kothari, J.; Rismani, A.; Scorer, H.; Nicholson, J.; El-Sharkawi, D.; D’SA, S.; et al. Patient reported outcome measures in Waldenström macroglobulinaemia: A real-world data analysis from the WMUK Rory Morrison Registry. EJHaem 2022, 4, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Sadullah, S.; Kastritis, E.; Tedeschi, A.; García-Sanz, R.; Bolkun, L.; Leleu, X.; Willenbacher, W.; Hájek, R.; Minnema, M.C.; et al. Treatment and outcome patterns in European patients with Waldenström’s macroglobulinaemia: A large, observational, retrospective chart review. Lancet Haematol. 2018, 5, e299–e309. [Google Scholar] [CrossRef] [PubMed]

| Quality-of-Life Instruments | Abbreviation |
|---|---|
| EORTC Core Quality-of-Life-questionnaire | EORTC QLQ-C30 |
| EuroQol 5-Dimension 5-level | EQ-5D-5L |
| Functional Assessment of Cancer Therapy—General | FACT-G |
| Functional Assessment of Chronic Illness Therapy—Fatigue | FACIT-F |
| Functional Assessment of Cancer Therapy—Anaemia | FACT-An |
| Functional Assessment of Cancer Therapy/Gynaecologic Oncology Group—Neurotoxicity | FACT-GOG/NTx |
| Functional Assessment of Chronic Illness Therapy—Lymphoma | FACT-Lym |
| 36-Item Short Form Health Survey questionnaire | SF-36 |
| Hospital Anxiety and Depression Scale | HADS |
| Depression, Anxiety and Stress Scale—21 Items | DASS-21 |
| Quality of Life in Adult Cancer Survivors | QLACS |
| Impact of Events Scale | IES |
| Fatigue Severity Scale | FSS |
| Interpretative Phenomenological Analysis | IPA |
| Characteristic, n (%) | n = 120 Ƚ |
|---|---|
| Age, years (n = 114) <50 51–60 61–70 >70 | 5 (4) 20 (18) 47 (41) 42 (37) |
| Gender (n = 114) Male Female | 60 (53) 54 (47) |
| Country (n = 110) UK Australia France Germany USA | 87 (79) 20 (18) 1 (1) 1 (1) 1 (1) |
| ECOG Performance status (n = 113) 0 1 2 3 | 56 (50) 49 (43) 5 (4) 3 (3) |
| Prior therapy exposure (n = 93) BR DRC Rituximab single-agent BTKi * RCHOP Fludarabine-based Clinical trial Mel-ASCT Bortezomib-based Other | 31 (33) 23 (25) 14 (15) 12 (13) 7 (8) 5 (5) 11 (12) 6 (6) 3 (3) 9 (10) |
| Current therapy (n = 43) BTKi BR DRC Rituximab | 30 ǂ (70) 8 (19) 4 (9) 1 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khwaja, J.; Bristogiannis, S.; Tohidi-Esfahani, I.; Lwin, Y.; Japzon, N.; Young, D.; Delibasi, S.; D’Sa, S.; Trotman, J.; Kyriakou, C. Patient Perception of the Relevance of Quality-of-Life Questionnaires in Waldenström Macroglobulinaemia: An International Survey. Cancers 2025, 17, 3609. https://doi.org/10.3390/cancers17223609
Khwaja J, Bristogiannis S, Tohidi-Esfahani I, Lwin Y, Japzon N, Young D, Delibasi S, D’Sa S, Trotman J, Kyriakou C. Patient Perception of the Relevance of Quality-of-Life Questionnaires in Waldenström Macroglobulinaemia: An International Survey. Cancers. 2025; 17(22):3609. https://doi.org/10.3390/cancers17223609
Chicago/Turabian StyleKhwaja, Jahanzaib, Sotirios Bristogiannis, Ibrahim Tohidi-Esfahani, Yadanar Lwin, Nicole Japzon, David Young, Sossana Delibasi, Shirley D’Sa, Judith Trotman, and Charalampia Kyriakou. 2025. "Patient Perception of the Relevance of Quality-of-Life Questionnaires in Waldenström Macroglobulinaemia: An International Survey" Cancers 17, no. 22: 3609. https://doi.org/10.3390/cancers17223609
APA StyleKhwaja, J., Bristogiannis, S., Tohidi-Esfahani, I., Lwin, Y., Japzon, N., Young, D., Delibasi, S., D’Sa, S., Trotman, J., & Kyriakou, C. (2025). Patient Perception of the Relevance of Quality-of-Life Questionnaires in Waldenström Macroglobulinaemia: An International Survey. Cancers, 17(22), 3609. https://doi.org/10.3390/cancers17223609






