Hot Topics in the Surgical Treatment of Intrahepatic Cholangiocarcinoma: A Narrative Review of Current Managements
Simple Summary
Abstract
1. Introduction
- The evidence supporting obligatory margin width and the effect of anatomical vs. non-anatomical resections on survival;
- Staging and possible therapeutic indications of regional lymphadenectomy;
- Present FLR optimization methods (PVE, LVD, ALPPS) and comparative outcomes;
- Selection and method for minimally invasive and robotic hepatectomy;
- Systemic therapy in adjuvant and neoadjuvant/conversion therapy, including immune checkpoint blockade and target therapies (IDH1, FGFR2);
- The judiciously expanding role of liver transplantation;
- Recurrence, monitoring, and treatment of recurrence.
2. Epidemiology, Risk Factors, and Natural History
3. Molecular Landscape and the Surgical Interface
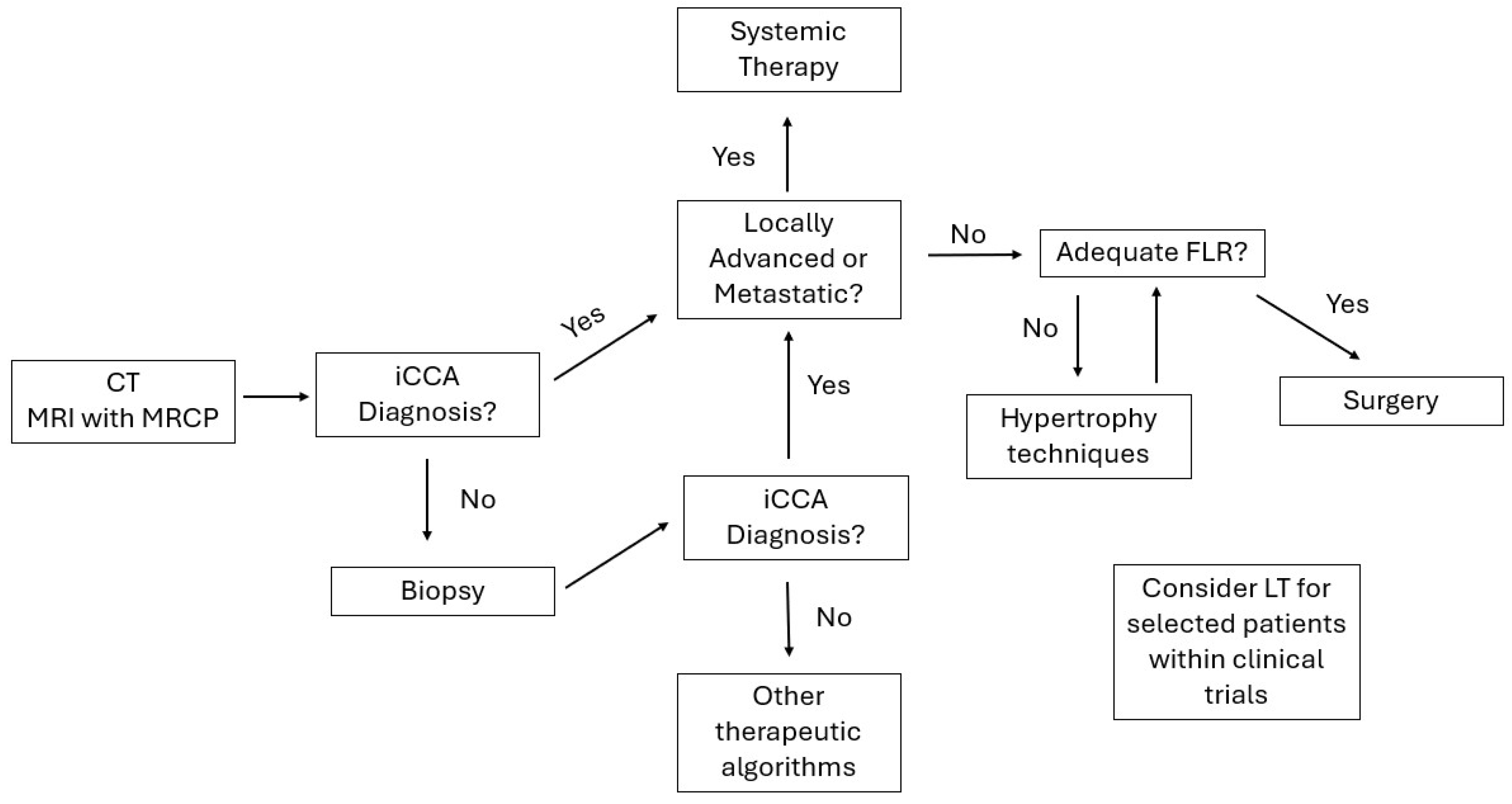
4. Diagnosis, Staging, and the Role of Staging Laparoscopy
5. Defining Resectability and Surgical Strategy
6. Margin Width: How Wide Is Wide Enough?
7. Regional Lymphadenectomy: Staging, Prognosis, and Potential Therapeutic Value
8. Optimizing the Future Liver Remnant: PVE, LVD, and ALPPS
9. Minimally Invasive and Robotic Hepatectomy
10. Complex Resections: Vascular and Biliary Reconstruction
11. Perioperative Care, ERAS, and Prevention of PHLF
12. The Role of Chemotherapy
13. Locoregional Therapies
14. Liver Transplantation: Conditional Options
15. Patterns of Recurrence, Treatment of Relapse, and Surveillance
16. Special Scenarios and Populations
- Cirrhosis and portal hypertension.
- 2.
- Elderly and frail.
- 3.
- Cholangitis and biliary sepsis.
- 4.
- Secondary hepatectomy.
17. Health Systems, Volume–Outcome Relationships, and Equity
18. Future Directions
19. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini–Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Fang, D.-Z.; Hu, Y.-T. Influence of surgical margin width on survival rate after resection of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. BMJ Open 2023, 13, e067222. [Google Scholar] [CrossRef]
- Liu, H.; Lin, L.; Lin, Z.; Chen, Y.; Huang, Q.; Ding, L.; Lou, J.; Zheng, S.; Bi, X.; Wang, J.; et al. Impact of surgical margin width on long-term outcomes for intrahepatic cholangiocarcinoma: A multicenter study. BMC Cancer 2021, 21, 840. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Zhang, X.; Beal, E.W.; Bagante, F.; Chakedis, J.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br. J. Surg. 2017, 105, 848–856. [Google Scholar] [CrossRef]
- Tan, J.C.C.; Coburn, N.G.; Baxter, N.N.; Kiss, A.; Law, C.H.L. Surgical Management of Intrahepatic Cholangiocarcinoma—A Population-Based Study. Ann. Surg. Oncol. 2007, 15, 600–608. [Google Scholar] [CrossRef]
- Si, A.; Li, J.; Yang, Z.; Xia, Y.; Yang, T.; Lei, Z.; Cheng, Z.; Pawlik, T.M.; Lau, W.Y.; Shen, F. Impact of Anatomical Versus Non-anatomical Liver Resection on Short- and Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 1841–1850. [Google Scholar] [CrossRef]
- Alvaro, D.; Gores, G.J.; Walicki, J.; Hassan, C.; Sapisochin, G.; Komuta, M.; Forner, A.; Valle, J.W.; Laghi, A.; Ilyas, S.I.; et al. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2022, 77, 659–702. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; McNamara, M.G.; A Hubner, R.; Nagino, M.; Bridgewater, J.; Primrose, J.; Valle, J.W. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat. Rev. 2020, 84, 101936. [Google Scholar] [CrossRef]
- Palmer, W.C.; Patel, T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J. Hepatol. 2012, 57, 69–76. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Ilyas, S.I.; Eaton, J.E.; Gores, G.J. Primary Sclerosing Cholangitis as a Premalignant Biliary Tract Disease: Surveillance and Management. Clin. Gastroenterol. Hepatol. 2015, 13, 2152–2165. [Google Scholar] [CrossRef]
- Giriyappa, V.H.; Bhavsar, M.S.; Vora, H.B. Choledochal cysts: A review of literature. Saudi J. Gastroenterol. 2012, 18, 230–236. [Google Scholar] [CrossRef]
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e194–e203. [Google Scholar] [CrossRef]
- Yuan, L.; Luo, X.; Lu, X.; Yi, B.; Chu, K.; Cai, Q.; Jiang, X. Comparison of clinicopathological characteristics between cirrhotic and non-cirrhotic patients with intrahepatic cholangiocarcinoma: A large-scale retrospective study. Mol. Clin. Oncol. 2017, 7, 615–622. [Google Scholar] [CrossRef][Green Version]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Graham, R.P.; Fritcher, E.G.B.; Pestova, E.; Schulz, J.; Sitailo, L.A.; Vasmatzis, G.; Murphy, S.J.; McWilliams, R.R.; Hart, S.N.; Halling, K.C.; et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum. Pathol. 2014, 45, 1630–1638. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2 -Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Ostojic, A.; Mahmud, N.; Reddy, K.R. Surgical risk stratification in patients with cirrhosis. Hepatol. Int. 2024, 18, 876–891. [Google Scholar] [CrossRef]
- Balzan, S.; Belghiti, J.; Farges, O.; Ogata, S.; Sauvanet, A.; Delefosse, D.; Durand, F. The “50-50 Criteria” on Postoperative Day 5. Ann. Surg. 2005, 242, 824–829. [Google Scholar] [CrossRef]
- Spolverato, G.; Yakoob, M.Y.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Pulitano, C.; et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 4020–4028. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 315–327. [Google Scholar] [CrossRef]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Farshidfar, F.; Zheng, S.; Gingras, M.-C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncology 2011, 17, 72–79. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Silverman, I.M.; Hollebecque, A.; Friboulet, L.; Owens, S.; Newton, R.C.; Zhen, H.; Feliz, L.; Zecchetto, C.; Melisi, D.; Burn, T.C. Clinicogenomic Analysis of FGFR2-Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discov. 2021, 11, 326–339. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Gonda, T.A.; Glick, M.P.; Sethi, A.; Poneros, J.M.; Palmas, W.; Iqbal, S.; Gonzalez, S.; Nandula, S.V.; Emond, J.C.; Brown, R.S.; et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest. Endosc. 2012, 75, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Ishiwatari, H.; Kawabata, T.; Kawashima, H.; Nakai, Y.; Miura, S.; Kato, H.; Shiomi, H.; Fujimori, N.; Ogura, T.; Inatomi, O.; et al. Endoscopic nasobiliary drainage versus endoscopic biliary stenting for preoperative biliary drainage in patients with malignant hilar biliary obstruction: Propensity score-matched multicenter comparative study. Dig. Endosc. 2023, 36, 726–734. [Google Scholar] [CrossRef]
- Maguchi, H.; Takahashi, K.; Katanuma, A.; Osanai, M.; Nakahara, K.; Matuzaki, S.; Urata, T.; Iwano, H. Preoperative biliary drainage for hilar cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 441–446. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614.e2. [Google Scholar] [CrossRef]
- Tang, R.S.Y. Endoscopic evaluation of indeterminate biliary strictures: Cholangioscopy, endoscopic ultrasound, or both? Dig. Endosc. 2023, 36, 778–788. [Google Scholar] [CrossRef]
- Pereira, P.; Santos, S.; Morais, R.; Gaspar, R.; Rodrigues-Pinto, E.; Vilas-Boas, F.; Macedo, G. Role of Peroral Cholangioscopy for Diagnosis and Staging of Biliary Tumors. Dig. Dis. 2020, 38, 431–440. [Google Scholar] [CrossRef]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Fujikura, K.; Ajiki, T.; Fukumoto, T.; Otani, K.; Azuma, T.; Itoh, T.; Ku, Y.; Zen, Y. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod. Pathol. 2017, 30, 986–997. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, H.; Yu, M.H.; Lee, E.S.; Yoon, J.H.; Joo, I.; Lee, J.M. Subtype Classification of Intrahepatic Cholangiocarcinoma Using Liver MR Imaging Features and Its Prognostic Value. Liver Cancer 2022, 11, 233–246. [Google Scholar] [CrossRef]
- Franken, L.C.; Coelen, R.J.S.; Roos, E.; Verheij, J.; Phoa, S.S.; Besselink, M.G.; Busch, O.R.; van Gulik, T.M. Staging Laparoscopy in Patients with Intrahepatic Cholangiocarcinoma: Is It Still Useful? Visc. Med. 2020, 36, 501–505. [Google Scholar] [CrossRef]
- Qin, D.; Chen, J.; Tang, Y.; Li, Z.; Geng, Z.; Wu, H.; Qiu, Y.; Song, T.; Mao, X.; He, Y.; et al. Evaluation of the outcomes of staging laparoscopy in intrahepatic cholangiocarcinoma patients undergoing surgical resection: A multicenter retrospective study in China. Hepatoma Res. 2024, 10, 40. [Google Scholar] [CrossRef]
- Ruys, A.T.; Busch, O.R.; Gouma, D.J.; van Gulik, T.M. Staging Laparoscopy for Hilar Cholangiocarcinoma: Is it Still Worthwhile? Ann. Surg. Oncol. 2011, 18, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Shoup, M.; Gonen, M.; D’ANgelica, M.; Jarnagin, W.R.; DeMatteo, R.P.; Schwartz, L.H.; Tuorto, S.; Blumgart, L.H.; Fong, Y. Volumetric Analysis Predicts Hepatic Dysfunction in Patients Undergoing Major Liver Resection. J. Gastrointest. Surg. 2003, 7, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, J.-N.; Chaoui, A.; Do, K.-A.; Bilimoria, M.M.; Fenstermacher, M.J.; Charnsangavej, C.; Hicks, M.; Alsfasser, G.; Lauwers, G.; Hawkins, I.F.; et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surgery 2000, 127, 512–519. [Google Scholar] [CrossRef]
- Rassam, F.; Olthof, P.B.; Takkenberg, B.; Besselink, M.G.; Busch, O.R.; Erdmann, J.I.; Swijnenburg, R.-J.; van Lienden, K.P.; Beuers, U.H.; Bennink, R.J.; et al. Functional assessment of liver regeneration after major hepatectomy. Hepatobiliary Surg. Nutr. 2021, 11, 530–538. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, L.; Lin, Z.; Liu, H.; Lou, J.; Zheng, S.; Bi, X.; Wang, J.; Guo, W.; Li, F.; et al. Anatomic versus non-anatomic resection for early-stage intrahepatic cholangiocarcinoma: A propensity score matching and stabilized inverse probability of treatment weighting analysis. BMC Cancer 2023, 23, 850. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Nimura, Y.; Nishio, H.; Ebata, T.; Igami, T.; Matsushita, M.; Nishikimi, N.; Kamei, Y. Hepatectomy with Simultaneous Resection of the Portal Vein and Hepatic Artery for Advanced Perihilar Cholangiocarcinoma. Ann. Surg. 2010, 252, 115–123. [Google Scholar] [CrossRef]
- Sevcik, J.; Massaro, M.S.; Palek, R.; Moulisova, V.; Liska, V. Anatomical biliary reconstruction as an ultimum refugium for selective cases—History and current state of knowledge. J. Hepato-Biliary-Pancreat. Sci. 2024, 31, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Serrablo, A.; Serrablo, L.; Alikhanov, R.; Tejedor, L. Vascular Resection in Perihilar Cholangiocarcinoma. Cancers 2021, 13, 5278. [Google Scholar] [CrossRef]
- Bennink, R.J.; Dinant, S.; Erdogan, D.; Heijnen, B.H.; Straatsburg, I.H.; Van Vliet, A.K.; Van Gulik, T.M. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J. Nucl. Med. 2004, 45, 965–971. [Google Scholar]
- Bagante, F.; Spolverato, G.; Weiss, M.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; Shen, F.; et al. Defining Long-Term Survivors Following Resection of Intrahepatic Cholangiocarcinoma. J. Gastrointest. Surg. 2017, 21, 1888–1897. [Google Scholar] [CrossRef]
- Dai, Y.-S.; Hu, H.-J.; Lv, T.-R.; Hu, Y.-F.; Zou, R.-Q.; Li, F.-Y. The influence of resection margin width in patients with intrahepatic cholangiocarcinoma: A meta-analysis. World J. Surg. Oncol. 2023, 21, 16. [Google Scholar] [CrossRef]
- Ribero, D.; Pinna, A.D.; Guglielmi, A.; Ponti, A.; Nuzzo, G.; Giulini, S.M.; Aldrighetti, L.; Calise, F.; Gerunda, G.E.; Tomatis, M.; et al. Surgical Approach for Long-term Survival of Patients with Intrahepatic Cholangiocarcinoma. Arch. Surg. 2012, 147, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef]
- Le Roy, B.; Gallon, A.; Cauchy, F.; Pereira, B.; Gagnière, J.; Lambert, C.; Yoh, T.; Boyer, L.; Pezet, D.; Buc, E.; et al. Combined biembolization induces higher hypertrophy than portal vein embolization before major liver resection. HPB 2020, 22, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right Portal Vein Ligation Combined with In Situ Splitting Induces Rapid Left Lateral Liver Lobe Hypertrophy Enabling 2-Staged Extended Right Hepatic Resection in Small-for-Size Settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Li, H.; Ren, H.; Cai, Y.; Lan, T.; Wu, H. Adequate lymph node dissection is essential for accurate nodal staging in intrahepatic cholangiocarcinoma: A population-based study. Cancer Med. 2023, 12, 8184–8198. [Google Scholar] [CrossRef]
- Lluís, N.; Asbun, D.; Wang, J.J.; Cao, H.S.T.; Jimenez, R.E.; Alseidi, A.; Asbun, H. Lymph Node Dissection in Intrahepatic Cholangiocarcinoma: A Critical and Updated Review of the Literature. J. Gastrointest. Surg. 2023, 27, 3001–3013. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Umeda, Y.; Takagi, K.; Matsuda, T.; Fuji, T.; Kojima, T.; Satoh, D.; Hioki, M.; Endo, Y.; Inagaki, M.; Oishi, M.; et al. Clinical implications and optimal extent of lymphadenectomy for intrahepatic cholangiocarcinoma: A multicenter analysis of the therapeutic index. Ann. Gastroenterol. Surg. 2022, 7, 512–522. [Google Scholar] [CrossRef]
- Hyder, O.; Marques, H.; Pulitano, C.; Marsh, J.W.; Alexandrescu, S.; Bauer, T.W.; Gamblin, T.C.; Sotiropoulos, G.C.; Paul, A.; Barroso, E.; et al. A Nomogram to Predict Long-term Survival After Resection for Intrahepatic Cholangiocarcinoma. JAMA Surg. 2014, 149, 432–438. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, F.; Tan, X.-G.; Guo, J.; Feng, W. Impact of lymph node dissection on the prognosis of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Front. Oncol. 2025, 15, 1590019. [Google Scholar] [CrossRef]
- Yasuda, K.; Kitano, S. Lymph node navigation for pancreatic and biliary malignancy by NOTES. J. Hepato-Biliary-Pancreat. Sci. 2009, 17, 617–621. [Google Scholar] [CrossRef][Green Version]
- Ribero, D.; Abdalla, E.K.; Madoff, D.C.; Donadon, M.; Loyer, E.M.; Vauthey, J. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br. J. Surg. 2007, 94, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Abulkhir, A.; Limongelli, P.; Healey, A.J.; Damrah, O.; Tait, P.; Jackson, J.; Habib, N.; Jiao, L.R. Preoperative Portal Vein Embolization for Major Liver Resection. Ann. Surg. 2008, 247, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Van Lienden, K.P.; van den Esschert, J.W.; De Graaf, W.; Bipat, S.; Lameris, J.S.; Van Gulik, T.M.; Van Delden, O.M. Portal Vein Embolization Before Liver Resection: A Systematic Review. Cardiovasc. Interv. Radiol. 2013, 36, 25–34. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Marangoni, G.; Ahmad, J.; Azoulay, D. Simultaneous portal and hepatic vein embolization is better than portal embolization or ALPPS for hypertrophy of future liver remnant before major hepatectomy: A systematic review and network meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2022, 22, 221–227. [Google Scholar] [CrossRef]
- Andriani, O.C. Long-Term Results with Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS). Ann. Surg. 2012, 256, e5. [Google Scholar] [CrossRef]
- Moris, D.; Ronnekleiv-Kelly, S.; Kostakis, I.D.; Tsilimigras, D.I.; Beal, E.W.; Papalampros, A.; Dimitroulis, D.; Felekouras, E.; Pawlik, T.M. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J. Surg. 2017, 42, 806–815. [Google Scholar] [CrossRef]
- Byrne, M.; Ruffolo, L.I.; Hernandez-Alejandro, R. ALPPS and the endless pursuit of hepatic resectability. Hepatobiliary Surg. Nutr. 2022, 11, 732–734. [Google Scholar] [CrossRef]
- Lai, Q.; Mennini, G.; Laureiro, Z.L.; Rossi, M. Uncommon indications for associating liver partition and portal vein ligation for staged hepatectomy: A systematic review. Hepatobiliary Surg. Nutr. 2021, 10, 210–225. [Google Scholar] [CrossRef]
- Ratti, F.; Maina, C.; Clocchiatti, L.; Marino, R.; Pedica, F.; Gardini, A.C.; De Cobelli, F.; Aldrighetti, L.A.M. Minimally Invasive Approach Provides Oncological Benefit in Patients with High Risk of Very Early Recurrence (VER) After Surgery for Intrahepatic Cholangiocarcinoma (iCCA). Ann. Surg. Oncol. 2024, 31, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; A Geller, D.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar]
- Chen, P.-D.; Wu, C.-Y.; Hu, R.-H.; Chen, C.-N.; Yuan, R.-H.; Liang, J.-T.; Lai, H.-S.; Wu, Y.-M. Robotic major hepatectomy: Is there a learning curve? Surgery 2017, 161, 642–649. [Google Scholar] [CrossRef]
- Hobeika, C.; Cauchy, F.; Fuks, D.; Barbier, L.; Fabre, J.M.; Boleslawski, E.; Regimbeau, J.M.; Farges, O.; Pruvot, F.R.; Pessaux, P.; et al. Laparoscopic versus open resection of intrahepatic cholangiocarcinoma: Nationwide analysis. Br. J. Surg. 2021, 108, 419–426. [Google Scholar] [CrossRef]
- Berardi, G. Minimally invasive liver resections for cancer: Moving forward. Laparosc. Surg. 2021, 5. [Google Scholar] [CrossRef]
- Nota, C.L.; Rinkes, I.H.B.; Molenaar, I.Q.; van Santvoort, H.C.; Fong, Y.; Hagendoorn, J. Robot-assisted laparoscopic liver resection: A systematic review and pooled analysis of minor and major hepatectomies. HPB 2016, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Radulova-Mauersberger, O.; Weitz, J.; Riediger, C. Vascular surgery in liver resection. Langenbeck’s Arch. Surg. 2021, 406, 2217–2248. [Google Scholar] [CrossRef]
- Franchi, E.; Donadon, M.; Torzilli, G. Effects of volume on outcome in hepatobiliary surgery: A review with guidelines proposal. Glob. Health Med. 2020, 2, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital Volume and Surgical Mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef]
- Joliat, G.; Kobayashi, K.; Hasegawa, K.; Thomson, J.; Padbury, R.; Scott, M.; Brustia, R.; Scatton, O.; Cao, H.S.T.; Vauthey, J.; et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations 2022. World J. Surg. 2022, 47, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tao, W.; Chen, Y.-Y.; Zhang, B.-H.; Tang, J.-M.; Zhong, S.; Chen, X.-X. Enhanced recovery after surgery programs versus traditional perioperative care in laparoscopic hepatectomy: A meta-analysis. Int. J. Surg. 2016, 36, 274–282. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Hall, T.; Dennison, A.; Bilku, D.; Metcalfe, M.S.; Garcea, G. Enhanced recovery programmes in hepatobiliary and pancreatic surgery: A systematic review. Ind. Mark. Manag. 2012, 94, 318–326. [Google Scholar] [CrossRef]
- Fojo, A.T.; I Neugut, A. Adjuvant Capecitabine for Biliary Cancer and the Importance of Looking Beyond P-Values. Oncol. 2023, 29, 102–105. [Google Scholar] [CrossRef]
- Edeline, J.; Hirano, S.; Bertaut, A.; Konishi, M.; Benabdelghani, M.; Uesaka, K.; Watelet, J.; Ohtsuka, M.; Hammel, P.; Kaneoka, Y.; et al. Individual patient data meta-analysis of adjuvant gemcitabine-based chemotherapy for biliary tract cancer: Combined analysis of the BCAT and PRODIGE-12 studies. Eur. J. Cancer 2022, 164, 80–87. [Google Scholar] [CrossRef]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant Therapy in the Treatment of Biliary Tract Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef]
- Pereira, M.F.I.; Buzatto, I.P.C.; Carrara, H.H.A.; Buono, F.d.O.; de Andrade, J.M.; Orlandini, L.F.; Tiezzi, D.G. Real-world data on adjuvant capecitabine after standard neoadjuvant chemotherapy for triple negative breast cancer. Rev. Bras. Hematol. Hemoter. 2024, 46, e-rbgo29. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Lindnér, P.; Rizell, M.; Hafström, L. The Impact of Changed Strategies for Patients with Cholangiocarcinoma in This Millenium. HPB Surg. 2015, 2015, 736049. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- André, T.; Tournigand, C.; Rosmorduc, O.; Provent, S.; Maindrault-Goebel, F.; Avenin, D.; Selle, F.; Paye, F.; Hannoun, L.; Houry, S.; et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: A GERCOR study. Ann. Oncol. 2004, 15, 1339–1343. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.-T.; Kitano, M.; Lee, C.-K.; Kim, J.W.; Chen, M.-H.; et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): Updated overall survival from a randomised phase 3 study. Lancet Gastroenterol. Hepatol. 2024, 9, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Zhou, T.-Y.; Zhou, G.-H.; Zhang, Y.-L.; Nie, C.-H.; Zhu, T.-Y.; Wang, H.-L.; Chen, S.-Q.; Wang, B.-Q.; Yu, Z.-N.; Wu, L.-M.; et al. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J. Cancer 2020, 11, 4534–4541. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Z.; Liu, B.; Chen, H.; Yin, T.; Zheng, J.; Li, W. Long-term outcome and prognostic nomogram for intrahepatic cholangiocarcinoma after thermal ablation: A retrospective study. Transl. Cancer Res. 2020, 9, 6743–6754. [Google Scholar] [CrossRef]
- Al-Adra, D.P.; Gill, R.S.; Axford, S.J.; Shi, X.; Kneteman, N.; Liau, S.-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: A systematic review and pooled analysis. Eur. J. Surg. Oncol. (EJSO) 2014, 41, 120–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, E.A.; Broadwell, S.R.; Bellavia, R.J.; Stein, J.P. Selective internal radiation therapy with SIR-Spheres in hepatocellular carcinoma and cholangiocarcinoma. J. Gastrointest. Oncol. 2017, 8, 266–278. [Google Scholar] [CrossRef]
- Hyder, O.; Marsh, J.W.; Salem, R.; Petre, E.N.; Kalva, S.; Liapi, E.; Cosgrove, D.; Neal, D.; Kamel, I.; Zhu, A.X.; et al. Intra-arterial Therapy for Advanced Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis. Ann. Surg. Oncol. 2013, 20, 3779–3786. [Google Scholar] [CrossRef]
- Mosconi, C.; Gramenzi, A.; Ascanio, S.; Cappelli, A.; Renzulli, M.; Pettinato, C.; Brandi, G.; Monari, F.; Cucchetti, A.; Trevisani, F.; et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: A survival, efficacy and safety study. Br. J. Cancer 2016, 115, 297–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, K.-A.; Kim, P.N. Radiofrequency Ablation for the Treatment of Primary Intrahepatic Cholangiocarcinoma. Am. J. Roentgenol. 2011, 196, W205–W209. [Google Scholar] [CrossRef]
- Pang, C.; Li, J.; Dou, J.; Li, Z.; Li, L.; Li, K.; Chen, Q.; An, C.; Zhou, Z.; He, G.; et al. Microwave ablation versus liver resection for primary intrahepatic cholangiocarcinoma within Milan criteria: A long-term multicenter cohort study. eClinicalMedicine 2024, 67, 102336. [Google Scholar] [CrossRef]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.J.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients with Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef]
- Kopek, N.; Holt, M.I.; Hansen, A.T.; Høyer, M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother. Oncol. 2010, 94, 47–52. [Google Scholar] [CrossRef]
- Massani, M.; Bonariol, L.; Stecca, T. Hepatic Arterial Infusion Chemotherapy for Unresectable Intrahepatic Cholangiocarcinoma, a Comprehensive Review. J. Clin. Med. 2021, 10, 2552. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Boerner, T.; Tan, B.R.; Chou, J.F.; Gönen, M.; Boucher, T.M.; Hauser, H.F.; Do, R.K.G.; Lowery, M.A.; Harding, J.J.; et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination with Systemic Gemcitabine and Oxaliplatin in Patients with Unresectable Intrahepatic Cholangiocarcinoma. JAMA Oncol. 2020, 6, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Z.; Wu, Y.; Gong, W.; Liao, X.; Li, X. Case report: Conversion therapy for advanced intrahepatic cholangiocarcinoma using PD-1 inhibitor plus S-1 and nab-paclitaxel. Front. Oncol. 2022, 12, 935817. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A phase 2 clinical trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- E Lunsford, K.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar] [CrossRef]
- De Martin, E.; Rayar, M.; Golse, N.; Dupeux, M.; Gelli, M.; Gnemmi, V.; Allard, M.A.; Cherqui, D.; Cunha, A.S.; Adam, R.; et al. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transplant. 2020, 26, 785–798. [Google Scholar] [CrossRef]
- Krendl, F.J.; Bellotti, R.; Sapisochin, G.; Schaefer, B.; Tilg, H.; Scheidl, S.; Margreiter, C.; Schneeberger, S.; Oberhuber, R.; Maglione, M. Transplant oncology—Current indications and strategies to advance the field. JHEP Rep. 2023, 6, 100965. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, H.; Xu, R.; Zhu, J.; Zhu, Y.; Zou, H. Comparative prognosis of liver transplantation versus liver resection in intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Chin. Clin. Oncol. 2025, 14, 3. [Google Scholar] [CrossRef]
- Safdar, N.Z.; Hakeem, A.R.; Faulkes, R.; James, F.; Mason, L.; Masson, S.; Powell, J.; Rowe, I.; Shetty, S.; Jones, R.; et al. Outcomes After Liver Transplantation with Incidental Cholangiocarcinoma. Transpl. Int. 2022, 35, 10802. [Google Scholar] [CrossRef]
- Luvira, V.; Eurboonyanun, C.; Bhudhisawasdi, V.; Pugkhem, A.; Pairojkul, C.; Luvira, V.; Sathitkarnmanee, E.; Somsap, K.; Kamsa-Ard, S. Patterns of Recurrence after Resection of Mass-Forming Type Intrahepatic Cholangiocarcinomas. Asian Pac. J. Cancer Prev. 2016, 17, 4735–4739. [Google Scholar] [CrossRef]
- Holzner, M.L.; Mazzaferro, V.; Busset, M.D.D.; Aldrighetti, L.; Ratti, F.; Hasegawa, K.; Arita, J.; Sapisochin, G.; Abreu, P.; Schoning, W.; et al. Is Repeat Resection for Recurrent Intrahepatic Cholangiocarcinoma Warranted? Outcomes of an International Analysis. Ann. Surg. Oncol. 2024, 31, 4397–4404. [Google Scholar] [CrossRef]
- Holster, J.J.; El Hassnaoui, M.; Franssen, S.; Ijzermans, J.N.M.; de Jonge, J.; Mostert, B.; Polak, W.G.; de Wilde, R.F.; Homs, M.Y.V.; Koerkamp, B.G. Hepatic Arterial Infusion Pump Chemotherapy for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2022, 29, 5528–5538. [Google Scholar] [CrossRef]
- Borakati, A.; Froghi, F.; Bhogal, R.H.; Mavroeidis, V.K. Stereotactic radiotherapy for intrahepatic cholangiocarcinoma. World J. Gastrointest. Oncol. 2022, 14, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Facciuto, M.; Rubbia-Brandt, L.; Marti, J.; Mehta, N.; Yao, F.; Vibert, E.; Cherqui, D.; Grant, D.; Hernandez-Alejandro, R.; et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016, 64, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Arrichiello, G.; Nacca, V.; Paragliola, F.; Giunta, E.F. Liquid biopsy in biliary tract cancer from blood and bile samples: Current knowledge and future perspectives. Explor. Target. Anti-Tumor Ther. 2022, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Nobrega, M.; Ferrier, T.; Dickinson, K.; Kaorey, N.; Nadeau, A.; Castillo, A.; Burnier, J.V. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. npj Precis. Oncol. 2025, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mei, W.; Ma, K.; Zeng, C. Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives. Front. Oncol. 2021, 11, 763790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, L.; Wang, M.; He, X.; Xu, W.; Zhu, W.; Zhao, Y.; Wang, L. Prognostic value of resection margin length after surgical resection for intrahepatic cholangiocarcinoma. Am. J. Surg. 2021, 222, 383–389. [Google Scholar] [CrossRef]
| TRIAL | TRIAL ID | STUDY | INCLUSION CRITERIA | PRE-TRANSPLANT | TYPE OF | PRIMARY | STATUS |
|---|---|---|---|---|---|---|---|
| LIRICA | NCT06098547 | Prospective, Exploratory, Single-arm | Unresectable, histologically confirmed iCCA; Stable or partial response after ≥6 months of chemotherapy; No extrahepatic or nodal disease | Gemcitabine + Cisplatin ≥ 6 months; PET-MRI; Staging laparotomy with lymphadenectomy | Deceased or living donor | 3-year and 5-year OS; DFS; Recurrence; Morbidity; QoL. | Ongoing Estimated end: 2028 |
| LIVINCA | NCT06539377 | Prospective, Non-randomized | Unresectable iCCA, stable or regressed after chemotherapy and/or locoregional therapy | GemCIS or FOLFOX; SIRT (Y-90 radioembolization) or ablation | Living donor only | Safety; DFS; OS; Dropout rate. | Ongoing Estimated end: 2030 |
| TESLA | NCT04556214 | Exploratory, Monocentric, Open-label | Unresectable iCCA, no vascular invasion, no extrahepatic or nodal metastases; Disease stability ≥ 6 months on systemic or locoregional therapy | GemCis or FOLFOX and/or SIRT or local ablation | Non-specified | OS from screening; OS from recurrence; DFS. | Ongoing Estimated end: 2030 |
| TORONTO (Early iCCA) | NCT02878473 | Prospective, Single-arm | iCCA ≤ 2 cm in cirrhotic patients; No extrahepatic spread; Negative lymph nodes | Active surveillance or selective downstaging | Deceased or living donor | 5-year OS and cumulative recurrence risk. | Ongoing Estimated end: 2029 |
| TORONTO (Advanced/ Stable iCCA) | NCT04195503 | Prospective, Single-arm, Open-label | Advanced unresectable iCCA; Disease stability ≥ 6 months under chemotherapy; No nodal or extrahepatic metastases | Gemcis or alternative regimens; Imaging every 3 months | Living donor only | 1-year and 5-year DFS and OS. | Ongoing Estimated end: 2031 |
| iCOLA | NCT06862934 | Multicentric | Unresectable iCCA with stable disease or response after downstaging with systemic and locoregional therapy | Chemotherapy + Radioembolization (Y-90); Patient selection post-treatment | Non-specified | 2-year and 5-year DFS and OS; LT conversion rate | Ongoing Estimated end: 2027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caringi, S.; Delvecchio, A.; Casella, A.; Ferraro, V.; Dezio, M.; Marini, S.; Calbi, R.; Cortese, F.; Filippo, R.; Stasi, M.; et al. Hot Topics in the Surgical Treatment of Intrahepatic Cholangiocarcinoma: A Narrative Review of Current Managements. Cancers 2025, 17, 3127. https://doi.org/10.3390/cancers17193127
Caringi S, Delvecchio A, Casella A, Ferraro V, Dezio M, Marini S, Calbi R, Cortese F, Filippo R, Stasi M, et al. Hot Topics in the Surgical Treatment of Intrahepatic Cholangiocarcinoma: A Narrative Review of Current Managements. Cancers. 2025; 17(19):3127. https://doi.org/10.3390/cancers17193127
Chicago/Turabian StyleCaringi, Silvio, Antonella Delvecchio, Annachiara Casella, Valentina Ferraro, Michele Dezio, Stefania Marini, Roberto Calbi, Francesco Cortese, Rosalinda Filippo, Matteo Stasi, and et al. 2025. "Hot Topics in the Surgical Treatment of Intrahepatic Cholangiocarcinoma: A Narrative Review of Current Managements" Cancers 17, no. 19: 3127. https://doi.org/10.3390/cancers17193127
APA StyleCaringi, S., Delvecchio, A., Casella, A., Ferraro, V., Dezio, M., Marini, S., Calbi, R., Cortese, F., Filippo, R., Stasi, M., Manzia, T. M., Tedeschi, M., Inchingolo, R., & Memeo, R. (2025). Hot Topics in the Surgical Treatment of Intrahepatic Cholangiocarcinoma: A Narrative Review of Current Managements. Cancers, 17(19), 3127. https://doi.org/10.3390/cancers17193127







