RBM39 Contributes to MGMT Maintenance in Response to Temozolomide-Induced DNA Damage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Chemicals, and Antibodies
2.2. Clonogenic Assay
2.3. Western Blot Analysis
2.4. siRNA Knockdown
3. Results
3.1. Snap-Capture Magnetic Beads Enable Affinity-Based Isolation of Active MGMT Complexes and Reveal Nuclear Interactors Following Temozolomide Treatment
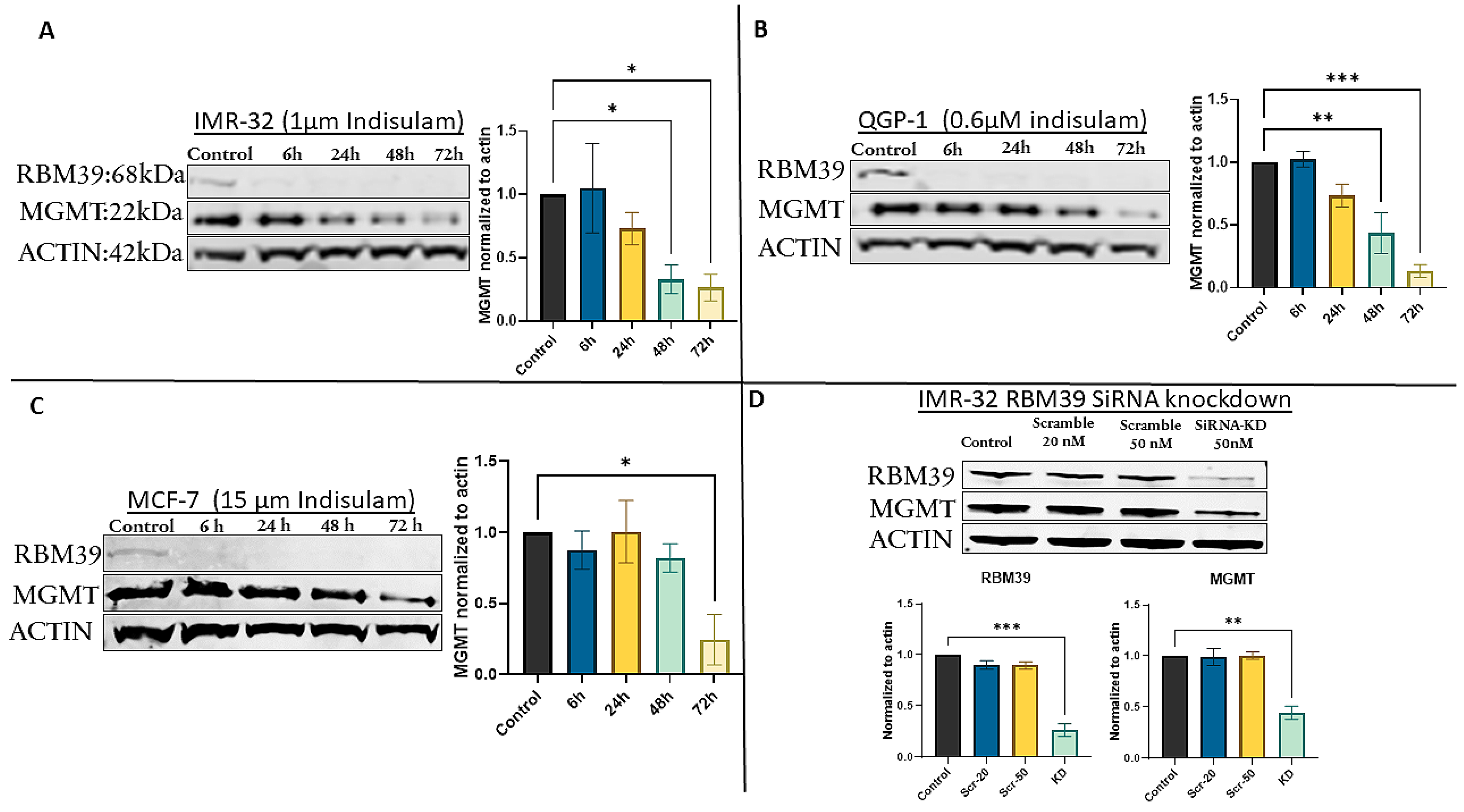
3.2. Indisulam Triggers Early RBM39 Depletion and Subsequent MGMT Suppression
3.3. siRNA-Mediated RBM39 Knockdown Confirms MGMT Downregulation Is a Direct Consequence of RBM39 Loss
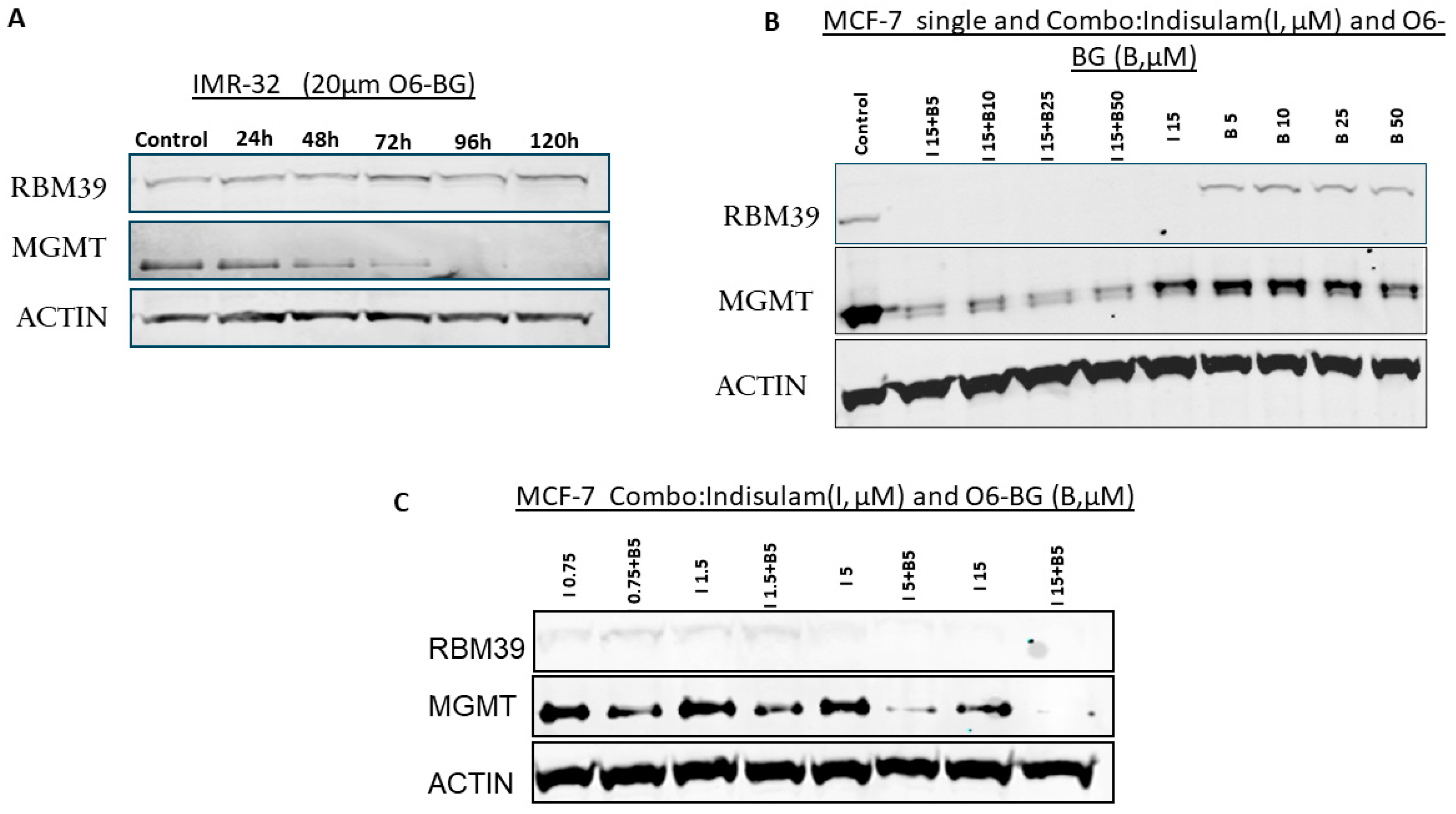
3.4. MGMT Inhibition Does Not Affect RBM39 Expression
3.5. Combination Treatment with Indisulam and O6-BG Synergistically Depletes MGMT
3.6. Co-Treatment with Indisulam and TMZ Enhances Apoptosis and Inhibits Clonogenic Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Obaide, M.A.I.; Srivenugopal, K.S. Transcriptional Pausing and Activation at Exons-1 and -2, Respectively, Mediate the MGMT Gene Expression in Human Glioblastoma Cells. Genes 2021, 12, 888. [Google Scholar] [CrossRef]
- Hazra, T.K.; Roy, R.; Biswas, T.; Grabowski, D.T.; Pegg, A.E.; Mitra, S. Specific recognition of O6-methylguanine in DNA by active site mutants of human O6-methylguanine-DNA methyltransferase. Biochemistry 1997, 36, 5769–5776. [Google Scholar] [CrossRef]
- Toorchen, D.; Lindamood, C.; Swenberg 3rd, J.A.; Topal, M.D. O6-Methylguanine-DNA transmethylase converts O6-methylguanine thymine base pairs to guanine thymine base pairs in DNA. Carcinogenesis 1984, 5, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Srivenugopal, K.S.; Yuan, X.H.; Friedman, H.S.; Ali-Osman, F. Ubiquitination-dependent proteolysis of O6-methylguanine-DNA methyltransferase in human and murine tumor cells following inactivation with O6-benzylguanine or 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochemistry 1996, 35, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, B.; Southgate, T.D.; Gilham, D.E.; Margisonet, G.P. O6-Methylguanine-DNA methyltransferase inactivation and chemotherapy. Br. Med. Bull. 2008, 85, 17–33. [Google Scholar] [CrossRef]
- Xu-Welliver, M.; Pegg, A.E. Degradation of the alkylated form of the DNA repair protein, O6-alkylguanine-DNA alkyltransferase. Carcinogenesis 2002, 23, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Barciszewska, A.M.; Gurda, D.; Głodowicz, P.; Nowak, S.; Naskręt-Barciszewskaet, M.Z. A New Epigenetic Mechanism of Temozolomide Action in Glioma Cells. PLoS ONE 2015, 10, e0136669. [Google Scholar] [CrossRef]
- Tano, K.; Shiota, S.; Collier, J.; Foote, S.; Mitra, S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc. Natl. Acad. Sci. USA 1990, 87, 686–690. [Google Scholar] [CrossRef]
- Koumarianou, A.; Kaltsas, G.; Kulke, M.H.; Oberg, K.; Strosberg, J.R.; Spada, F.; Galdy, S.; Barberis, M.; Fumagalli, C.; Berruti, A.; et al. Temozolomide in Advanced Neuroendocrine Neoplasms: Pharmacological and Clinical Aspects. Neuroendocrinology 2015, 101, 274–288. [Google Scholar] [CrossRef]
- Pandith, A.A.; Qasim, I.; Zahoor, W.; Shah, P.; Bhat, A.R.; Sanadhya, D.; Shah, Z.A.; Naikoo, N.A. Concordant association validates MGMT methylation and protein expression as favorable prognostic factors in glioma patients on alkylating chemotherapy (Temozolomide). Sci. Rep. 2018, 8, 6704. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Monika, E.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef]
- Kitange, G.J.; Carlson, B.L.; Schroeder, M.A.; Grogan, P.T.; Lamont, J.D.; Decker, P.A.; Wu, W.; James, C.D.; Sarkaria, J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009, 11, 281–291. [Google Scholar] [CrossRef]
- Ortiz, R.; Perazzoli, G.; Cabeza, L.; Jiménez-Luna, C.; Luque, R.; Prados, J.; Melguizo, C. Temozolomide: An Updated Overview of Resistance Mechanisms, Nanotechnology Advances and Clinical Applications. Curr. Neuropharmacol. 2021, 19, 513–537. [Google Scholar]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair, and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lou, X.; Wang, F.; Zhang, W.; Xu, X.; Ye, Z.; Zhuo, Q.; Wang, Y.; Jing, D.; Fan, G.; et al. MEN1 Deficiency-Driven Activation of the β-Catenin-MGMT Axis Promotes Pancreatic Neuroendocrine Tumor Growth and Confers Temozolomide Resistance. Adv. Sci. 2024, 11, e2308417. [Google Scholar] [CrossRef] [PubMed]
- Brighi, N.; Lamberti, G.; Andrini, E.; Mosconi, C.; Manuzzi, L.; Donati, G.; Lisotti, A.; Campana, D. Prospective Evaluation of MGMT-Promoter Methylation Status and Correlations with Outcomes to Temozolomide-Based Chemotherapy in Well-Differentiated Neuroendocrine Tumors. Curr. Oncol. 2023, 30, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Tacelli, M.; Gentiluomo, M.; Biamonte, P.; Castano, J.P.; Berković, M.C.; Cives, M.; Kapitanović, S.; Marinoni, I.; Marinovic, S.; Nikas, I.; et al. Pancreatic neuroendocrine neoplasms (pNENs): Genetic and environmental biomarkers for risk of occurrence and prognosis. Semin. Cancer Biol. 2025, 112, 112–125. [Google Scholar] [CrossRef]
- Yagi, K.; Ono, H.; Kudo, A.; Kinowaki, Y.; Asano, D.; Watanabe, S.; Ishikawa, Y.; Ueda, H.; Akahoshi, K.; Tanaka, S.; et al. MGMT is frequently inactivated in pancreatic NET-G2 and is associated with the therapeutic activity of STZ-based regimens. Sci. Rep. 2023, 13, 7535. [Google Scholar] [CrossRef]
- Kulke, M.H.; Hornick, J.L.; Frauenhoffer, C.; Hooshmand, S.; Ryan, D.P.; Enzinger, P.C.; Meyerhardt, J.A.; Clark, J.W.; Stuart, K.; Fuchs, C.S.; et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin. Cancer Res. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Trillo Aliaga, P.; Spada, F.; Peveri, G.; Bagnardi, V.; Fumagalli, C.; Laffi, A.; Rubino, M.; Gervaso, L.; Guerini Rocco, E.; Pisa, E.; et al. Should temozolomide be used on the basis of O6-methylguanine DNA methyltransferase status in patients with advanced neuroendocrine tumors? A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 99, 102261. [Google Scholar] [CrossRef]
- Sun, G.; Bai, P.; Fan, T.; Zhao, L.; Zhong, R.; McElhinney, R.S.; McMurry, T.B.H.; Donnelly, D.J.; McCormick, J.E.; Kelly, J.; et al. QSAR and Chemical Read-Across Analysis of 370 Potential MGMT Inactivators to Identify the Structural Features Influencing Inactivation Potency. Pharmaceutics 2023, 15, 2170. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, L.; Zhong, R.; Peng, Y. The specific role of O6-methylguanine-DNA methyltransferase inhibitors in cancer chemotherapy. Future Med. Chem. 2018, 10, 1971–1996. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.A.; Jiang, S.X.; Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Rich, J.N.; Gururangan, S.; Friedman, A.H.; Bigner, D.D.; Sampson, J.H.; et al. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J. Clin. Oncol. 2009, 27, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Ranson, M.; Middleton, M.R.; Bridgewater, J.; Lee, S.M.; Dawson, M.; Jowle, D.; Halbert, G.; Waller, S.; McGrath, H.; Gumbrell, L.; et al. Lomeguatrib, a potent inhibitor of O6-alkylguanine-DNA-alkyltransferase: Phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 2006, 12, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, G.; Ye, S.; Tian, S.; Li, H.; Zuo, F.; Wan, J.; Cai, H. Regulatory mechanisms of O6-methylguanine methyltransferase expression in glioma cells. Sci. Prog. 2025, 108, 368504251345014. [Google Scholar] [CrossRef]
- Gonzalez-Aponte, M.F.; Damato, A.R.; Trebucq, L.L.; Simon, T.; Cárdenas-García, S.P.; Cho, K.; Patti, G.J.; Golombek, D.A.; Chiesa, J.J.; Rubin, J.B.; et al. Circadian regulation of MGMT expression and promoter methylation underlies daily rhythms in TMZ sensitivity in glioblastoma. J. Neuro-Oncol. 2024, 166, 419–430. [Google Scholar] [CrossRef]
- Tancredi, A.; Gusyatiner, O.; Bady, P.; Buri, M.C.; Lomazzi, R.; Chiesi, D.; Messerer, M.; Hegi, M.E. BET protein inhibition sensitizes glioblastoma cells to temozolomide treatment by attenuating MGMT expression. Cell Death Dis. 2022, 13, 1037. [Google Scholar] [CrossRef]
- Cropper, J.D.; Alimbetov, D.S.; Brown, K.T.G.; Likhotvorik, R.I.; Robles, A.J.; Guerra, J.T.; He, B.; Chen, Y.; Kwon, Y.; Kurmasheva, R.T. PARP1-MGMT complex underpins pathway crosstalk in O6-methylguanine repair. J. Hematol. Oncol. 2022, 15, 146. [Google Scholar] [CrossRef]
- Khalaj, V.; AghaAmiri, S.; Ghosh, S.C.; Vargas, S.H.; Momeny, M.; Azhdarinia, A. A simple and rapid in vitro assay for identification of direct inhibitors of O6-methylguanine-DNA methyltransferase. Biotechniques 2024, 76, 343–351. [Google Scholar] [CrossRef]
- Xu, Y.; Spear, S.; Ma, Y.; Lorentzen, M.P.; Gruet, M.; McKinney, F.; Xu, Y.; Wickremesinghe, C.; Shepherd, M.R.; McNeish, I.; et al. Pharmacological depletion of RNA splicing factor RBM39 by indisulam synergizes with PARP inhibitors in high-grade serous ovarian carcinoma. Cell Rep. 2023, 42, 113307. [Google Scholar] [CrossRef]
- Bussiere, D.E.; Xie, L.; Srinivas, H.; Shu, W.; Burke, A.; Be, C.; Zhao, J.; Godbole, A.; King, D.; Karki, R.G.; et al. Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Nat. Chem. Biol. 2020, 16, 15–23. [Google Scholar] [CrossRef]
- Nijhuis, A.; Sikka, A.; Yogev, O.; Herendi, L.; Balcells, C.; Ma, Y.; Poon, E.; Eckold, C.; Valbuena, G.N.; Xu, Y.; et al. Indisulam targets RNA splicing and metabolism to serve as a therapeutic strategy for high-risk neuroblastoma. Nat. Commun. 2022, 13, 1380. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Goralski, M.; Gaskill, N.; Capota, E.; Kim, J.; Ting, T.C.; Xie, Y.; Williams, N.S.; Nijhawan, D. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 2017, 356, 6336. [Google Scholar] [CrossRef]
- Faust, T.B.; Yoon, H.; Nowak, R.P.; Donovan, K.A.; Li, Z.; Cai, Q.; Eleuteri, N.A.; Zhang, T.; Gray, N.S.; Fischer, E.S. Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nat. Chem. Biol. 2020, 16, 7–14. [Google Scholar] [CrossRef]
- Bobustuc, G.C.; Kassam, A.B.; Rovin, R.A.; Jeudy, S.; Smith, J.S.; Isley, B.; Singh, M.; Sassoon, D.; Srivenugopal, K.S.; Konduri, S.D. MGMT inhibition in ER positive breast cancer leads to CDC2, TOP2A, AURKB, CDC20, KIF20A, Cyclin A2, Cyclin B2, Cyclin D1, ERα and Survivin inhibition and enhances response to temozolomide. Oncotarget 2018, 9, 29727–29742. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; Middleton, M.R.; McGown, G.; Thorncroft, M.; Ranson, M.; Herse, P.; McArthur, G.; Davis, I.D.; Thomson, D.; Beith, J.; et al. O6-methylguanine-DNA methyltransferase depletion and DNA damage in patients with melanoma treated with temozolomide alone or with lomeguatrib. Br. J. Cancer 2009, 100, 1250–1256. [Google Scholar] [CrossRef]
- Mai, S.; Qu, X.; Li, P.; Ma, Q.; Cao, C.; Liu, X. Global regulation of alternative RNA splicing by the SR-rich protein RBM39. Biochim. Biophys Acta 2016, 1859, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Yang, Y.; Yu, J.; Yin, H.; Chu, X.; Yang, P.; Xu, L.; Wang, X.; Hu, S.; Li, Y.; et al. Targeting RBM39 through indisulam induced mis-splicing of mRNA to exert anti-cancer effects in T-cell acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2024, 43, 205. [Google Scholar] [CrossRef]
- Bai, P.; Fan, T.; Sun, G.; Wang, X.; Zhao, L.; Zhong, R. The dual role of DNA repair protein MGMT in cancer prevention and treatment. DNA Repair. 2023, 123, 103449. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Wei, Q.; Shao, A. O6-Methylguanine-DNA Methyltransferase (MGMT): Challenges and New Opportunities in Glioma Chemotherapy. Front. Oncol. 2019, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, Y.; Xu, X.; Xu, G.; Wu, Z.; Wu, Q.; Zeng, Q.; Yang, J.; Lv, T.; Yang, J. RBM39 promotes hepatocarcinogenesis by regulating RFX1’s alternative splicing and subsequent activation of integrin signaling pathway. Oncogene 2025, 44, 1488–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.; Liu, X.; Nie, Z.; Liu, M.; Wang, T.; Lu, Y.; Pan, Y.; Zhan, Y.; Wang, Z.; et al. Regulatory role of RBM39 in acute myeloid leukemia: Mediation through the PI3K/AKT pathway. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119607. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Yang, Z.; Chen, Z.; Jiang, H.; Wang, Y.; Shen, D.; Su, G. RBM39 Functions as a Potential Oncogene Through the NF-κB Signaling Pathway in Colorectal Cancer Cells. J. Cancer 2025, 16, 2233–2249. [Google Scholar] [CrossRef]
- Wang, X.; Jia, L.; Jin, X.; Liu, Q.; Cao, W.; Gao, X.; Yang, M.; Sun, B. NF-κB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol. Lett. 2015, 9, 2586–2590. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, W.; Zhang, N.; Chen, X.; Liu, W.; Zhang, L.; Liu, N. Systematic pan-cancer analysis identifies RBM39 as an immunological and prognostic biomarker. J. Cell Mol. Med. 2022, 26, 4859–4871. [Google Scholar] [CrossRef]
- Medarova, Z.; Pantazopoulos, P.; Yoo, B. Screening of potential miRNA therapeutics for the prevention of multi-drug resistance in cancer cells. Sci. Rep. 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Talbot, D.; Norbury, C.; Slade, M.; von Pawel, J.; Bosquee, L.; Ellis, P.A.; Gatzemeier, U.; Ravic, M. A phase II and pharmacodynamic study of E7070 in patients with non-small cell lung cancer (NSCLC) who have failed platinum-based chemotherapy. Proc. Am. Soc. Clin. Oncol. 2002, 21, 327a. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaj, V.; Adams, J.T.; AghaAmiri, S.; Hernandez Vargas, S.; Bateman, T.M.; Ghosh, S.C.; Momeny, M.; Azhdarinia, A. RBM39 Contributes to MGMT Maintenance in Response to Temozolomide-Induced DNA Damage. Cancers 2025, 17, 3604. https://doi.org/10.3390/cancers17223604
Khalaj V, Adams JT, AghaAmiri S, Hernandez Vargas S, Bateman TM, Ghosh SC, Momeny M, Azhdarinia A. RBM39 Contributes to MGMT Maintenance in Response to Temozolomide-Induced DNA Damage. Cancers. 2025; 17(22):3604. https://doi.org/10.3390/cancers17223604
Chicago/Turabian StyleKhalaj, Vahid, Jack T. Adams, Solmaz AghaAmiri, Servando Hernandez Vargas, Tyler M. Bateman, Sukhen C. Ghosh, Majid Momeny, and Ali Azhdarinia. 2025. "RBM39 Contributes to MGMT Maintenance in Response to Temozolomide-Induced DNA Damage" Cancers 17, no. 22: 3604. https://doi.org/10.3390/cancers17223604
APA StyleKhalaj, V., Adams, J. T., AghaAmiri, S., Hernandez Vargas, S., Bateman, T. M., Ghosh, S. C., Momeny, M., & Azhdarinia, A. (2025). RBM39 Contributes to MGMT Maintenance in Response to Temozolomide-Induced DNA Damage. Cancers, 17(22), 3604. https://doi.org/10.3390/cancers17223604





