Association Between MMR Status and Prognostic Pathological Factors in Endometrioid Endometrial Cancer—A Single-Center Retrospective Study
Simple Summary
Abstract
1. Introduction
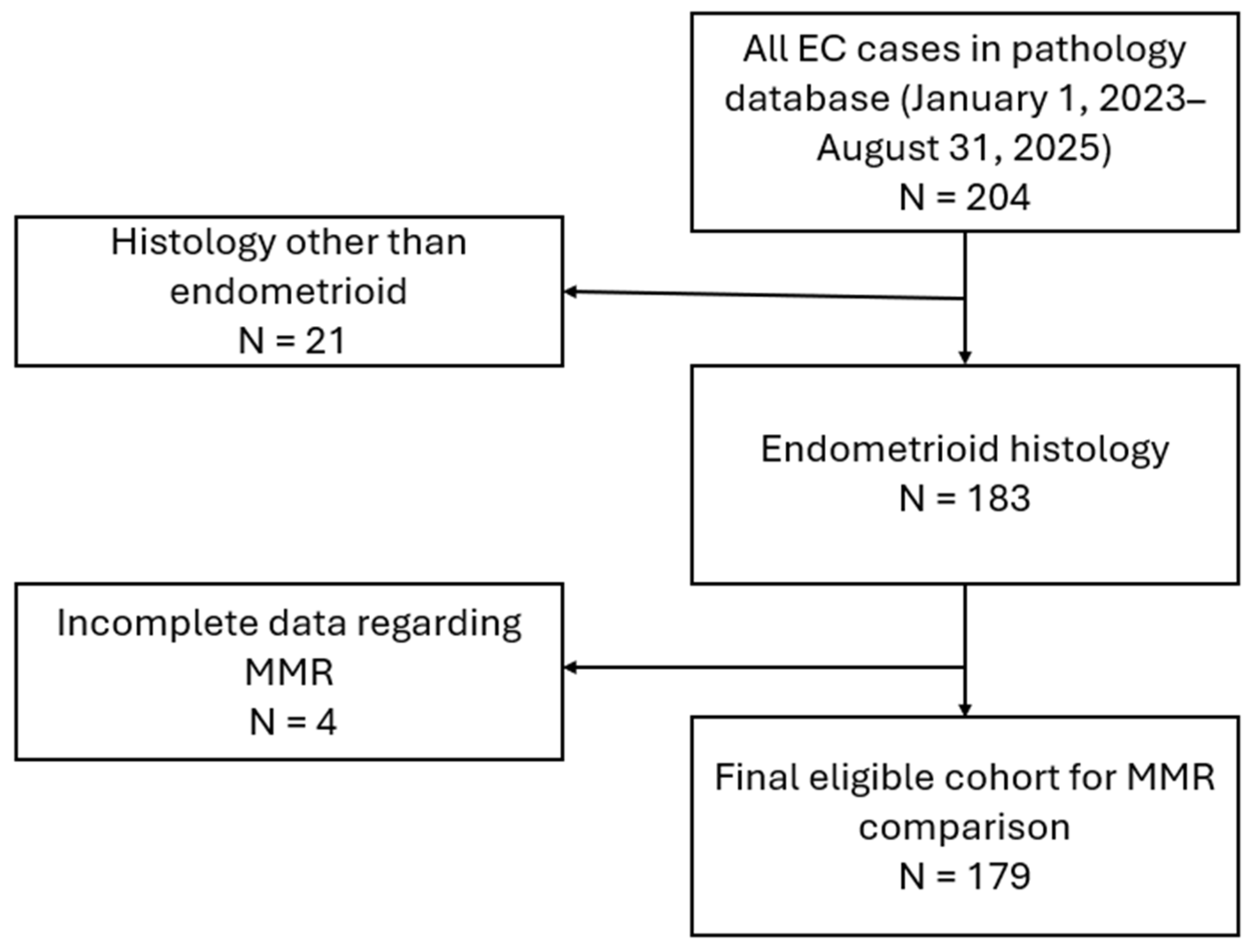
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tejerizo-García, Á.; Jiménez-López, J.S.; Muñoz-González, J.L.; Bartolomé-Sotillos, S.; Marqueta-Marqués, L.; López-González, G.; Gómez, J.F.P.-R. Overall Survival and Disease-Free Survival in Endometrial Cancer: Prognostic Factors in 276 Patients. OncoTargets Ther. 2013, 6, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Coll-de la Rubia, E.; Martinez-Garcia, E.; Dittmar, G.; Gil-Moreno, A.; Cabrera, S.; Colas, E. Prognostic Biomarkers in Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1900. [Google Scholar] [CrossRef]
- Wang, X.; Aziz, A.u.R.; Wang, D.; Wang, Y.; Liu, M.; Yu, X.; Wang, D. Prognostic Factors and Survival Outcomes of Immunohistochemically Detection Based-Molecular Subtypes of Endometrial Cancer–Analysis of 576 Clinical Cases. Diagn. Pathol. 2024, 19, 162. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Galant, N.; Krawczyk, P.; Monist, M.; Obara, A.; Gajek, Ł.; Grenda, A.; Nicoś, M.; Kalinka, E.; Milanowski, J. Molecular Classification of Endometrial Cancer and Its Impact on Therapy Selection. Int. J. Mol. Sci. 2024, 25, 5893. [Google Scholar] [CrossRef]
- Corr, B.; Cosgrove, C.; Spinosa, D.; Guntupalli, S. Endometrial Cancer: Molecular Classification and Future Treatments. BMJ Med. 2022, 1, e000152. [Google Scholar] [CrossRef]
- Adorisio, R.; Troncone, G.; Barberis, M.; Pepe, F. Molecular Profiling of H-MSI/dMMR/for Endometrial Cancer Patients: “New Challenges in Diagnostic Routine Practice”. J. Mol. Pathol. 2024, 5, 187–198. [Google Scholar] [CrossRef]
- Paranjpe, R.; Chen, C.; Sun, Y.; Hong, J.; Prabhu, V.S.; Ojha, J.; Meng, R.; Duska, L.R. Molecular Heterogeneity in dMMR/MSI-H Endometrial Cancer in the US. J. Clin. Oncol. 2025, 43, e17643. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Cibula, D.; Colombo, N.; Creutzberg, C.L.; Ledermann, J.; Mirza, M.R.; Vergote, I.; Abu-Rustum, N.R.; Bosse, T.; et al. ESGO-ESTRO-ESP Guidelines for the Management of Patients with Endometrial Carcinoma: Update 2025. Lancet Oncol. 2025, 26, e423–e435. [Google Scholar] [CrossRef]
- Plotkin, A.; Olkhov-Mitsel, E.; Nofech-Mozes, S. MLH1 Methylation Testing as an Integral Component of Universal Endometrial Cancer Screening—A Critical Appraisal. Cancers 2023, 15, 5188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Vakiani, E.; Shia, J. Detecting Mismatch Repair Deficiency in Solid Neoplasms: Immunohistochemistry, Microsatellite Instability, or Both? Mod. Pathol. 2022, 35, 1515–1528. [Google Scholar] [CrossRef]
- Ahmad Fauzi, M.F.; Wan Ahmad, W.S.H.M.; Jamaluddin, M.F.; Lee, J.T.H.; Khor, S.Y.; Looi, L.M.; Abas, F.S.; Aldahoul, N. Allred Scoring of ER-IHC Stained Whole-Slide Images for Hormone Receptor Status in Breast Carcinoma. Diagnostics 2022, 12, 3093. [Google Scholar] [CrossRef]
- Armbruster, H.; Schotte, T.; Götting, I.; Overkamp, M.; Granai, M.; Volmer, L.L.; Bahlinger, V.; Matovina, S.; Koch, A.; Dannehl, D.; et al. Aberrant P53 Immunostaining Patterns in Breast Carcinoma of No Special Type Strongly Correlate with Presence and Type of TP53 Mutations. Virchows Arch. 2024, 485, 631–642. [Google Scholar] [CrossRef]
- Mcgregor, M.J.; Fadhil, W.; Wharton, R.; Yanagisawa, Y.; Presz, M.; Pritchard, A.; Womack, C.; Dutton, S.; Kerr, R.S.; Kerr, D.J.; et al. Aberrant P53 Expression Lacks Prognostic or Predictive Significance in Colorectal Cancer: Results from the VICTOR Trial. Anticancer Res. 2015, 35, 1641–1645. [Google Scholar]
- Lorenzi, M.; Amonkar, M.; Zhang, J.; Mehta, S.; Liaw, K.-L. Epidemiology of Microsatellite Instability High (MSI-H) and Deficient Mismatch Repair (dMMR) in Solid Tumors: A Structured Literature Review. J. Oncol. 2020, 2020, 1807929. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Cibula, D.; Lv, W.; Ortaç, F.; Hietanen, S.; Backes, F.; Kikuchi, A.; Lorusso, D.; Dańska-Bidzińska, A.; Samouëlian, V.; et al. Pembrolizumab or Placebo Plus Adjuvant Chemotherapy With or Without Radiotherapy for Newly Diagnosed, High-Risk Endometrial Cancer: Results in Mismatch Repair-Deficient Tumors. J. Clin. Oncol. 2025, 43, 251–259. [Google Scholar] [CrossRef]
- Jumaah, A.S.; Al-Haddad, H.S.; Salem, M.M.; McAllister, K.A.; Yasseen, A.A. Mismatch Repair Deficiency and Clinicopathological Characteristics in Endometrial Carcinoma: A Systematic Review and Meta-Analysis. J. Pathol. Transl. Med. 2021, 55, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Ferrari, J.; Vezzaro, T.; Masatti, L.; Tasca, G.; Maggino, T.; Tozzi, R.; Saccardi, C.; Noventa, M.; Spagnol, G. The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives. J. Clin. Med. 2024, 13, 7041. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Connor, E.V.; Atkins, K.A.; Ricketts, W.; Kashlan, B.; Modesitt, S.C. Women 50 Years or Younger with Endometrial Cancer: The Argument for Universal Mismatch Repair Screening and Potential for Targeted Therapeutics. Int. J. Gynecol. Cancer 2013, 23, 853–860. [Google Scholar] [CrossRef]
- Gordhandas, S.; Kahn, R.M.; Gamble, C.; Talukdar, N.; Maddy, B.; Nelson, B.B.; Askin, G.; Christos, P.J.; Holcomb, K.; Caputo, T.A.; et al. Clinicopathologic Features of Endometrial Cancer with Mismatch Repair Deficiency. Ecancermedicalscience 2020, 14, 1061. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, E.; Sato, N.; Sugawara, T.; Noto, A.; Takahashi, K.; Makino, K.; Terada, Y. MLH1 Promoter Hypermethylation Predicts Poorer Prognosis in Mismatch Repair Deficiency Endometrial Carcinomas. J. Gynecol. Oncol. 2021, 32, e79. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, A.; Loukovaara, M.; Bützow, R. Clinicopathological Significance of Deficient DNA Mismatch Repair and MLH1 Promoter Methylation in Endometrioid Endometrial Carcinoma. Mod. Pathol. 2020, 33, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Hathout, L.; Sherwani, Z.K.; Alegun, J.; Ohri, N.; Fields, E.C.; Shah, S.; Beriwal, S.; Horne, Z.D.; Kidd, E.A.; Leung, E.W.; et al. Prognostic Effect of Mismatch Repair Status in Early-Stage Endometrial Cancer Treated With Adjuvant Radiation: A Multi-Institutional Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Keverline, K.J.; Hill, B.; Hom-Tedla, M.; Jacobs, M.; Eskander, R.N. Impact of Mismatch Repair (MMR) Status on Recurrence in High Intermediate Risk Endometrial Cancer. Gynecol. Oncol. 2025, 197, 116–120. [Google Scholar] [CrossRef]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in Endometrial Cancer. Int. J. Gynecol. Cancer 2023, 33, 351–357. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Carvalho, J.P. Unraveling the Heterogeneity of Deficiency of Mismatch Repair Proteins in Endometrial Cancer: Predictive Biomarkers and Assessment Challenges. Cancers 2024, 16, 3452. [Google Scholar] [CrossRef]
- Yarandi, F.; Shirali, E.; Akhavan, S.; Nili, F.; Ramhormozian, S. The Impact of Lymphovascular Space Invasion on Survival in Early Stage Low-Grade Endometrioid Endometrial Cancer. Eur. J. Med. Res. 2023, 28, 118. [Google Scholar] [CrossRef]
- Backes, F.J.; Haag, J.; Cosgrove, C.M.; Suarez, A.; Cohn, D.E.; Goodfellow, P.J. Mismatch Repair Deficiency Identifies Patients with High-Intermediate–Risk (HIR) Endometrioid Endometrial Cancer at the Highest Risk of Recurrence: A Prognostic Biomarker. Cancer 2019, 125, 398–405. [Google Scholar] [CrossRef]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef]
- Fountzilas, E.; Kotoula, V.; Pentheroudakis, G.; Manousou, K.; Polychronidou, G.; Vrettou, E.; Poulios, C.; Papadopoulou, E.; Raptou, G.; Pectasides, E.; et al. Prognostic Implications of Mismatch Repair Deficiency in Patients with Nonmetastatic Colorectal and Endometrial Cancer. ESMO Open 2019, 4, e000474. [Google Scholar] [CrossRef]
- Boer, S.M.d.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant Chemoradiotherapy versus Radiotherapy Alone for Women with High-Risk Endometrial Cancer (PORTEC-3): Final Results of an International, Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Saharti, S.; Altaf, F. Mismatch Repair Proteins Evaluation in Endometrial Carcinoma: Clinicopathological Features and Prognostic Implications. Available online: https://www.ejgo.net/articles/10.22514/ejgo.2024.076?utm_source=chatgpt.com (accessed on 8 September 2025).
- Vrede, S.W.; Weelden, W.J.V.; Bulten, J.; Gilks, C.B.; Teerenstra, S.; Huvila, J.; Matias-Guiu, X.; Gil-Moreno, A.; Asberger, J.; Sweegers, S.; et al. Hormonal Biomarkers Remain Prognostically Relevant within the Molecular Subgroups in Endometrial Cancer. Gynecol. Oncol. 2025, 192, 15–23. [Google Scholar] [CrossRef]
- Yadav, A.; Sistla, A.; Swain, M.; Gowrishankar, S.; Padua, M.d.; Modi, T.; Himabindu, R.; Agarwal, N.; Kulkarni, A.; Bhandari, T.; et al. To Study the Expression of Estrogen, Progesterone Receptor and P53 Immunohistochemistry Markers in Subtyping Endometrial Carcinoma. Indian J. Pathol. Microbiol. 2024, 67, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Wood, R. Optimizing Immunohistochemistry Reporting in Endometrial Cancer. Cureus 2024, 16, e65810. [Google Scholar] [CrossRef]
- Nádorvári, M.L.; Kenessey, I.; Kiss, A.; Barbai, T.; Kulka, J.; Rásó, E.; Tímár, J. Comparison of Standard Mismatch Repair Deficiency and Microsatellite Instability Tests in a Large Cancer Series. J. Transl. Med. 2024, 22, 150. [Google Scholar] [CrossRef]
- Aytekin, O.; Cesur, N.; Gozel, S.; Karsli, S.E.; Tokalioglu, A.A.; Kilic, F.; Cuylan, Z.F.; Selcuk, I.; Comert, G.K.; Erdogan, F.; et al. Mismatch Repair Protein Deficiency and Its Relationship with Clinicopathological Factors in Endometrial Cancer: A Retrospective Study. J. Cancer 2025, 16, 2778–2786. [Google Scholar] [CrossRef]
- Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Available online: https://www.mdpi.com/1422-0067/25/2/1056?utm_source=chatgpt.com (accessed on 7 September 2025).
- Lachit, K.; Vinita, P. Study of Mismatch Repair Protein Expression by Using Immunohistochemistry in Various Carcinomas with Special Reference to Colorectal Adenocarcinomas—At a Tertiary Referral Laboratory in India. Asian Pac. J. Cancer Biol. 2022, 7, 341–347. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, N.; Xie, X. The Clinicopathological Characteristics of POLE-Mutated/Ultramutated Endometrial Carcinoma and Prognostic Value of POLE Status: A Meta-Analysis Based on 49 Articles Incorporating 12,120 Patients. BMC Cancer 2022, 22, 1157. [Google Scholar] [CrossRef]
- Eich, M.-L.; Siemanowsk-Hrach, J.; Drebber, U.; Friedrichs, N.; Mallmann, P.; Domröse, C.; Ratiu, D.; Merkelbach-Bruse, S.; Büttner, R.; Quaas, A.; et al. Assessment of P53 in Endometrial Carcinoma Biopsy and Corresponding Hysterectomy Cases in a Real-World Setting: Which Cases Need Molecular Work-Up? Cancers 2025, 17, 1506. [Google Scholar] [CrossRef] [PubMed]
- Thiel, K.W.; Devor, E.J.; Filiaci, V.L.; Mutch, D.; Moxley, K.; Alvarez Secord, A.; Tewari, K.S.; McDonald, M.E.; Mathews, C.; Cosgrove, C.; et al. TP53 Sequencing and P53 Immunohistochemistry Predict Outcomes When Bevacizumab Is Added to Frontline Chemotherapy in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2022, 40, 3289–3300. [Google Scholar] [CrossRef]
- Vermij, L.; Léon-Castillo, A.; Singh, N.; Powell, M.E.; Edmondson, R.J.; Genestie, C.; Khaw, P.; Pyman, J.; McLachlin, C.M.; Ghatage, P.; et al. P53 Immunohistochemistry in Endometrial Cancer: Clinical and Molecular Correlates in the PORTEC-3 Trial. Mod. Pathol. 2022, 35, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
| Target | Antibody | Clone |
|---|---|---|
| P53 | Flex RTU Monoclonal Mouse Anti-Human, P53 Protein | DO-7 |
| ER | Flex RTU Monoclonal Rabbit Anti-Human, Estrogen Receptor alfa | EP1 |
| PR | Flex RTU Monoclonal Mouse Anti-Human, Progesterone Receptor | PgR 636 |
| MLH1 | Flex RTU Monoclonal Mouse Anti-Human, MutL Protein Homolog 1, MLH1 | ES05 |
| PMS2 | Flex RTU Monoclonal Rabbit Anti-Human, Postmeiotic Segregation Increased 2, PMS2 | EP51 |
| MSH2 | Flex RTU Monoclonal Mouse Anti-Human, MutS Protein Homolog 2, MSH2 | FE11 |
| MSH6 | Flex RTU Monoclonal Rabbit Anti-Human, MutS Protein Homolog 6, MSH6 | EP49 |
| Variable | Total (n = 179) | pMMR (n = 127) | dMMR (n = 52) | p-Value | HL | 95% CI |
|---|---|---|---|---|---|---|
| Age [years] mean ± SD | 63.76 ± 10.43 | 63.31 ± 10.21 | 64.85 ± 10.97 | 0.255 | 2.00 | −1.00–5.00 |
| Body weight [kg] median, IQR | 77 (67–93) | 77 (67–93) | 76.5 (68–92) | 0.709 | 1.00 | −7.00–4.00 |
| BMI [kg/m2] median, IQR | 29.67 (25.71–34.85) | 29.62 (25.86–34.29) | 30.18 (25.06–35.3) | 0.868 | −0.19 | −2.55–1.89 |
| Variable | Total (n = 179) | pMMR (n = 127) | dMMR (n = 52) | p-Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| FIGO | 0.036 * | 2.63 | 1.04–6.63 | |||
| 150 (83.80%) | 111 (87.40%) | 39 (75.00%) | |||
| 27 (15.08%) | 14 (11.02%) | 13 (25.00%) | |||
| 2 (1.12%) | 2 (1.57%) | 0 (0.00%) | |||
| Grade | 0.422 | 1.57 | 0.58–4.04 | |||
| 148 (82.68%) | 106 (83.46%) | 42 (80.77%) | |||
| 26 (14.35%) | 16 (12.60%) | 10 (19.23%) | |||
| 5 (2.79%) | 5 (3.94%) | 0 (0.00%) | |||
| LVSI | 0.008 * | 2.52 | 1.24–5.21 | |||
| 77 (43.02%) | 46 (36.22%) | 31 (59.62%) | |||
| 100 (55.87%) | 79 (62.20%) | 21 (40.38%) | |||
| 2 (1.12%) | 2 (1.57%) | 0 (0.00%) | |||
| Estrogen receptors | 0.765 | 2.25 | 0.24–109.25 | |||
| 135 (75.42%) | 93 (73.23%) | 42 (80.77%) | |||
| 6 (3.35%) | 5 (3.94%) | 1 (1.92%) | |||
| 38 (21.23%) | 29 (22.83%) | 9 (17.31%) | |||
| Progesterone receptors | 0.352 | 3.71 | 0.47–169.42 | |||
| 132 (73.74%) | 90 (70.87%) | 42 (80.77%) | |||
| 9 (5.03%) | 8 (6.30%) | 1 (1.92%) | |||
| 38 (21.23%) | 29 (22.83%) | 9 (17.31%) | |||
| P53 | 0.335 | 0.50 | 0.11–1.64 | |||
| 22 (12.29%) | 18 (14.17%) | 4 (7.69%) | |||
| 156 (87.15%) | 108 (85.04%) | 48 (92.31%) | |||
| 1 (0.56%) | 1 (0.79%) | 0 (0.00%) | |||
| Myometrial invasion | 0.902 | 1.10 | 0.54–2.28 | |||
| 104 (58.10%) | 73 (57.48%) | 31 (59.62%) | |||
| 72 (40.22%) | 52 (40.94%) | 20 (38.46%) | |||
| 3 (1.68%) | 2 (1.57%) | 1 (1.92%) |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| FIGO (III/IV) | 1.95 | 0.72–5.25 | 0.187 |
| Grade (high) | 1.26 | 0.48–3.28 | 0.642 |
| LVSI (present) | 2.96 | 1.25–7.04 | 0.014 * |
| P53 (aberrant) | 0.88 | 0.25–3.03 | 0.838 |
| Myometrial invasion (deep) | 0.50 | 0.21–1.21 | 0.123 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| MMR (dMMR) | 3.38 | 1.46–7.81 | 0.004 * |
| Grade (high) | 1.74 | 0.64–4.75 | 0.281 |
| P53 (aberrant) | 0.53 | 0.16–1.72 | 0.289 |
| Myometrial invasion (deep) | 12.45 | 5.15–30.11 | <0.001 * |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| MMR (dMMR) | 2.68 | 1.11–6.47 | 0.028 * |
| Grade (high) | 3.85 | 1.45–10.19 | 0.007 * |
| P53 (aberrant) | 1.37 | 0.35–5.37 | 0.654 |
| Pattern of MMR Protein Loss | Number of Cases (Percent) | Pattern of MMR Protein Loss | Number of Cases (Percent) |
|---|---|---|---|
| MLH1, PMS2 | 41 (78.8%) | MLH1, PMS2, MSH6 | 1 (1.9%) |
| MSH6 | 4 (7.7%) | MSH2 | 1 (1.9%) |
| MLH1 | 2 (3.8%) | PMS2, MSH2, MSH6 | 1 (1.9%) |
| MSH2, MSH6 | 2 (3.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedziarek, C.; Bochyński, H.; Bociańska, K.; Potograbski, M.; Tyburski, P.; Zaborowski, M.P.; Nowak-Markwitz, E. Association Between MMR Status and Prognostic Pathological Factors in Endometrioid Endometrial Cancer—A Single-Center Retrospective Study. Cancers 2025, 17, 3605. https://doi.org/10.3390/cancers17223605
Miedziarek C, Bochyński H, Bociańska K, Potograbski M, Tyburski P, Zaborowski MP, Nowak-Markwitz E. Association Between MMR Status and Prognostic Pathological Factors in Endometrioid Endometrial Cancer—A Single-Center Retrospective Study. Cancers. 2025; 17(22):3605. https://doi.org/10.3390/cancers17223605
Chicago/Turabian StyleMiedziarek, Cezary, Hubert Bochyński, Katarzyna Bociańska, Michał Potograbski, Piotr Tyburski, Mikołaj Piotr Zaborowski, and Ewa Nowak-Markwitz. 2025. "Association Between MMR Status and Prognostic Pathological Factors in Endometrioid Endometrial Cancer—A Single-Center Retrospective Study" Cancers 17, no. 22: 3605. https://doi.org/10.3390/cancers17223605
APA StyleMiedziarek, C., Bochyński, H., Bociańska, K., Potograbski, M., Tyburski, P., Zaborowski, M. P., & Nowak-Markwitz, E. (2025). Association Between MMR Status and Prognostic Pathological Factors in Endometrioid Endometrial Cancer—A Single-Center Retrospective Study. Cancers, 17(22), 3605. https://doi.org/10.3390/cancers17223605





