Causes of Childhood Cancer: A Literature Review (2014–2021)—Part 3: Environmental and Occupational Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Air Quality
3.1. Outdoor Exposures
3.2. Indoor Exposures
4. Radiation
4.1. Radiation from Outdoor Sources
4.1.1. Ionizing Radiation
4.1.2. Non-Ionizing Radiation
4.2. Radiation from Indoor Sources
5. Occupational Exposures
5.1. Occupational Exposures to Benzene
5.2. Miscellaneous Occupational Exposures
5.3. Agricultural Animals
5.4. Agricultural Pesticides
6. Limitations
7. Summary of the Three-Part Series
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, A.M.; Emeny, R.T.; Bagley, P.J.; Blunt, H.B.; Butow, M.E.; Morgan, A.; Alford-Teaster, J.A.; Titus, L.; Walston, R.R., 3rd; Rees, J.R. Causes of Childhood Cancer: A Review of the Recent Literature: Part I-Childhood Factors. Cancers 2024, 16, 1297. [Google Scholar] [CrossRef] [PubMed]
- Emeny, R.T.; Ricci, A.M.; Titus, L.; Morgan, A.; Bagley, P.J.; Blunt, H.B.; Butow, M.E.; Alford-Teaster, J.A.; Walston, R.R., 3rd; Rees, J.R. Causes of Childhood Cancer: A Review of Literature (2014–2021). Part 2—Pregnancy and Birth-Related Factors. Cancers 2025, 17, 2499. [Google Scholar] [CrossRef] [PubMed]
- WHO. Outdoor Air Pollution. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2015; Volume 109. [Google Scholar]
- Sun, X.-X.; Zhang, S.-S.; Ma, X.-L. No association between traffic density and risk of childhood leukemia: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 5229–5232. [Google Scholar] [CrossRef]
- Boothe, V.L.; Boehmer, T.K.; Wendel, A.M.; Yip, F.Y. Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am. J. Prev. Med. 2014, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Wallace, F.M.; Zhang, L.; Smith, M.T.; Rader, G.; Steinmaus, C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am. J. Epidemiol. 2016, 183, 1–14. [Google Scholar] [CrossRef]
- Gong, Z.-H.; Li, J.; Wang, X.-Y.; Yu, Y.; Ren, M.-M.; Zhou, J. A Meta-analysis of Traffic-related Air Pollution and Risk of Childhood Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 267–274. [Google Scholar] [CrossRef]
- Filippini, T.; Hatch, E.E.; Rothman, K.J.; Heck, J.E.; Park, A.S.; Crippa, A.; Orsini, N.; Vinceti, M. Association between Outdoor Air Pollution and Childhood Leukemia: A Systematic Review and Dose-Response Meta-Analysis. Environ. Health Perspect. 2019, 127, 46002. [Google Scholar] [CrossRef]
- Filippini, T.; Heck, J.E.; Malagoli, C.; Del Giovane, C.; Vinceti, M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J. Environ. Sci. Health Part C 2015, 33, 36–66. [Google Scholar] [CrossRef]
- Steinmaus, C.; Smith, M.T. Steinmaus and Smith respond to “proximity to gasoline stations and childhood leukemia”. Am. J. Epidemiol. 2017, 185, 5–7. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Painting, Firefighting, and Shiftwork. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 98, pp. 9–764. [Google Scholar]
- Bailey, H.D.; Metayer, C.; Milne, E.; Petridou, E.T.; Infante-Rivard, C.; Spector, L.G.; Clavel, J.; Dockerty, J.D.; Zhang, L.; Armstrong, B.K.; et al. Home paint exposures and risk of childhood acute lymphoblastic leukemia: Findings from the Childhood Leukemia International Consortium. Cancer Causes Control 2015, 26, 1257–1270. [Google Scholar] [CrossRef]
- Volk, J.; Heck, J.E.; Schmiegelow, K.; Hansen, J. Risk of selected childhood cancers and parental employment in painting and printing industries: A register-based case-control study in Denmark 1968–2015. Scand. J. Work Environ. Health 2019, 45, 475–482. [Google Scholar] [CrossRef]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans. International Agency for Research on Cancer. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 26 October 2025).
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Household exposure to pesticides and risk of leukemia in children and adolescents: Updated systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2019, 222, 49–67. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef]
- Zumel-Marne, A.; Castano-Vinyals, G.; Kundi, M.; Alguacil, J.; Cardis, E. Environmental Factors and the Risk of Brain Tumours in Young People: A Systematic Review. Neuroepidemiology 2019, 53, 121–141. [Google Scholar] [CrossRef]
- WHO. Benzene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, December 2018; Volume 120. [Google Scholar]
- Nicolle-Mir, L. Exposure to benzene and risk of childhood leukemia: Synthesis of the literature. Environ. Risques Sante 2016, 15, 299–301. [Google Scholar]
- Zhou, Y.; Zhang, S.; Li, Z.; Zhu, J.; Bi, Y.; Bai, Y.; Wang, H. Maternal benzene exposure during pregnancy and risk of childhood acute lymphoblastic leukemia: A meta-analysis of epidemiologic studies. PLoS ONE 2014, 9, e110466. [Google Scholar] [CrossRef]
- Mosallanejad, Z.; Fakhri, Y.; Ferrante, M.; Zandsalimi, Y.; Amirhajeloo, L.R.; Amanidaz, N.; Moradi, B.; Keramati, H. Association between benzene exposure and childhood leukemia: A systematic review and meta-analysis updated to July 2016. Intl. J. Pharm. Technol. 2016, 8, 4640–4652. [Google Scholar]
- Spycher, B.D.; Lupatsch, J.E.; Huss, A.; Rischewski, J.; Schindera, C.; Spoerri, A.; Vermeulen, R.; Kuehni, C.E.; Claudia Elisabeth Kuehni for the Swiss Paediatric Oncology Group; The Swiss National Cohort Study Group. Parental occupational exposure to benzene and the risk of childhood cancer: A census-based cohort study. Environ. Int. 2017, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; He, D.; Contreras, Z.A.; Ritz, B.; Olsen, J.; Hansen, J. Parental occupational exposure to benzene and the risk of childhood and adolescent acute lymphoblastic leukaemia: A population-based study. Occup. Environ. Med. 2019, 76, 527–529. [Google Scholar] [CrossRef]
- Metayer, C.; Scelo, G.; Kang, A.Y.; Gunier, R.B.; Reinier, K.; Lea, S.; Chang, J.S.; Selvin, S.; Kirsch, J.; Crouse, V.; et al. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ. Res. 2016, 151, 174–183. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Hoet, P.; Lison, D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: A systematic review and meta-analysis. Environ. Int. 2013, 56, 19–31. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ionizing Radiation, Part 2: Some Internally Deposited Radionuclides. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2000; Volume 78. [Google Scholar]
- Lu, Y.; Liu, L.; Chen, Q.; Wei, J.; Cao, G.; Zhang, J. Domestic radon exposure and risk of childhood leukemia: A meta-analysis. J. Buon 2020, 25, 1035–1041. [Google Scholar]
- Nelson, L.; Valle, J.; King, G.; Mills, P.K.; Richardson, M.J.; Roberts, E.M.; Smith, D.; English, P. Estimating the Proportion of Childhood Cancer Cases and Costs Attributable to the Environment in California. Am. J. Public Health 2017, 107, 756–762. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ionizing Radiation, Part 1: X- and Gamma (γ)-Radiation, and Neutrons. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2000; Volume 75. [Google Scholar]
- Stram, D.O. Analysis of cancer risks in populations near nuclear facilities: Phase I. A report by the National Academies Nuclear and Radiation Studies Board. Health Phys. 2014, 106, 305–306. [Google Scholar] [CrossRef]
- Zaki, A.M.; Rahim, M.A.A.; Zaidun, Z.; Ramdzan, A.R.; Isa, Z.M. Exposure to non-ionizing radiation and childhood cancer: A meta-analysis. Middle East J. Cancer 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Wang, C.; Yan, K.; Lin, X.; Li, S.; Bao, H.; Liu, X. Magnetic fields exposure and childhood leukemia risk: A meta-analysis based on 11,699 cases and 13,194 controls. Leuk. Res. 2014, 38, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.M.; Swanson, J.; Vergara, X.P.; Kheifets, L. Childhood leukemia risk in the California Power Line Study: Magnetic fields versus distance from power lines. Environ. Res. 2019, 171, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Slusky, D.A.; Does, M.; Metayer, C.; Mezei, G.; Selvin, S.; Buffler, P.A. Potential role of selection bias in the association between childhood leukemia and residential magnetic fields exposure: A population-based assessment. Cancer Epidemiol. 2014, 38, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Enriquez, J.C.; Correa-Correa, V.; Flores-Lujano, J.; Perez-Saldivar, M.L.; Jimenez-Hernandez, E.; Martin-Trejo, J.A.; Espinoza-Hernandez, L.E.; Medina-Sanson, A.; Cardenas-Cardos, R.; Flores-Villegas, L.V.; et al. Extremely Low-Frequency Magnetic Fields and the Risk of Childhood B-Lineage Acute Lymphoblastic Leukemia in a City With High Incidence of Leukemia and Elevated Exposure to ELF Magnetic Fields. Bioelectromagnetics 2020, 41, 581–597. [Google Scholar] [CrossRef]
- Booth, B.J.; Jones, R.R.; Turyk, M.E.; Freels, S.; Patel, D.M.; Stayner, L.T.; Ward, M.H. Livestock and poultry density and childhood cancer incidence in nine states in the USA. Environ. Res. 2017, 159, 444–451. [Google Scholar] [CrossRef]
- Hall, C.; Hansen, J.; von Ehrenstein, O.S.; He, D.; Olsen, J.; Ritz, B.; Heck, J.E. Occupational livestock or animal dust exposure and offspring cancer risk in Denmark, 1968–2016. Int. Arch. Occup. Environ. Health 2020, 93, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Jones, R.R.; Booth, B.J.; Olsson, A.C.; Kromhout, H.; Straif, K.; Vermeulen, R.; Tikellis, G.; Paltiel, O.; Golding, J.; et al. Parental occupational exposure to pesticides, animals and organic dust and risk of childhood leukemia and central nervous system tumors: Findings from the International Childhood Cancer Cohort Consortium (I4C). Int. J. Cancer 2020, 146, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Dessypris, N.; Papadakis, V.; Tragiannidis, A.; Bouka, E.; Hatzipantelis, E.; Moschovi, M.; Papakonstantinou, E.; Polychronopoulou, S.; Sgouros, S.; et al. Perinatal and early life risk factors for childhood brain tumors: Is instrument-assisted delivery associated with higher risk? Cancer Epidemiol. 2019, 59, 178–184. [Google Scholar] [CrossRef]
- Lupo, P.J.; Spector, L.G. Cancer progress and priorities: Childhood cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1081–1094. [Google Scholar] [CrossRef]
- Danysh, H.E.; Mitchell, L.E.; Zhang, K.; Scheurer, M.E.; Lupo, P.J. Traffic-related air pollution and the incidence of childhood central nervous system tumors: Texas, 2001–2009. Pediatr Blood Cancer 2015, 62, 1572–1578. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Erdmann, F.; Urhoj, S.K.; Brandt, J.; Geels, C.; Ketzel, M.; Frohn, L.M.; Christensen, J.H.; Sorensen, M.; Raaschou-Nielsen, O. Air pollution exposure at the residence and risk of childhood cancers in Denmark: A nationwide register-based case-control study. EClinicalMedicine 2020, 28, 100569. [Google Scholar] [CrossRef] [PubMed]
- Danysh, H.E.; Zhang, K.; Mitchell, L.E.; Scheurer, M.E.; Lupo, P.J. Maternal residential proximity to major roadways at delivery and childhood central nervous system tumors. Environ. Res. 2016, 146, 315–322. [Google Scholar] [CrossRef]
- Kumar, S.V.; Lupo, P.J.; Pompeii, L.A.; Danysh, H.E. Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring. Int. J. Environ. Res. Public Health 2018, 15, 505. [Google Scholar] [CrossRef]
- Peckham-Gregory, E.C.; Ton, M.; Rabin, K.R.; Danysh, H.E.; Scheurer, M.E.; Lupo, P.J. Maternal Residential Proximity to Major Roadways and the Risk of Childhood Acute Leukemia: A Population-Based Case-Control Study in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2019, 16, 2029. [Google Scholar] [CrossRef]
- Shrestha, A.; Ritz, B.; Wilhelm, M.; Qiu, J.; Cockburn, M.; Heck, J.E. Prenatal exposure to air toxics and risk of Wilms’ tumor in 0- to 5-year-old children. J. Occup. Environ. Med. 2014, 56, 573–578. [Google Scholar] [CrossRef]
- Park, A.S.; Ritz, B.; Ling, C.; Cockburn, M.; Heck, J.E. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int. J. Hyg. Environ. Health 2017, 220, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Heck, J.E.; Ritz, B.; Cockburn, M.; Escobedo, L.A.; von Ehrenstein, O.S. Prenatal Exposure to Air Toxics and Malignant Germ Cell Tumors in Young Children. J. Occup. Environ. Med. 2019, 61, 529–534. [Google Scholar] [CrossRef]
- Lavigne, E.; Lima, I.; Hatzopoulou, M.; Van Ryswyk, K.; van Donkelaar, A.; Martin, R.V.; Chen, H.; Stieb, D.M.; Crighton, E.; Burnett, R.T.; et al. Ambient ultrafine particle concentrations and incidence of childhood cancers. Environ. Int. 2020, 145, 106135. [Google Scholar] [CrossRef]
- Kirkeleit, J.; Riise, T.; Bjorge, T.; Christiani, D.C.; Bratveit, M.; Baccarelli, A.; Mattioli, S.; Hollund, B.E.; Gjertsen, B.T. Maternal exposure to gasoline and exhaust increases the risk of childhood leukaemia in offspring—A prospective study in the Norwegian Mother and Child Cohort Study. Br. J. Cancer 2018, 119, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Greenop, K.R.; Hinwood, A.L.; Fritschi, L.; Scott, R.J.; Attia, J.; Ashton, L.J.; Heath, J.A.; Armstrong, B.K.; Milne, E. Vehicle refuelling, use of domestic wood heaters and the risk of childhood brain tumours: Results from an Australian case-control study. Pediatr. Blood Cancer 2015, 62, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, J.; Gomez-Barroso, D.; Tamayo-Uria, I.; Ramis, R. Methodological approaches to the study of cancer risk in the vicinity of pollution sources: The experience of a population-based case-control study of childhood cancer. Int. J. Health Geogr. 2019, 18, 12. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Lopez-Abente, G.; Gomez-Barroso, D.; Morales-Piga, A.; Romaguera, E.P.; Tamayo, I.; Fernandez-Navarro, P.; Ramis, R. Childhood leukemia and residential proximity to industrial and urban sites. Environ. Res. 2015, 140, 542–553. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Morales-Piga, A.; Gomez, J.; Gomez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; Fernandez-Navarro, P.; Lopez-Abente, G.; Ramis, R. Association between residential proximity to environmental pollution sources and childhood renal tumors. Environ. Res. 2016, 147, 405–414. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Morales-Piga, A.; Gomez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; Fernandez-Navarro, P.; Lopez-Abente, G.; Ramis, R. Risk of neuroblastoma and residential proximity to industrial and urban sites: A case-control study. Environ. Int. 2016, 92–93, 269–275. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Morales-Piga, A.; Gomez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; Lopez-Abente, G.; Ramis, R. Risk of bone tumors in children and residential proximity to industrial and urban areas: New findings from a case-control study. Sci. Total Environ. 2017, 579, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Niazi, S.; Johnson, G.; Nodehi, V.; Yunesian, M. Exposure to ambient air pollution and risk of childhood cancers: A population-based study in Tehran, Iran. Sci. Total Environ. 2019, 646, 105–110. [Google Scholar] [CrossRef]
- McKenzie, L.M.; Allshouse, W.B.; Byers, T.E.; Bedrick, E.J.; Serdar, B.; Adgate, J.L. Childhood hematologic cancer and residential proximity to oil and gas development. PLoS ONE 2017, 12, e0170423. [Google Scholar] [CrossRef]
- Malagoli, C.; Malavolti, M.; Costanzini, S.; Fabbri, S.; Tezzi, S.; Palazzi, G.; Arcolin, E.; Vinceti, M. Increased incidence of childhood leukemia in urban areas: A population-based case-control study. Epidemiol. Prev. 2015, 39 (Suppl. S1), 102–107. [Google Scholar]
- Irigaray, P.; Newby, J.A.; Clapp, R.; Hardell, L.; Howard, V.; Montagnier, L.; Epstein, S.; Belpomme, D. Lifestyle-related factors and environmental agents causing cancer: An overview. Biomed. Pharmacother. 2007, 61, 640–658. [Google Scholar] [CrossRef]
- Xia, C.; Diamond, M.L.; Peaslee, G.F.; Peng, H.; Blum, A.; Wang, Z.; Shalin, A.; Whitehead, H.D.; Green, M.; Schwartz-Narbonne, H.; et al. Per- and Polyfluoroalkyl Substances in North American School Uniforms. Environ. Sci. Technol 2022, 56, 13845–13857. [Google Scholar] [CrossRef] [PubMed]
- Black, D. Investigation of the Possible Increased Incidence of Cancer in West Cumbria; Report of the Independent Advisory Group; HMSO: London, UK, 1984. [Google Scholar]
- United States Environmental Protection Agency. A Citizen’s Guide to Radon: The Guide to Protecting Yourself and Your Family from Radon. 2016. Available online: https://www.epa.gov/sites/default/files/2016-12/documents/2016_a_citizens_guide_to_radon.pdf (accessed on 26 October 2025).
- Omidakhsh, N.; Ganguly, A.; Bunin, G.R.; von Ehrenstein, O.S.; Ritz, B.; Heck, J.E. Residential Pesticide Exposures in Pregnancy and the Risk of Sporadic Retinoblastoma: A Report From the Children’s Oncology Group. Am. J. Ophthalmol. 2017, 176, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Deziel, N.C.; Rull, R.P.; Colt, J.S.; Reynolds, P.; Whitehead, T.P.; Gunier, R.B.; Month, S.R.; Taggart, D.R.; Buffler, P.; Ward, M.H.; et al. Polycyclic aromatic hydrocarbons in residential dust and risk of childhood acute lymphoblastic leukemia. Environ. Res. 2014, 133, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Colt, J.S.; Deziel, N.C.; Whitehead, T.P.; Reynolds, P.; Gunier, R.B.; Nishioka, M.; Dahl, G.V.; Rappaport, S.M.; Buffler, P.A.; et al. Residential levels of polybrominated diphenyl ethers and risk of childhood acute lymphoblastic leukemia in California. Environ. Health Perspect. 2014, 122, 1110–1116. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Adhatamsoontra, P.; Wang, Y.; Arcolin, E.; Sender, L.; Selvin, S.; Metayer, C. Home remodeling and risk of childhood leukemia. Ann. Epidemiol. 2017, 27, 140–144.e4. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Ward, M.H.; Colt, J.S.; Dahl, G.; Ducore, J.; Reinier, K.; Gunier, R.B.; Katharine Hammond, S.; Rappaport, S.M.; Metayer, C. Dust metal loadings and the risk of childhood acute lymphoblastic leukemia. J. Exp. Sci. Environ. Epidemiol. 2015, 25, 593–598. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Liang, G.; Zhang, N.; Wang, C.; Wang, Y.; Nie, L.; Lv, X.; Li, W.; Guo, Q.; et al. Maternal prenatal exposure to environmental factors and risk of childhood acute lymphocytic leukemia: A hospital-based case-control study in China. Cancer Epidemiol. 2019, 58, 146–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Shi, R.; Chen, D.; Wang, X.; Kamijima, M.; Sakai, K.; Nakajima, T.; Khalequzzaman, M.; Zhou, Y.; et al. Household pesticide exposure and the risk of childhood acute leukemia in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 11755–11763. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Shi, R.; Kamijima, M.; Sakai, K.; Tian, Y.; Gao, Y. Indoor volatile organic compounds exposures and risk of childhood acute leukemia: A case-control study in shanghai. J. Environ. Sci. Health Part A Tox Hazard. Subst. Environ. Eng. 2021, 56, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Kamijima, M.; Sakai, K.; Khalequzzaman, M.; Nakajima, T.; Shi, R.; Wang, X.; Chen, D.; Ji, X.; et al. Quantitative assessments of indoor air pollution and the risk of childhood acute leukemia in Shanghai. Environ. Pollut. 2014, 187, 81–89. [Google Scholar] [CrossRef]
- Perez-Saldivar, M.L.; Fajardo-Gutierrez, A.; Sierra-Ramirez, J.A.; Nunez-Villegas, N.; Perez-Lorenzana, H.; Dorantes-Acosta, E.M.; Roman-Zepeda, P.F.; Rodriguez-Zepeda, M.D.C.; Gonzalez-Ulivarri, J.E.; Lopez-Santiago, N.; et al. Parental Exposure to Workplace Carcinogens and the Risk of Development of Acute Leukemia in Infants. Case-Control Study. Arch. Med. Res. 2016, 47, 684–693. [Google Scholar] [CrossRef]
- ACR–SPR Practice Parameter for Imaging Pregnant or Potentially Pregnant Patients with Ionizing Radiation. 2017. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Pregnant-Pts.pdf (accessed on 8 September 2025).
- Dauer, L.T.; Miller, D.L.; Schueler, B.; Silberzweig, J.; Balter, S.; Bartal, G.; Chambers, C.; Collins, J.D.; Damilakis, J.; Dixon, R.G.; et al. Occupational radiation protection of pregnant or potentially pregnant workers in IR: A joint guideline of the Society of Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2015, 26, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Vock, P. Clinical perspective on diagnostic X-ray examinations of pregnant patients—What to take into account. Phys. Med. 2017, 43, 165–171. [Google Scholar] [CrossRef] [PubMed]
- US Nuclear Regulatory Commission. Safety Requirements. 2025. Available online: https://www.nrc.gov/reactors/operating/ops-experience/tritium/safety-requirements.html (accessed on 9 September 2025).
- Ghirga, G. Cancer in children residing near nuclear power plants: An open question. Ital. J. Pediatr. 2010, 36, 60. [Google Scholar] [CrossRef]
- Baker, P.J.; Hoel, D.G. Meta-analysis of standardized incidence and mortality rates of childhood leukaemia in proximity to nuclear facilities. Eur. J. Cancer Care 2007, 16, 355–363. [Google Scholar] [CrossRef]
- Kinlen, L. Evidence for an infective cause of childhood leukaemia: Comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet 1988, 2, 1323–1327. [Google Scholar] [CrossRef]
- Backgrounder on Analysis of Cancer Risks in Populations Near Nuclear Facilities. 2020. Available online: https://www.nrc.gov/reading-rm/doc-collections/fact-sheets/bg-analys-cancer-risk-study#closeout (accessed on 29 October 2025).
- Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2000; Volume 80. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2000; Volume 102. [Google Scholar]
- Electromagnetic Fields and Cancer. National Cancer Institute, National Institutes of Health. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/electromagnetic-fields-fact-sheet (accessed on 10 October 2023).
- Amoon, A.T.; Arah, O.A.; Kheifets, L. The sensitivity of reported effects of EMF on childhood leukemia to uncontrolled confounding by residential mobility: A hybrid simulation study and an empirical analysis using CAPS data. Cancer Causes Control 2019, 30, 901–908. [Google Scholar] [CrossRef]
- Swanson, J.; Kheifets, L.; Vergara, X. Changes over time in the reported risk for childhood leukaemia and magnetic fields. J. Radiol. Prot. 2019, 39, 470–488. [Google Scholar] [CrossRef]
- Tabrizi, M.M.; Hosseini, S.A. Role of electromagnetic field exposure in childhood acute lymphoblastic leukemia and no impact of urinary alpha-amylase—A case control study in Tehran, Iran. Asian Pac. J. Cancer Preven. 2015, 16, 7613–7618. [Google Scholar] [CrossRef]
- Kheifets, L.; Crespi, C.M.; Hooper, C.; Cockburn, M.; Amoon, A.T.; Vergara, X.P. Residential magnetic fields exposure and childhood leukemia: A population-based case-control study in California. Cancer Causes Control 2017, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fei, Y.; Wei, X.; Guo, J.; Jiang, X.; Lu, L.; Chen, G. Associations of parental occupational exposure to extremely low-frequency magnetic fields with childhood leukemia risk. Leuk Lymphoma 2016, 57, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Raaschou-Nielsen, O.; Rod, N.H.; Frei, P.; Poulsen, A.H.; Johansen, C.; Schuz, J. Distance from residence to power line and risk of childhood leukemia: A population-based case-control study in Denmark. Cancer Causes Control 2014, 25, 171–177. [Google Scholar] [CrossRef]
- Bunch, K.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Magnetic fields and childhood cancer: An epidemiological investigation of the effects of high-voltage underground cables. J. Radiol. Prot. 2015, 35, 695–705. [Google Scholar] [CrossRef]
- Salvan, A.; Ranucci, A.; Lagorio, S.; Magnani, C.; Group, S.R. Childhood leukemia and 50 Hz magnetic fields: Findings from the Italian SETIL case-control study. Int. J. Environ. Res. Public Health 2015, 12, 2184–2204. [Google Scholar] [CrossRef]
- Hauri, D.D.; Spycher, B.; Huss, A.; Zimmermann, F.; Grotzer, M.; von der Weid, N.; Spoerri, A.; Kuehni, C.E.; Roosli, M.; Swiss National, C.; et al. Exposure to radio-frequency electromagnetic fields from broadcast transmitters and risk of childhood cancer: A census-based cohort study. Am. J. Epidemiol. 2014, 179, 843–851. [Google Scholar] [CrossRef]
- Radon: What is Radon? National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/radon (accessed on 10 October 2023).
- United States. Environmental Protection Agency. Office of Radiation Programs. Technical Support Document for the 1992 Citizen’s Guide to Radon; Air and Radon ANR-464. EPA 400-R-92-011; Radon Division, Office of Radiation Programs, US Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective. World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154767-3-2009. Available online: https://www.who.int/publications/i/item/9789241547673 (accessed on 29 October 2025).
- Stanley, F.K.T.; Irvine, J.L.; Jacques, W.R.; Salgia, S.R.; Innes, D.G.; Winquist, B.D.; Torr, D.; Brenner, D.R.; Goodarzi, A.A. Radon exposure is rising steadily within the modern North American residential environment, and is increasingly uniform across seasons. Sci. Rep. 2019, 9, 18472. [Google Scholar] [CrossRef] [PubMed]
- EPA. EPA Assessment of Risks from Radon in Homes; EPA 402-R-03-003; United States Environmental Protection Agency Office of Radiation and Indoor Air: Washington, DC, USA, 2003. [Google Scholar]
- Bailey, H.D.; Fritschi, L.; Metayer, C.; Infante-Rivard, C.; Magnani, C.; Petridou, E.; Roman, E.; Spector, L.G.; Kaatsch, P.; Clavel, J.; et al. Parental occupational paint exposure and risk of childhood leukemia in the offspring: Findings from the Childhood Leukemia International Consortium. Cancer Causes Control 2014, 25, 1351–1367. [Google Scholar] [CrossRef]
- Bunch, K.J.; Kendall, G.M.; Stiller, C.A.; Vincent, T.J.; Murphy, M.F.G. Case-control study of paternal occupational exposures and childhood lymphoma in Great Britain, 1962–2010. Br. J. Cancer 2019, 120, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.; Heck, J.E.; Schmiegelow, K.; Hansen, J. Parental occupational exposure to diesel engine exhaust in relation to childhood leukaemia and central nervous system cancers: A register-based nested case-control study in Denmark 1968–2016. Occup. Environ. Med. 2019, 76, 809–817. [Google Scholar] [CrossRef]
- Kendall, G.M.; Bunch, K.J.; Stiller, C.A.; Vincent, T.J.; Murphy, M.F.G. Case-control study of paternal occupational exposures and childhood bone tumours and soft-tissue sarcomas in Great Britain, 1962-2010. Br. J. Cancer 2020, 122, 8. [Google Scholar] [CrossRef]
- Peters, S.; Glass, D.C.; Greenop, K.R.; Armstrong, B.K.; Kirby, M.; Milne, E.; Fritschi, L. Childhood brain tumours: Associations with parental occupational exposure to solvents. Br. J. Cancer 2014, 111, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Omidakhsh, N.; Hansen, J.; Ritz, B.; Coleman, A.L.; McKean-Cowdin, R.; Olsen, J.; Heck, J.E. Parental Occupation and Risk of Childhood Retinoblastoma in Denmark. J. Occup. Environ. Med. 2021, 63, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Omidakhsh, N.; Bunin, G.R.; Ganguly, A.; Ritz, B.; Kennedy, N.; von Ehrenstein, O.S.; Krause, N.; Heck, J.E. Parental occupational exposures and the risk of childhood sporadic retinoblastoma: A report from the Children’s Oncology Group. Occup. Environ. Med. 2018, 75, 205–211. [Google Scholar] [CrossRef]
- Janitz, A.E.; Ramachandran, G.; Tomlinson, G.E.; Krailo, M.; Richardson, M.; Spector, L. Maternal and paternal occupational exposures and hepatoblastoma: Results from the HOPE study through the Children’s Oncology Group. J. Expos. Sci. Environ. Epidemiol. 2017, 27, 359–364. [Google Scholar] [CrossRef]
- Beane Freeman, L.E.; Deroos, A.J.; Koutros, S.; Blair, A.; Ward, M.H.; Alavanja, M.; Hoppin, J.A. Poultry and livestock exposure and cancer risk among farmers in the agricultural health study. Cancer Causes Control 2012, 23, 663–670. [Google Scholar] [CrossRef]
- Gomez-Barroso, D.; Garcia-Perez, J.; Lopez-Abente, G.; Tamayo-Uria, I.; Morales-Piga, A.; Pardo Romaguera, E.; Ramis, R. Agricultural crop exposure and risk of childhood cancer: New findings from a case-control study in Spain. Int. J. Health Geogr. 2016, 15, 18. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer, World Health Organization. IARC Monographs Volume 112: Evaluation of Five Organophosphate Insecticides and Herbicides. In IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer, World Health Organization: Lyon, France, 2015. [Google Scholar]
- Glyphosate: Proposed Interim Registration Review Decision; Case Number 0178. Docket Number EPA-HQ-OPP-2009-0361; United States Environmental Protection Agency. 2019. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2009-0361-2344 (accessed on 29 October 2025).
- United States Environmental Protection Agency. Glyphosate. Available online: https://www.epa.gov/ingredients-used-pesticide-products/glyphosate (accessed on 26 October 2025).
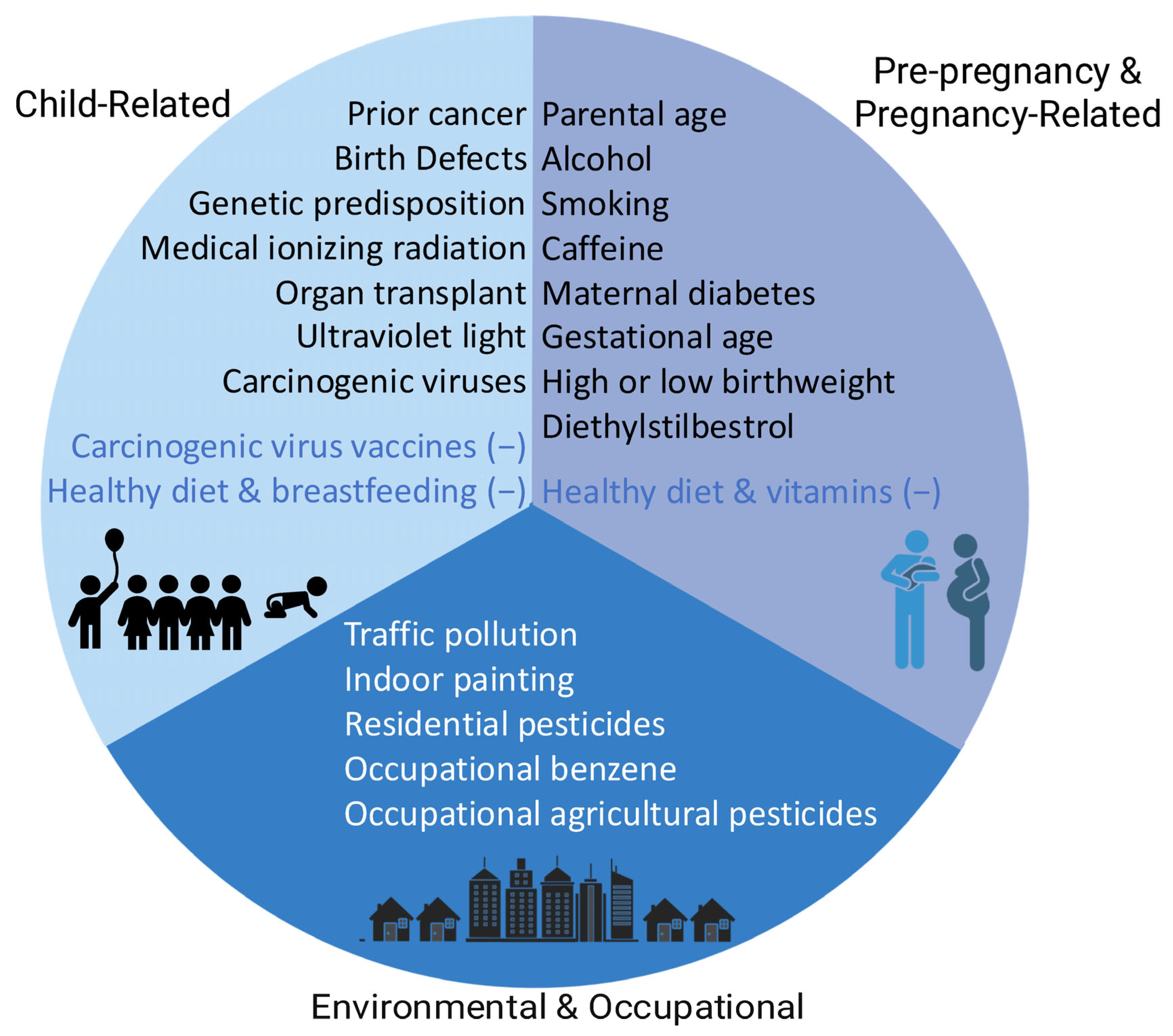
| Part 1 [1]. Child Factors | Part 2 [2]. Parental Pre-Pregnancy and Pregnancy Factors | Part 3 Environmental and Occupational Factors |
|---|---|---|
| Genetic predisposition | Alcohol | Air quality: |
| Birth defects | Smoking |
|
| Prior cancer and associated treatments | Diet and vitamins |
|
| Medical ionizing radiation | Caffeine | Radiation: |
| Ultraviolet (UV) light | Parental age |
|
| Organ transplantation | Maternal diabetes |
|
| Medications in childhood | Maternal obesity |
|
| Diet and breastfeeding | Birth characteristics and obstetric history: |
|
| Body mass index |
| Occupational exposures: |
| Infections |
|
|
| Vaccinations |
|
|
| Allergies |
|
|
|
| |
| ||
| Assistive reproductive technologies | ||
Medications during pregnancy:
| ||
| Medical ionizing radiation during pregnancy |
| Exposure | Notes |
|---|---|
| Strong Evidence of Association with Childhood Cancer | |
| Traffic pollution | The International Agency for Research on Cancer (IARC) classifies outdoor air pollution as Group 1 “carcinogenic to humans” [3]. Traffic pollution exposure to both parents and children is associated with increased childhood cancer risk. Outdoor pollution is most often associated with benzene (see below) in ambient air. Children’s residential proximity to traffic is associated with leukemia [4,5,6,7,8,9,10]. |
| Indoor paint | IARC classifies some components of paint as a Group 1 carcinogen to humans [11]. Indoor paint exposure to parents and children is associated with leukemia [12,13]. |
| Residential pesticides | IARC classifies several pesticides as either “probably carcinogenic to humans” (Group 2A) or “possibly carcinogenic to humans” (Group 2B) [14]. Pesticide exposure to both parents and children is associated with childhood leukemia and brain cancers [15,16,17]. |
| Occupational/Nonoccupational Benzene | IARC classifies benzene as a human carcinogen (Group 1) [18]. Parental occupational exposure to benzene is associated with increased risk of leukemia, ALL, and AML in their children [19,20,21,22,23,24]. |
| Agricultural pesticides | Parental occupational exposure to agricultural pesticides is associated with increased risk of brain cancer in their children [25]. |
| Mixed evidence (inconclusive) of association with childhood cancer | |
| Radon | IARC classifies radon as a known human carcinogen (Group 1) that causes lung cancer in adults [26], but associations with childhood leukemia are mixed [27,28]. |
| Ionizing radiation | IARC classifies ionizing radiation as a Group 1 known human carcinogen due to strong evidence for adult lung cancer [26,29]. However, evidence is mixed for an association between childhood leukemia and residential proximity to nuclear power facilities and a causal association seems difficult to prove [30]. |
| Low and medium electromagnetic fields | IARC classifies both extremely low frequency (ELF) magnetic fields and radiofrequency electromagnetic fields (RF-EMFs) as Group 2B, which means they are “possibly carcinogenic to humans” [31,32,33,34,35]. Sources include personal computers, cell phones, and domestic wireless internet (wifi) networks. Measuring these exposures is challenging and evidence is mixed for an association with childhood leukemia. |
| Weak or no evidence of association with childhood cancer | |
| Agricultural animals | A systematic review found little association between childhood brain cancer and mothers who lived/worked on a farm while pregnant [17]; although a few observational studies have reported associations of parental exposures to livestock and animal dust with childhood CNS cancers and leukemia, evidence is weak [36,37,38,39]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emeny, R.T.; Butow, M.E.; Titus, L.; Ricci, A.M.; Bagley, P.J.; Blunt, H.B.; Morgan, A.; Alford-Teaster, J.A.; Walston, R.R., III; Rees, J.R. Causes of Childhood Cancer: A Literature Review (2014–2021)—Part 3: Environmental and Occupational Factors. Cancers 2025, 17, 3516. https://doi.org/10.3390/cancers17213516
Emeny RT, Butow ME, Titus L, Ricci AM, Bagley PJ, Blunt HB, Morgan A, Alford-Teaster JA, Walston RR III, Rees JR. Causes of Childhood Cancer: A Literature Review (2014–2021)—Part 3: Environmental and Occupational Factors. Cancers. 2025; 17(21):3516. https://doi.org/10.3390/cancers17213516
Chicago/Turabian StyleEmeny, Rebecca T., Mary E. Butow, Linda Titus, Angela M. Ricci, Pamela J. Bagley, Heather B. Blunt, Alexandra Morgan, Jennifer A. Alford-Teaster, Raymond R. Walston, III, and Judy R. Rees. 2025. "Causes of Childhood Cancer: A Literature Review (2014–2021)—Part 3: Environmental and Occupational Factors" Cancers 17, no. 21: 3516. https://doi.org/10.3390/cancers17213516
APA StyleEmeny, R. T., Butow, M. E., Titus, L., Ricci, A. M., Bagley, P. J., Blunt, H. B., Morgan, A., Alford-Teaster, J. A., Walston, R. R., III, & Rees, J. R. (2025). Causes of Childhood Cancer: A Literature Review (2014–2021)—Part 3: Environmental and Occupational Factors. Cancers, 17(21), 3516. https://doi.org/10.3390/cancers17213516






