Pediatric Non-Down Syndrome Acute Megakaryoblastic Leukemia Patients Have Dismal Outcomes Irrespective of Allogeneic Hematopoietic Stem Cell Transplant: A Single-Center Experience
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
2.2. Patient and Disease Characteristics
2.3. Donor, Graft, and Transplant Conditioning Regimen Characteristics
2.4. Supportive Care
2.5. Study Endpoints
2.6. Definitions
2.7. Statistics
3. Results
3.1. Patients and Disease Characteristics
3.2. Initial Chemotherapy Regimens and Outcomes
3.3. Pre-Transplant, Donor, Stem Cell Source, Graft, and Conditioning Regimen Characteristics
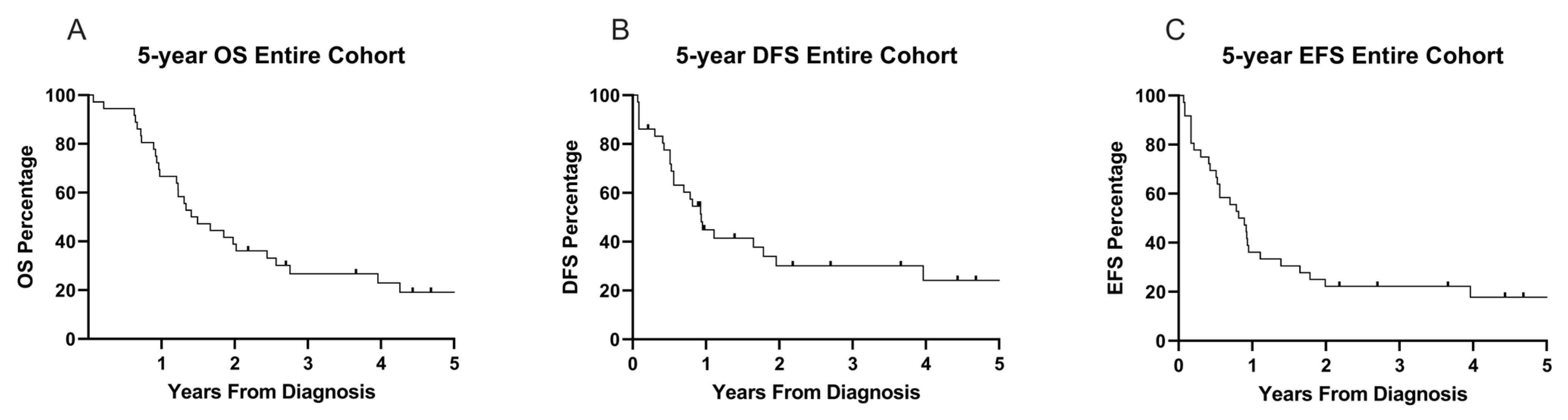
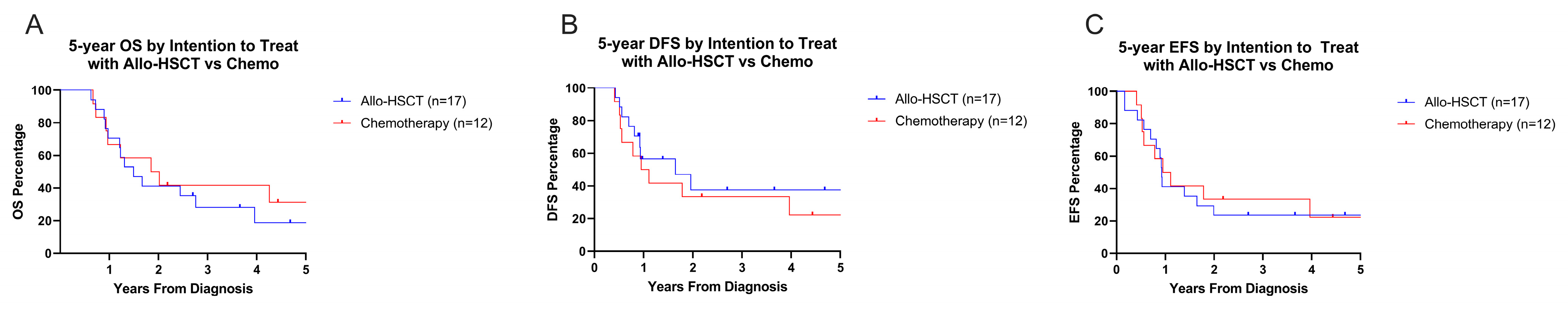
3.4. Survival Outcomes
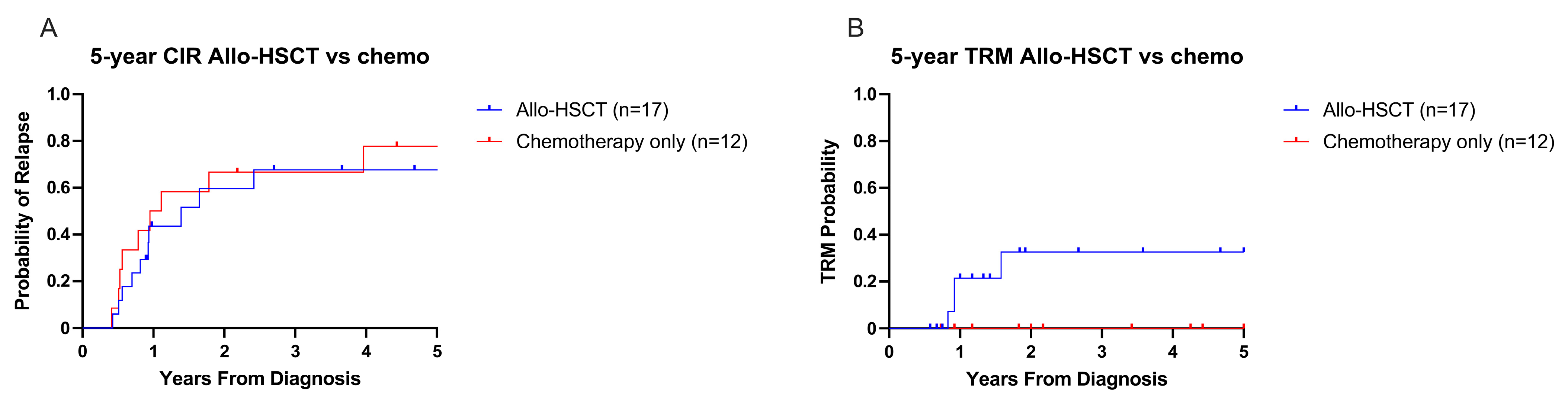
3.5. Relapse- and Treatment-Related Mortality
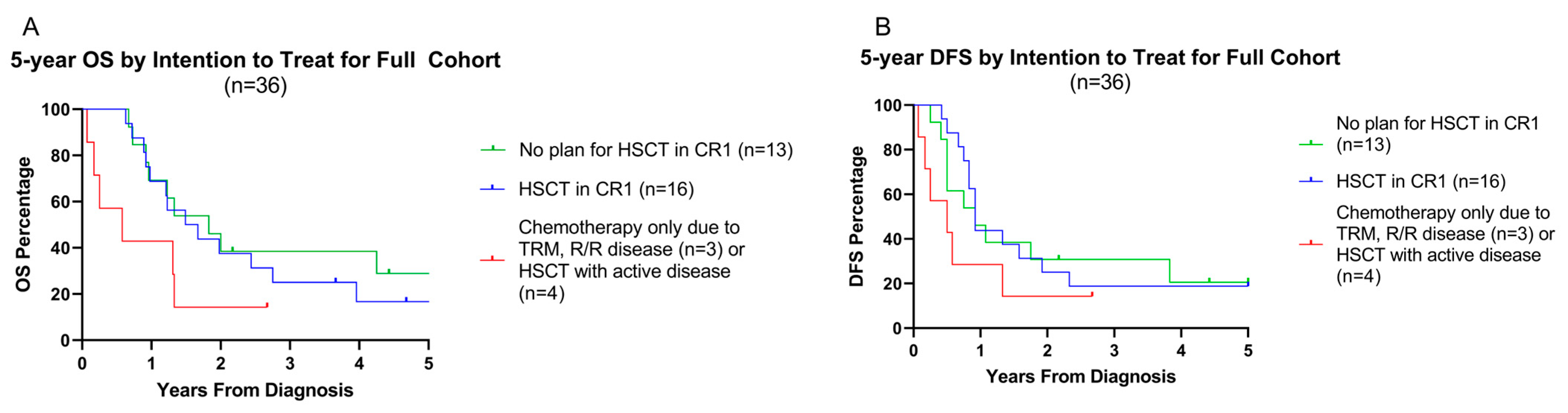
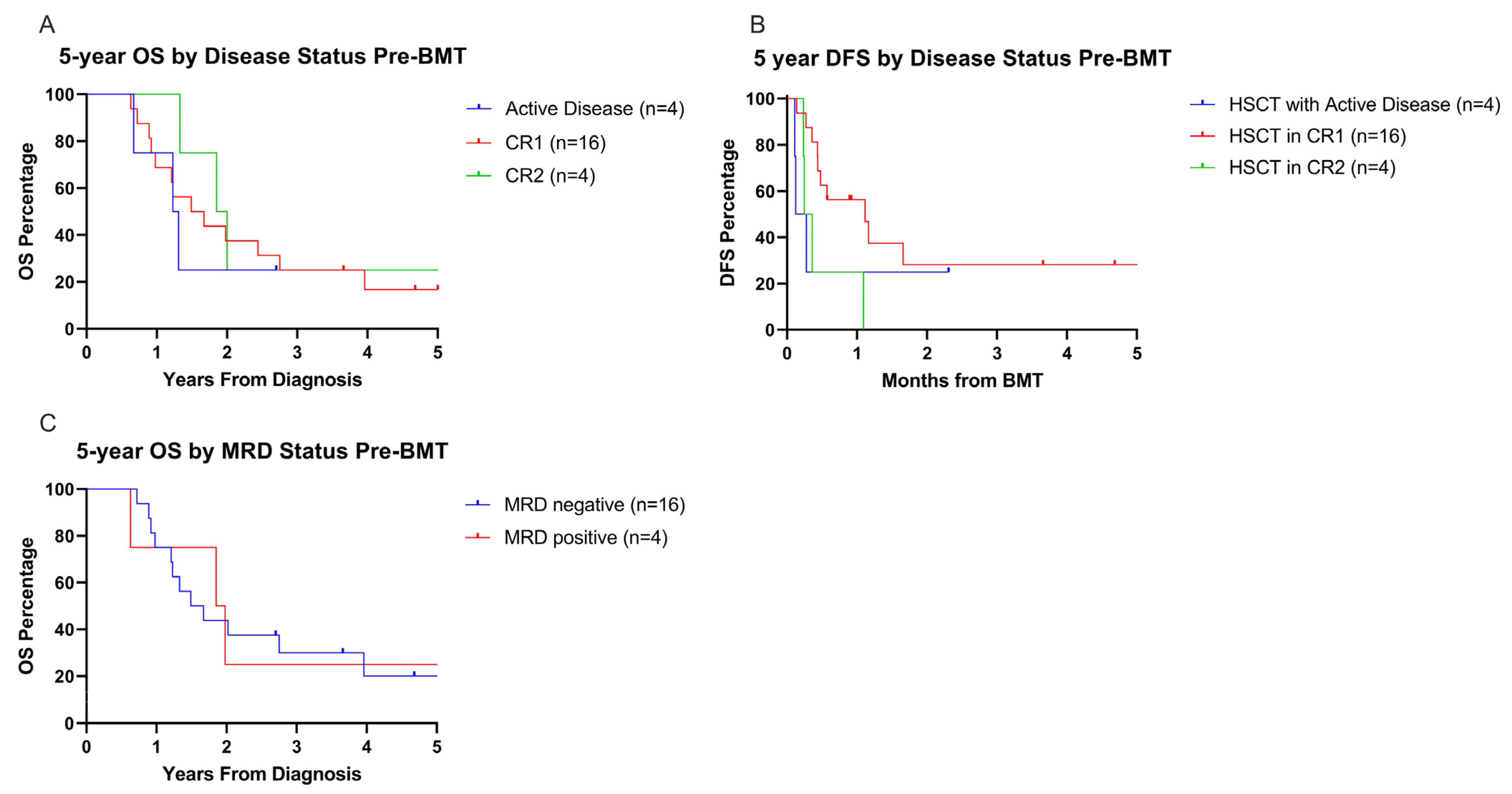
3.6. Influence of Pre-HSCT Disease Status on Overall and Disease-Free Survival
3.7. Post-HSCT Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’BRien, M.M.; Cao, X.; Pounds, S.; Dahl, G.V.; Raimondi, S.C.; Lacayo, N.J.; Taub, J.; Chang, M.; Weinstein, H.J.; Ravindranath, Y.; et al. Prognostic features in acute megakaryoblastic leukemia in children without Down syndrome: A report from the AML02 multicenter trial and the Children’s Oncology Group Study POG 9421. Leukemia 2012, 27, 731–734. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Araki, M.; Komatsu, N. Molecular features, prognosis, and novel treatment options for pediatric acute megakaryoblastic leukemia. Expert Rev. Hematol. 2019, 12, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Athale, U.H.; Razzouk, B.I.; Raimondi, S.C.; Tong, X.; Behm, F.G.; Head, D.R.; Srivastava, D.K.; Rubnitz, J.E.; Bowman, L.; Pui, C.-H.; et al. Biology and outcome of childhood acute megakaryoblastic leukemia: A single institution’s experience. Blood 2001, 97, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, W.; Wang, X.; Gu, J. Clinical diagnosis of adult patients with acute megakaryocytic leukemia. Oncol. Lett. 2018, 16, 6988–6997. [Google Scholar] [CrossRef]
- Brodersen, L.E.; A Alonzo, T.; Menssen, A.J.; Gerbing, R.B.; Pardo, L.; Voigt, A.P.; Kahwash, S.B.; Hirsch, B.; Raimondi, S.; Gamis, A.S.; et al. A recurrent immunophenotype at diagnosis independently identifies high-risk pediatric acute myeloid leukemia: A report from Children’s Oncology Group. Leukemia 2016, 30, 2077–2080. [Google Scholar] [CrossRef]
- Gruber, T.A.; Downing, J.R. The biology of pediatric acute megakaryoblastic leukemia. Blood 2015, 126, 943–949. [Google Scholar] [CrossRef]
- Gruber, T.A.; Gedman, A.L.; Zhang, J.; Koss, C.S.; Marada, S.; Ta, H.Q.; Chen, S.C.; Su, X.; Ogden, S.K.; Dang, J.; et al. An Inv(16)(p13.3q24.3)-Encoded CBFA2T3-GLIS2 Fusion Protein Defines an Aggressive Subtype of Pediatric Acute Megakaryoblastic Leukemia. Cancer Cell 2012, 22, 683–697. [Google Scholar] [CrossRef]
- Pagano, L.; Pulsoni, A.; Vignetti, M.; Mele, L.; Fianchi, L.; Petti, M.; Mirto, S.; Falcucci, P.; Fazi, P.; Broccia, G.; et al. Acute megakaryoblastic leukemia: Experience of GIMEMA trials. Leukemia 2002, 16, 1622–1626. [Google Scholar] [CrossRef]
- Giri, S.; Pathak, R.; Prouet, P.; Li, B.; Martin, M.G. Acute megakaryocytic leukemia is associated with worse outcomes than other types of acute myeloid leukemia. Blood 2014, 124, 3833–3834. [Google Scholar] [CrossRef]
- Teyssier, A.C.; Lapillonne, H.; Pasquet, M.; Ballerini, P.; Baruchel, A.; Ducassou, S.; Fenneteau, O.; Petit, A.; Cuccuini, W.; Ragu, C.; et al. Acute megakaryoblastic leukemia (excluding Down syndrome) remains an acute myeloid subgroup with inferior outcome in the French ELAM02 trial. Pediatr. Hematol. Oncol. 2017, 34, 425–427. [Google Scholar] [CrossRef]
- Taub, J.W.; Berman, J.N.; Hitzler, J.K.; Sorrell, A.D.; Lacayo, N.J.; Mast, K.; Head, D.; Raimondi, S.; Hirsch, B.; Ge, Y.; et al. Improved outcomes for myeloid leukemia of Down syndrome: A report from the Children’s Oncology Group AAML0431 trial. Blood 2017, 129, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, K.M.; Smith, J.; Heerema-McKenney, A.E.; Choi, J.K.; Ries, R.E.; Hirsch, B.A.; Raimondi, S.C.; Wang, Y.; Dang, A.; Alonzo, T.A.; et al. Pathologic, cytogenetic, and molecular features of acute myeloid leukemia with megakaryocytic differentiation: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2023, 70, e30251. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.C.; Oliveira, M.S.; Fairclough, D.; Hurwitz, C.; Mirro, J.; Behm, F.G.; Head, D.; Silva, M.L.; Raimondi, S.C.; Crist, W.M.; et al. Acute Megakaryoblastic Leukemia in Children and Adolescents: A Retrospective Analysis of 24 Cases. Leuk. Lymphoma 1993, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Taga, T.; Tomizawa, D.; Muramatsu, H.; Hasegawa, D.; Adachi, S.; Yoshida, N.; Noguchi, M.; Sato, M.; Okada, K.; et al. Haematopoietic cell transplantation for children with acute megakaryoblastic leukaemia without Down syndrome. Br. J. Haematol. 2023, 201, 747–756. [Google Scholar] [CrossRef]
- Schweitzer, J.; Zimmermann, M.; Rasche, M.; von Neuhoff, C.; Creutzig, U.; Dworzak, M.; Reinhardt, D.; Klusmann, J.H. Improved outcome of pediatric patients with acute megakaryoblastic leukemia in the AML-BFM 04 trial. Ann. Hematol. 2015, 94, 1327–1336. [Google Scholar] [CrossRef]
- Reinhardt, D.; Diekamp, S.; Langebrake, C.; Ritter, J.; Stary, J.; Dworzak, M.; Schrauder, A.; Zimmermann, M.; Fleischhack, G.; Ludwig, W.D.; et al. Acute megakaryoblastic leukemia in children and adolescents, excluding Down’s syndrome: Improved outcome with intensified induction treatment. Leukemia 2005, 19, 1495–1496. [Google Scholar] [CrossRef]
- de Rooij, J.D.E.; Masetti, R.; Heuvel-Eibrink, M.M.v.D.; Cayuela, J.M.; Trka, J.; Reinhardt, D.; Rasche, M.; Sonneveld, E.; Alonzo, T.A.; Fornerod, M.; et al. Recurrent abnormalities can be used for risk group stratification in pediatric AMKL: A retrospective intergroup study. Blood 2016, 127, 3424–3430. [Google Scholar] [CrossRef]
- de Rooij, J.D.E.; Branstetter, C.; Ma, J.; Li, Y.; Walsh, M.P.; Cheng, J.; Obulkasim, A.; Dang, J.; Easton, J.; Verboon, L.J.; et al. Pediatric non–Down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nat. Genet. 2017, 49, 451–456. [Google Scholar] [CrossRef]
- Cooper, T.M.; Ries, R.E.; Alonzo, T.A.; Gerbing, R.B.; Loken, M.R.; Broderson, L.E.; Raimondi, S.C.; Hirsch, B.A.; Aplenc, R.; Gamis, A.S.; et al. Revised Risk Stratification Criteria for Children with Newly Diagnosed Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Blood 2017, 130, 407. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. CLINICAL MANIFESTATIONS OF GRAFT-VERSUS-HOST DISEASE IN HUMAN RECIPIENTS OF MARROW FROM HL-A-MATCHED SIBLING DONOR,S. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Doherty, E.E.; Redell, M.; Sasa, G.; Yassine, K.; John, T.D.; Craddock, J.; Wu, M.; Wang, T.; Martinez, C.A.; Krance, R.A.; et al. Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation for Pediatric Acute Myeloid Leukemia in the Contemporary Era. Blood 2019, 134, 2056. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, A.; Jia, Y.; Zuo, Y.; Zhang, L. Outcome and Prognostic Features in Pediatric Acute Megakaryoblastic Leukemia Without Down Syndrome: A Retrospective Study in China. Clin. Lymphoma Myeloma Leuk. 2021, 21, e301–e308. [Google Scholar] [CrossRef]
- Getz, K.D.; Alonzo, T.A.; Sung, L.; Meshinchi, S.; Gerbing, R.B.; Raimondi, S.; Hirsch, B.; Loken, M.; Brodersen, L.E.; Kahwash, S.; et al. Cytarabine dose reduction in patients with low-risk acute myeloid leukemia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2021, 69, e29313. [Google Scholar] [CrossRef]
- Foster, J.H.; Williams, C.L.; Elghetany, M.T.; Liu, P.; Krance, R.A.; Bertuch, A.A.; Gramatges, M.M. Monozygotic twins with non-Down syndrome associated MLL-rearranged hematologic malignancy and megakaryoblastic differentiation. Leuk. Lymphoma 2018, 60, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.S.; E Sale, G.; Bryant, E.M.; Sanders, J.; Buckner, C.D. Acute megakaryoblastic leukemia in children: Treatment with bone marrow transplantation. Bone Marrow Transplant. 1992, 10, 399–403. [Google Scholar] [PubMed]
- Sharma, A.; Kang, G.; Cunningham, L.; Madden, R.; Qudeimat, A.; Triplett, B.M. Allogeneic Hematopoietic Cell Transplantation for Acute Megakaryoblastic Leukemia: A Single Center Experience. Biol. Blood Marrow Transplant. 2017, 23, S299–S300. [Google Scholar] [CrossRef]
- Harney, S.M.; Jin, Z.; Ricci, A.M.; Kahn, J.; Wong, P.; McKetta, S.; Satwani, P. Race and socoioeconomic status in pediatric allogeneic hematopoietic cell transplantation: A single-center study. J. Clin. Oncol. 2018, 36, e18659. [Google Scholar] [CrossRef]
- Conneely, S.E.; McAtee, C.L.; Gupta, R.; Lubega, J.; Scheurer, M.E.; Rau, R.E. Association of race and ethnicity with clinical phenotype, genetics, and survival in pediatric acute myeloid leukemia. Blood Adv. 2021, 5, 4992–5001. [Google Scholar] [CrossRef]
- Lamba, J.K.; Marrero, R.; Wu, H.; Cao, X.; Parcha, P.K.; Karol, S.E.; Inaba, H.; Kuo, D.J.; Degar, B.A.; Heym, K.; et al. Pharmacogenomics, Race, and Treatment Outcome in Pediatric Acute Myeloid Leukemia. JAMA Netw. Open 2024, 7, e2411726. [Google Scholar] [CrossRef]
- Garderet, L.; Labopin, M.; Gorin, N.C.; Polge, E.; Baruchel, A.; Meloni, G.; Ortega, J.; Vossen, J.; Bunjes, D.; Leverger, G.; et al. Hematopoietic stem cell transplantation for de novo acute megakaryocytic leukemia in first complete remission: A retrospective study of the European Group for Blood and Marrow Transplantation (EBMT). Blood 2005, 105, 405–409. [Google Scholar] [CrossRef]
- Lamble, A.J.; Tasian, S.K. Opportunities for immunotherapy in childhood acute myeloid leukemia. Blood Adv. 2019, 3, 3750–3758. [Google Scholar] [CrossRef]
- Krivtsov, A.V.; Evans, K.; Gadrey, J.Y.; Eschle, B.K.; Hatton, C.; Uckelmann, H.J.; Ross, K.N.; Perner, F.; Olsen, S.N.; Pritchard, T.; et al. A Menin-MLL Inhibitor Induces Specific Chromatin Changes and Eradicates Disease in Models of MLL-Rearranged Leukemia. Cancer Cell 2019, 36, 660–673.e11. [Google Scholar] [CrossRef]
- Tang, T.; Le, Q.; Castro, S.; Pardo, L.; McKay, C.N.; Perkins, L.; Smith, J.; Kirkey, D.; Abrahams, C.; Bedard, K.; et al. Targeting FOLR1 in high-risk CBF2AT3-GLIS2 pediatric AML with STRO-002 FOLR1–antibody-drug conjugate. Blood Adv. 2022, 6, 5933–5937. [Google Scholar] [CrossRef]

| Patient and Disease Characteristics—By Intention to Treat | |||
|---|---|---|---|
| Chemotherapy Alone n = 12 | Chemotherapy and Allo-HSCT (n = 17) | p-Value | |
| Gender | Number (%) | Number (%) | 0.67 |
| Male | 4 (33) | 7 (41) | |
| Female | 8 (67) | 10 (59) | |
| Self-Described Race/Ethnicity | 0.13 | ||
| Hispanic White | 8 (67) | 4 (24) | |
| Non-Hispanic African American | 3 (25) | 7 (41) | |
| Non-Hispanic White | 1 (8) | 4 (24) | |
| Other * | 0 | 2 (12) | |
| Age At Diagnosis (years) | 0.60 | ||
| Median | 2 | 2 | |
| Range | 0.7–17 | 0.6–15 | |
| 0 to 5 years | 10 (75) | 13 (76) | |
| 6 to 10 years | 0 | 2 (12) | |
| 11 to 15 years | 1 (8) | 2 (12) | |
| >15 years | 1 (8) | 0 | |
| CNS Disease Status at Diagnosis | 0.25 | ||
| Positive | 1 (8) | 4 (24) | |
| Negative | 11 (92) | 12 (71) | |
| Unknown | 0 | 1 (6) | |
| Cytogenetics | 0.09 | ||
| Monosomy 7 | 0 | 2 (12) | |
| KMT2A-rearranged | 1 (8) | 5 (29) | |
| CBFA2T3::GLIS2 fusion | 1 (8) | 3 (18) | |
| Normal | 3 (25) | 0 | |
| Other | 7 (58) | 6 (35) | |
| Not available | 0 | 1 (6) | |
| Risk Stratification | 0.015 | ||
| Low Risk | 9 (75) | 5 (29) | |
| High Risk | 3 (25) | 12 (71) | |
| Initial Chemotherapy Regimen ** | 0.38 | ||
| POG 9421 | 1 (8) | 4 (24) | |
| AML 2002 | 1 (8) | 2 (12) | |
| AAML0531 | 5 (42) | 2 (12) | |
| AAML1031 | 5 (42) | 8 (47) | |
| AAML1831 | 0 | 1 (6) | |
| Patient and Transplant Characteristics for Patients Treated with Chemotherapy and Allo-HSCT, n = 24 | ||||
|---|---|---|---|---|
| Disease status before allo-HSCT | Number (%) | Conditioning regimen | Number (%) | |
| CR 1 | 16 (67) | Busulfan/Cyclophosphamide based ^ | 12 (50) | |
| CR2 | 4 (16) | Cytarabine/Cyclophosphamide based & | 7 (29) | |
| Active | 4 (16) | Fludarabine/Cyclophosphamide | 2 (8) | |
| MRD status by flow cytometry before allo-HSCT (n = 20) | Other # | 3 (13) | ||
| Positive | 4 (20) | Relapsed after allo-HSCT | ||
| Negative | 16 (80) | Yes | 17 (71) | |
| Year of allo-HSCT | No | 7 (29) | ||
| 2000–2009 | 9 (38) | Post-HSCT day at relapse | ||
| 2010–2022 | 15 (62) | Median | 131 | |
| Age at time of allo-HSCT (years) | Range | 40–425 | ||
| Median | 3 | Acute graft-versus-host disease grade | ||
| Range | 0.8–19 | None | 18 (75) | |
| Time from diagnosis to allo-HSCT (months) | Grade I–II | 3 (13) | ||
| Median | 5 | Grade III–IV | 2 (8) | |
| Range | 2.7–23.2 | Unknown | 1 (4) | |
| Indication for allo-HSCT | Chronic graft-versus-host disease | |||
| Induction failure * | 3 (13) | None | 22 (92) | |
| Considered high-risk disease | 8 (33) | Limited | 0 | |
| MRD+ disease after induction I/II | 8 (33) | Extensive | 1 (4) | |
| Relapsed disease after chemotherapy only | 5 (21) | Unknown | 1 (4) | |
| Stem Cell Source | Censored for Second Allo-HSCT | |||
| Bone marrow | 15 (62) | Yes | 9 (38) | |
| Peripheral blood stem cells ** | 5 (21) | No | 15 (62) | |
| Umbilical cord blood | 3 (13) | Indication for Second Allo-HSCT | ||
| Unavailable | 1 (4) | Post-HSCT Relapse | 7 (78) | |
| Donor Type | Poor Graft Function | 2 (22) | ||
| Matched sibling donor | 4 (16) | |||
| Matched unrelated donor | 9 (38) | |||
| Mismatched unrelated donor | 2 (8) | |||
| Haploidentical | 9 (38) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llaurador, G.; Willis, M.; Redell, M.S.; Gramatges, M.M.; Marcogliese, A.N.; Naik, S.; Krance, R.; Doherty, E.; Stevens, A.M. Pediatric Non-Down Syndrome Acute Megakaryoblastic Leukemia Patients Have Dismal Outcomes Irrespective of Allogeneic Hematopoietic Stem Cell Transplant: A Single-Center Experience. Cancers 2025, 17, 3511. https://doi.org/10.3390/cancers17213511
Llaurador G, Willis M, Redell MS, Gramatges MM, Marcogliese AN, Naik S, Krance R, Doherty E, Stevens AM. Pediatric Non-Down Syndrome Acute Megakaryoblastic Leukemia Patients Have Dismal Outcomes Irrespective of Allogeneic Hematopoietic Stem Cell Transplant: A Single-Center Experience. Cancers. 2025; 17(21):3511. https://doi.org/10.3390/cancers17213511
Chicago/Turabian StyleLlaurador, Gabriela, Matthew Willis, Michele S. Redell, M. Monica Gramatges, Andrea N. Marcogliese, Swati Naik, Robert Krance, Erin Doherty, and Alexandra M. Stevens. 2025. "Pediatric Non-Down Syndrome Acute Megakaryoblastic Leukemia Patients Have Dismal Outcomes Irrespective of Allogeneic Hematopoietic Stem Cell Transplant: A Single-Center Experience" Cancers 17, no. 21: 3511. https://doi.org/10.3390/cancers17213511
APA StyleLlaurador, G., Willis, M., Redell, M. S., Gramatges, M. M., Marcogliese, A. N., Naik, S., Krance, R., Doherty, E., & Stevens, A. M. (2025). Pediatric Non-Down Syndrome Acute Megakaryoblastic Leukemia Patients Have Dismal Outcomes Irrespective of Allogeneic Hematopoietic Stem Cell Transplant: A Single-Center Experience. Cancers, 17(21), 3511. https://doi.org/10.3390/cancers17213511







