The Unfolded Protein Response in Sarcomas: From Proteostasis to Therapy Resistance
Simple Summary
Abstract
1. Introduction
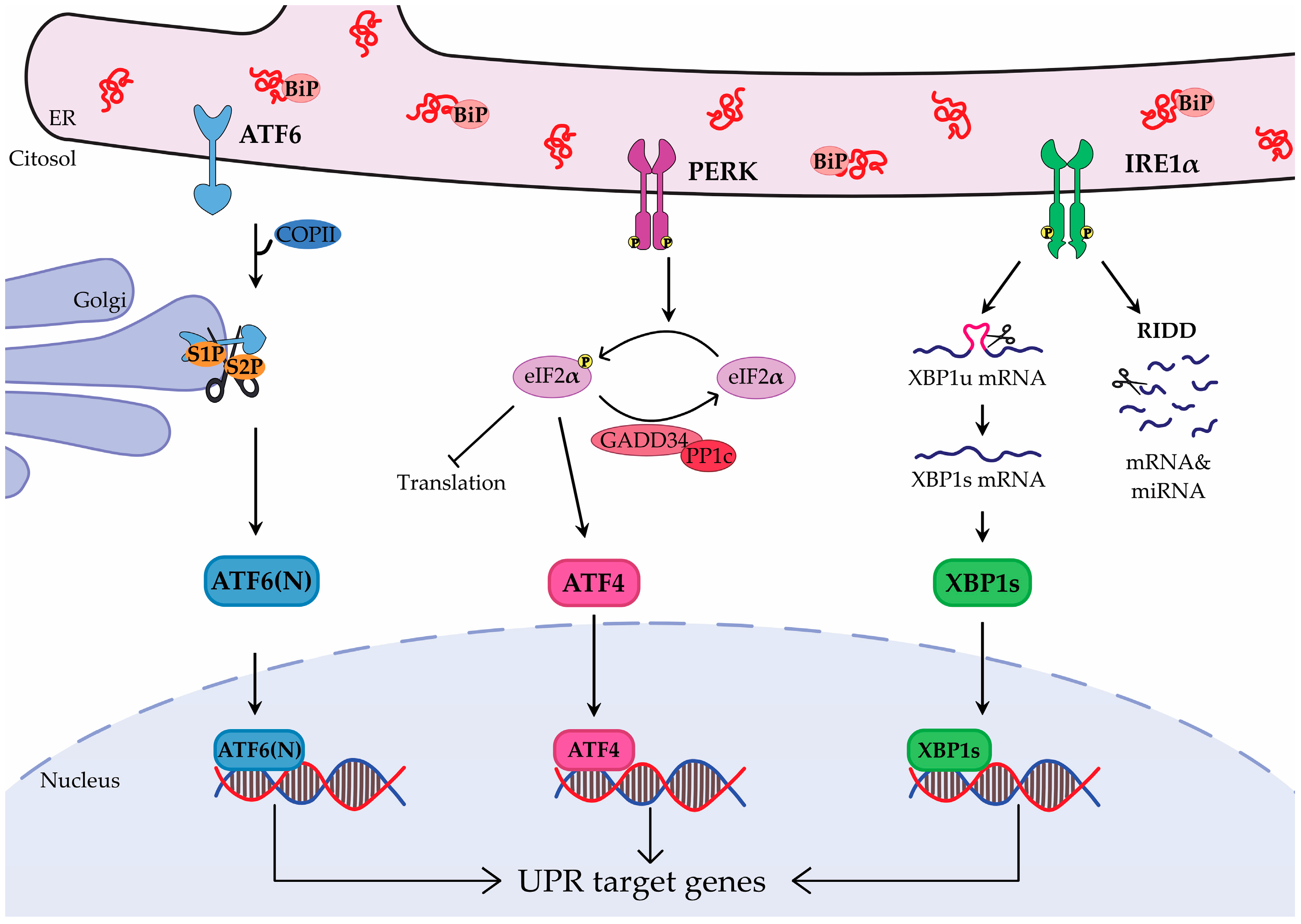
2. Unfolded Protein Response
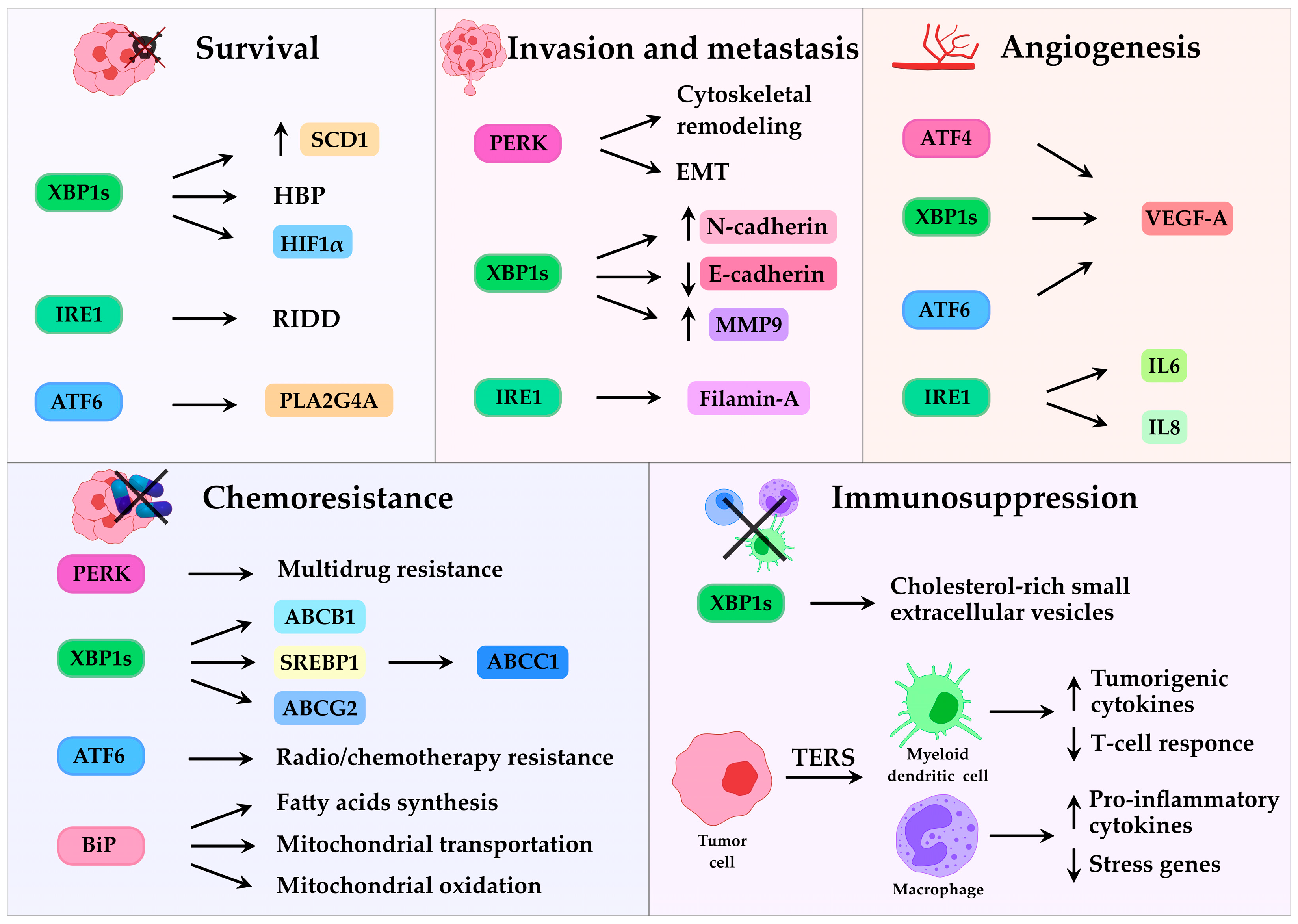
3. UPR in Cancer
3.1. UPR and Tumor Progression
3.2. UPR and Lipid Metabolism
3.3. UPR and Resistance to Therapy
3.4. UPR and Tumor Microenvironment
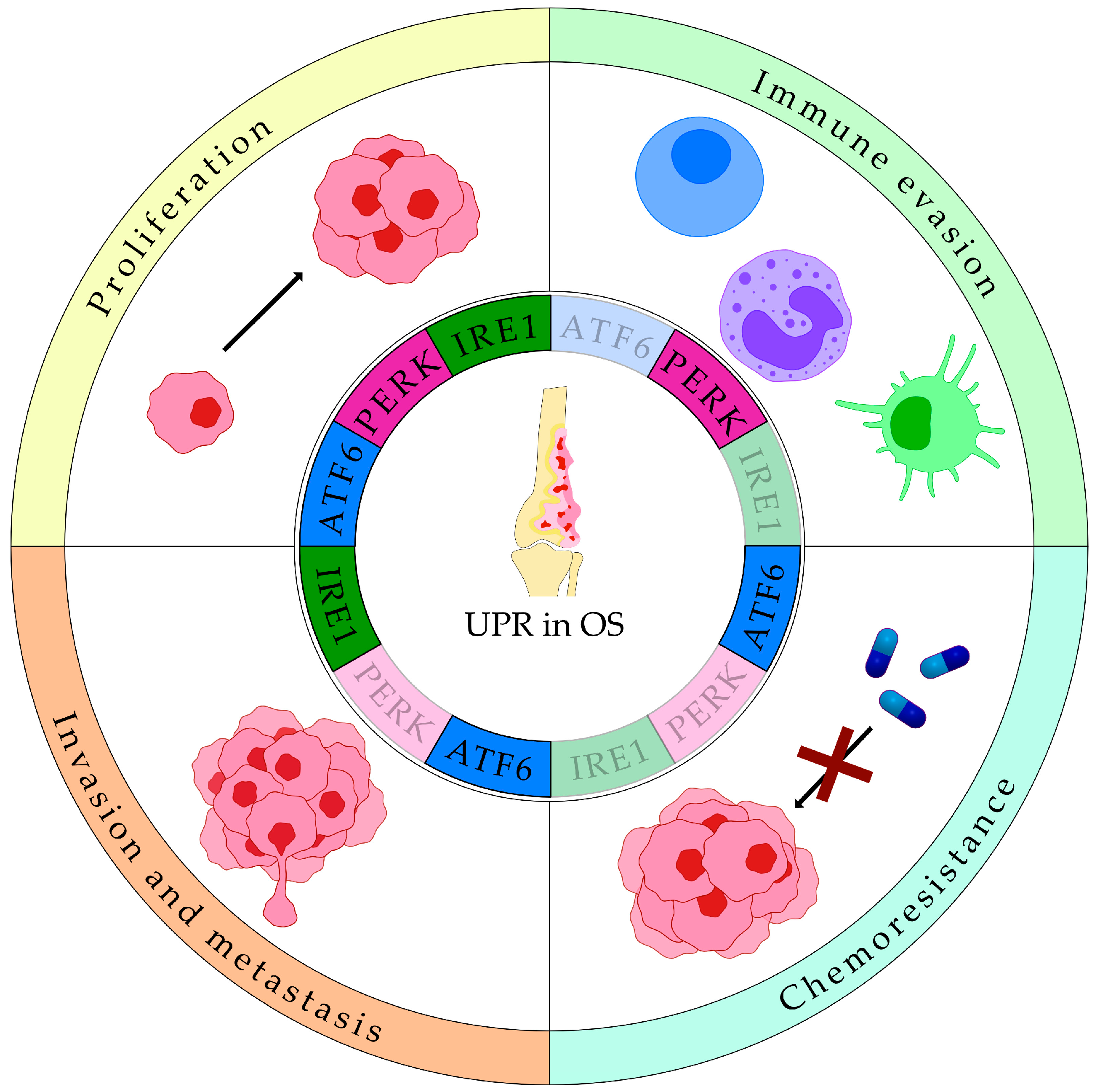
4. UPR in Sarcomas
4.1. UPR in Bone Sarcomas
4.1.1. UPR in Osteosarcoma
| UPR Markers | Datasets | Normal Tissue Control | Clinical Correlation | Total Number of Patients/Samples | Reference |
|---|---|---|---|---|---|
| BiP, XBP1s, ATF4 and ATF6α | GSE99671, GSE126209, GSE21257, TARGET, Zhengzhou, TCGA sarcoma dataset | Yes | Tumor growth, low immune infiltration, reduced immunotherapy response, poor survival | 512 | [13] |
| UPR related genes (STC2, PREB, TSPYL2, and ATP6V0D1) | GSE21257, TARGET | Yes | immune infiltration and prognosis | 138 | [107] |
| ATF6α | GSE152048 and GSE162454 | Yes | OS progression and poor prognosis | 110,000 single cells from 17 samples | [15] |
| BiP | Maharaj Nakorn Chiang Mai Hospital | Yes | Chemoresistance | 20 | [12] |
| ATF6α(N) | Phoenix Children’s Hospital | Yes | Metastatic disease and poor response to chemotherapy | 40 | [17] |
| XBP1s | Shanghai Jiao Tong University Affiliated Sixth People’s Hospital | Yes | Disease progression and low tumor necrosis rate | 40 | [111] |
| STC2 | GSE21257, GSE33382 and TARGET | Yes | Poor survival | 225 | [113] |
4.1.2. UPR in Ewing’s Sarcoma
4.2. UPR in Soft-Tissue Sarcomas
5. Targeting the UPR in Cancer
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCB1 | ABC subfamily B member 1 |
| ABCG2 | ABC subfamily G member 2 |
| ATF4 | activating transcription factor 4 |
| ATF6 | activating transcription factor 6 |
| ASK1 | apoptosis signal-regulating kinase 1 |
| BMAL1 | basic helix-loop-helix ARNT like 1 |
| BAK | BCL2 antagonist/killer 1 |
| BCL-2 | BCL2 apoptosis regulator |
| BAX | BCL2 associated X protein |
| BIM | BCL-2 interacting mediator of cell death |
| BiP | binding immunoglobulin protein |
| BTZ | bortezomib |
| CHOP | C/EBP homologous protein |
| JNK | c-Jun N-terminal kinase |
| CLOCK | clock circadian regulator |
| PLA2G4A | cytosolic phospholipase A2 |
| DGAT2 | diacylglycerol O-acyltransferase 2 |
| PKR | double-stranded RNA-activated protein kinase |
| DOX | doxorubicin |
| eIF2α | α-subunit of eukaryotic initiation factor 2 |
| ER | endoplasmic reticulum |
| EMT | epithelial–mesenchymal transition |
| ERAD | ER-associated protein degradation |
| ES | Ewing’s sarcoma |
| EWS | Ewing sarcoma breakpoint region 1 |
| FGF | fibroblast growth factor |
| FLI1 | friend leukemia integration 1 transcription factor |
| GNL3 | G protein nucleolar 3 |
| GGDPS | geranylgeranyl diphosphate synthase |
| GRP78 | glucose-regulated protein 78 |
| GADD45GIP1 | growth arrest and DNA damage-inducible gamma interacting protein 1 |
| GADD34 | growth arrest and DNA damage–inducible gene 34 |
| HSP70 | heat shock 70 kDa protein |
| HSP90B1 | heat shock protein 90 beta family member 1 |
| HBP | hexosamine biosynthetic pathway |
| HHV-8 | human herpesvirus 8 |
| HIV | human immunodeficiency virus |
| HIF1α | hypoxia-inducible factor 1 alpha |
| IRE1 | inositol-requiring enzyme 1 |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| KLF4 | Kruppel-like factor 4 |
| MPNSTs | malignant peripheral nerve sheath tumors |
| MMP9 | matrix metalloproteinase-9 |
| LAMA4 | laminin subunit alpha 4 |
| mTOR | mechanistic target of rapamycin kinase |
| MRP1 | multidrug resistance-associated protein 1 |
| O-GlcNAcylation | N-linked glycosylation |
| NLRP3 | NLR family pyrin domain containing 3 |
| ATF6(N) | N-terminal ATF6 fragment |
| oHSV | oncolytic herpes simplex virus-1 |
| OS | osteosarcoma |
| PUMA | p53 upregulated modulator of apoptosis |
| NOXA | phorbol-12-myristate-13-acetate-induced protein 1 |
| PTEN | phosphatase and tensin homolog |
| PIK3R3 | phosphoinositide-3-kinase regulatory subunit 3 |
| PERK | PKR-like ER kinase |
| PDGF | platelet-derived growth factor |
| PRP-1 | proline-rich polypeptide |
| PDI | protein disulfide isomerase |
| PP1C | protein phosphatase 1 |
| RIDD | regulated IRE1-dependent decay |
| RMS | rhabdomyosarcoma |
| S1P | site-1 protease |
| S2P | site-2 protease |
| STC2 | stanniocalcin 2 |
| SCD1 | stearoyl-CoA desaturase 1 |
| SREBP1 | sterol regulatory element-binding protein 1 |
| TXNIP | thioredoxin interacting protein |
| TGF-β | transforming growth factor beta |
| TERS | transmissible ER stress |
| TNBC | triple-negative breast cancer |
| TRDMT1 | tRNA aspartic acid methyltransferase 1 |
| TRAF2 | tumor necrosis factor receptor-associated factor 2 |
| TME | tumor microenvironment |
| p53 | tumor protein P53 |
| UPR | unfolded protein response |
| UDP-GlcNAc | uridine diphosphate-N-acetylglucosamine |
| p97 | valosin containing protein |
| VEGF-A | vascular endothelial growth factor-A |
| VEGFR2 | VEGF receptor 2 |
| XBP1 | X-box binding protein 1 |
References
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- Al Shihabi, A.; Tebon, P.J.; Nguyen, H.T.L.; Chantharasamee, J.; Sartini, S.; Davarifar, A.; Jensen, A.Y.; Diaz-Infante, M.; Cox, H.; Gonzalez, A.E.; et al. The landscape of drug sensitivity and resistance in sarcoma. Cell Stem Cell 2024, 31, 1524–1542.e4. [Google Scholar] [CrossRef] [PubMed]
- Damerell, V.; Pepper, M.S.; Prince, S. Molecular mechanisms underpinning sarcomas and implications for current and future therapy. Signal Transduct. Target. Ther. 2021, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Dancsok, A.R.; Asleh-Aburaya, K.; Nielsen, T.O. Advances in sarcoma diagnostics and treatment. Oncotarget 2016, 8, 7068–7093. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Avril, T.; Chevet, E.; Hetz, C. Endoplasmic Reticulum Stress and the Hallmarks of Cancer. Trends Cancer 2016, 2, 252–262. [Google Scholar] [CrossRef]
- Le Reste, P.-J.; Avril, T.; Quillien, V.; Morandi, X.; Chevet, E. Signaling the Unfolded Protein Response in primary brain cancers. Brain Res. 2016, 1642, 59–69. [Google Scholar] [CrossRef]
- Rajapaksa, G.; Thomas, C.; Gustafsson, J. Estrogen signaling and unfolded protein response in breast cancer. J. Steroid Biochem. Mol. Biol. 2016, 163, 45–50. [Google Scholar] [CrossRef]
- Rufo, N.; Yang, Y.; De Vleeschouwer, S.; Agostinis, P. The “Yin and Yang” of Unfolded Protein Response in Cancer and Immunogenic Cell Death. Cells 2022, 11, 2899. [Google Scholar] [CrossRef] [PubMed]
- Soumya, V.V.; Jisna, B.; Anu, D.; Binoy, C.F.; Babu, T.D. IRE1α-mediated UPR activation in gastrointestinal cancers: Adaptive mechanisms and therapeutic potential. Drug Discov. Today 2025, 30, 104335. [Google Scholar] [CrossRef]
- Storm, M.; Sheng, X.; Arnoldussen, Y.J.; Saatcioglu, F. Prostate cancer and the unfolded protein response. Oncotarget 2016, 7, 54051–54066. [Google Scholar] [CrossRef]
- Vincenz, L.; Jäger, R.; O’Dwyer, M.; Samali, A. Endoplasmic Reticulum Stress and the Unfolded Protein Response: Targeting the Achilles Heel of Multiple Myeloma. Mol. Cancer Ther. 2013, 12, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Chaiyawat, P.; Sungngam, P.; Teeyakasem, P.; Sirikaew, N.; Klangjorhor, J.; Settakorn, J.; Diskul-Na-Ayudthaya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Svasti, J.; et al. Protein profiling of osteosarcoma tissue and soft callus unveils activation of the unfolded protein response pathway. Int. J. Oncol. 2019, 54, 1704–1718. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, F.; Zhang, T.; Xu, D.; Hao, Z.; Cui, F.; Shi, J.; Jin, Y.; Li, N.; Yang, C.; et al. A novel prognostic signature in osteosarcoma characterised from the perspective of unfolded protein response. Clin. Transl. Med. 2022, 12, e750. [Google Scholar] [CrossRef]
- Tanabe, Y.; Suehara, Y.; Kohsaka, S.; Hayashi, T.; Akaike, K.; Mukaihara, K.; Kurihara, T.; Kim, Y.; Okubo, T.; Ishii, M.; et al. IRE1α-XBP1 inhibitors exerted anti-tumor activities in Ewing’s sarcoma. Oncotarget 2018, 9, 14428–14443. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Yang, Y.; Wang, K.; Wei, J.; Shi, J.-H.; Zhang, D.; Sheng, X.; Zhang, Y.; Zhou, J.; et al. Integrated analysis of single-cell and bulk transcriptomics reveals cellular subtypes and molecular features associated with osteosarcoma prognosis. BMC Cancer 2025, 25, 280. [Google Scholar] [CrossRef]
- McCarthy, N.; Dolgikh, N.; Logue, S.; Patterson, J.B.; Zeng, Q.; Gorman, A.M.; Samali, A.; Fulda, S. The IRE1 and PERK arms of the unfolded protein response promote survival of rhabdomyosarcoma cells. Cancer Lett. 2020, 490, 76–88. [Google Scholar] [CrossRef]
- Yarapureddy, S.; Abril, J.; Foote, J.; Kumar, S.; Asad, O.; Sharath, V.; Faraj, J.; Daniel, D.; Dickman, P.; White-Collins, A.; et al. ATF6α Activation Enhances Survival against Chemotherapy and Serves as a Prognostic Indicator in Osteosarcoma. Neoplasia 2019, 21, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, X.; Wang, W.; Liu, P. Inhibition of PERK pathway promotes TAMs M1 polarization and ER stress in Osteosarcoma cells hindering tumor progression. Int. Immunopharmacol. 2025, 163, 115293. [Google Scholar] [CrossRef]
- Anspach, L.; Tsaryk, R.; Seidmann, L.; Unger, R.E.; Jayasinghe, C.; Simiantonaki, N.; Kirkpatrick, C.J.; Pröls, F. Function and mutual interaction of BiP-, PERK-, and IRE1α-dependent signalling pathways in vascular tumours. J. Pathol. 2020, 251, 123–134. [Google Scholar] [CrossRef]
- Mori, K. Signalling Pathways in the Unfolded Protein Response: Development from Yeast to Mammals. J. Biochem. 2009, 146, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Hien, L.T.; Back, S.H. Establishment of a reporter system for monitoring activation of the ER stress transducer ATF6β. Biochem. Biophys. Res. Commun. 2021, 558, 1–7. [Google Scholar] [CrossRef]
- Luo, H.; Gong, W.-Y.; Zhang, Y.-Y.; Liu, Y.-Y.; Chen, Z.; Feng, X.-L.; Jiao, Q.-B.; Zhang, X.-W. IRE1β evolves to be a guardian of respiratory and gastrointestinal mucosa. Heliyon 2024, 10, e39011. [Google Scholar] [CrossRef]
- Worby, C.A.; Dixon, J.E. Unpacking the Unfolded Protein Response. Cell 2014, 158, 1221–1224. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Schindler, A.J.; Schekman, R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc. Natl. Acad. Sci. USA 2009, 106, 17775–17780. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER Stress Induces Cleavage of Membrane-Bound ATF6 by the Same Proteases that Process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Wu, J.; Rutkowski, D.T.; Dubois, M.; Swathirajan, J.; Saunders, T.; Wang, J.; Song, B.; Yau, G.D.-Y.; Kaufman, R.J. ATF6α Optimizes Long-Term Endoplasmic Reticulum Function to Protect Cells from Chronic Stress. Dev. Cell 2007, 13, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Carrara, M.; Prischi, F.; Nowak, P.R.; Kopp, M.C.; Ali, M.M. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. eLife 2015, 4, e03522. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, J.; Tao, J.; Sha, B. The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J. Biol. Chem. 2018, 293, 4110–4121. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk Is Essential for Translational Regulation and Cell Survival during the Unfolded Protein Response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.S.; et al. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback Inhibition of the Unfolded Protein Response by GADD34-Mediated Dephosphorylation of eIF2α. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef]
- Gardner, B.M.; Walter, P. Unfolded Proteins Are Ire1-Activating Ligands That Directly Induce the Unfolded Protein Response. Science 2011, 333, 1891–1894. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Ryno, L.M.; Genereux, J.C.; Moresco, J.J.; Tu, P.G.; Wu, C.; Yates, J.R., III; Su, A.I.; Kelly, J.W.; Wiseman, R.L. Stress-Independent Activation of XBP1s and/or ATF6 Reveals Three Functionally Diverse ER Proteostasis Environments. Cell Rep. 2013, 3, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Kang, T.-I.; So, J.-S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Hollien, J.; Lin, J.H.; Li, H.; Stevens, N.; Walter, P.; Weissman, J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009, 186, 323–331. [Google Scholar] [CrossRef]
- Upton, J.-P.; Wang, L.; Han, D.; Wang, E.S.; Huskey, N.E.; Lim, L.; Truitt, M.; McManus, M.T.; Ruggero, D.; Goga, A.; et al. IRE1α Cleaves Select microRNAs During ER Stress to Derepress Translation of Proapoptotic Caspase-2. Science 2012, 338, 818–822. [Google Scholar] [CrossRef]
- Le Thomas, A.; Ferri, E.; Marsters, S.; Harnoss, J.M.; Lawrence, D.A.; Zuazo-Gaztelu, I.; Modrusan, Z.; Chan, S.; Solon, M.; Chalouni, C.; et al. Decoding non-canonical mRNA decay by the endoplasmic-reticulum stress sensor IRE1α. Nat. Commun. 2021, 12, 7310. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013, 1833, 3507–3517. [Google Scholar] [CrossRef]
- Han, D.; Lerner, A.G.; Walle, L.V.; Upton, J.-P.; Xu, W.; Hagen, A.; Backes, B.J.; Oakes, S.A.; Papa, F.R. IRE1α Kinase Activation Modes Control Alternate Endoribonuclease Outputs to Determine Divergent Cell Fates. Cell 2009, 138, 562–575. [Google Scholar] [CrossRef]
- Bronner, D.N.; Abuaita, B.H.; Chen, X.; Fitzgerald, K.A.; Nuñez, G.; He, Y.; Yin, X.-M.; O’riordan, M.X. Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage. Immunity 2015, 43, 451–462. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. The Unfolded Protein Response: At the Intersection between Endoplasmic Reticulum Function and Mitochondrial Bioenergetics. Front. Oncol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ronai, Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef]
- Urra, H.; Henriquez, D.R.; Cánovas, J.; Villarroel-Campos, D.; Carreras-Sureda, A.; Pulgar, E.; Molina, E.; Hazari, Y.M.; Limia, C.M.; Alvarez-Rojas, S.; et al. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol. 2018, 20, 942–953. [Google Scholar] [CrossRef]
- van Vliet, A.R.; Agostinis, P. PERK and filamin A in actin cytoskeleton remodeling at ER-plasma membrane contact sites. Mol. Cell. Oncol. 2017, 4, e1340105. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.E.L.; Tang, B.L. Linking membrane dynamics and trafficking to autophagy and the unfolded protein response. J. Cell. Physiol. 2013, 228, 1638–1640. [Google Scholar] [CrossRef]
- Horiuchi, K.; Tohmonda, T.; Morioka, H. The unfolded protein response in skeletal development and homeostasis. Cell. Mol. Life Sci. 2016, 73, 2851–2869. [Google Scholar] [CrossRef]
- Aronov, M.; Tirosh, B. Metabolic Control of Plasma Cell Differentiation- What We Know and What We Don’t Know. J. Clin. Immunol. 2016, 36, 12–17. [Google Scholar] [CrossRef]
- Lee, A.-H.; Chu, G.C.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005, 24, 4368–4380. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Moncan, M.; Mnich, K.; Blomme, A.; Almanza, A.; Samali, A.; Gorman, A.M. Regulation of lipid metabolism by the unfolded protein response. J. Cell. Mol. Med. 2021, 25, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Khatiwada, S.; Costa-Mattioli, M.; Hetz, C. ER Proteostasis Control of Neuronal Physiology and Synaptic Function. Trends Neurosci. 2018, 41, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Karali, E.; Bellou, S.; Stellas, D.; Klinakis, A.; Murphy, C.; Fotsis, T. VEGF Signals through ATF6 and PERK to Promote Endothelial Cell Survival and Angiogenesis in the Absence of ER Stress. Mol. Cell 2014, 54, 559–572. [Google Scholar] [CrossRef]
- Urra, H.; Aravena, R.; González-Johnson, L.; Hetz, C. The UPRising connection between endoplasmic reticulum stress and the tumor microenvironment. Trends Cancer 2024, 10, 1161–1173. [Google Scholar] [CrossRef]
- Obacz, J.; Avril, T.; Rubio-Patiño, C.; Bossowski, J.P.; Igbaria, A.; Ricci, J.; Chevet, E. Regulation of tumor–stroma interactions by the unfolded protein response. FEBS J. 2017, 286, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Chevet, E.; Hetz, C.; Samali, A. Endoplasmic Reticulum Stress–Activated Cell Reprogramming in Oncogenesis. Cancer Discov. 2015, 5, 586–597. [Google Scholar] [CrossRef]
- Oakes, S.A. Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am. J. Pathol. 2020, 190, 934–946. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Sokol, E.S.; Del Vecchio, C.A.; Sanduja, S.; Claessen, J.H.; Proia, T.A.; Jin, D.X.; Reinhardt, F.; Ploegh, H.L.; Wang, Q.; et al. Epithelial-to-Mesenchymal Transition Activates PERK–eIF2α and Sensitizes Cells to Endoplasmic Reticulum Stress. Cancer Discov. 2014, 4, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Gao, Y.; Wu, J.; Zeng, F.; Song, F. XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell. Signal. 2015, 27, 82–89. [Google Scholar] [CrossRef]
- Jin, C.; Jin, Z.; Chen, N.-Z.; Lu, M.; Liu, C.-B.; Hu, W.-L.; Zheng, C.-G. Activation of IRE1α-XBP1 pathway induces cell proliferation and invasion in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2016, 470, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, G.; Xian, X.; Li, J.; Zhang, D.; Guo, J.; Zhang, A.; Chen, S.; Yan, D.; Yang, B.; et al. Cancer cell-derived migrasomes harboring ATF6 promote breast cancer brain metastasis via endoplasmic reticulum stress-mediated disruption of the blood-brain barrier. Cancer Biol. Med. 2025, 22, 1–24. [Google Scholar] [CrossRef]
- Xia, T.; Tong, S.; Fan, K.; Zhai, W.; Fang, B.; Wang, S.-H.; Wang, J.-J. XBP1 induces MMP-9 expression to promote proliferation and invasion in human esophageal squamous cell carcinoma. Am. J. Cancer Res. 2016, 6, 2031–2040. [Google Scholar]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol. Cell 2019, 111, 1–17. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The role of lipids in cancer progression and metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef]
- Pinto, B.A.S.; França, L.M.; Laurindo, F.R.M.; Paes, A.M.d.A. Unfolded Protein Response: Cause or Consequence of Lipid and Lipoprotein Metabolism Disturbances? Adv. Exp. Med. Biol. 2019, 1127, 67–82. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, Y.; Feng, T.; Zhang, R.; Ge, L.; Pan, J.; Han, B.; Song, G.; Wang, L. ATF6α promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. Prostate 2022, 82, 617–629. [Google Scholar] [CrossRef]
- Almanza, A.; Mnich, K.; Blomme, A.; Robinson, C.M.; Rodriguez-Blanco, G.; Kierszniowska, S.; McGrath, E.P.; Le Gallo, M.; Pilalis, E.; Swinnen, J.V.; et al. Regulated IRE1α-dependent decay (RIDD)-mediated reprograming of lipid metabolism in cancer. Nat. Commun. 2022, 13, 2493. [Google Scholar] [CrossRef]
- Xu, Y.; Gui, F.; Zhang, Z.; Chen, Z.; Zhang, T.; Hu, Y.; Wei, H.; Fu, Y.; Chen, X.; Wu, Z. IRE1α-XBP1s axis regulates SREBP1-dependent MRP1 expression to promote chemoresistance in non-small cell lung cancer cells. Thorac. Cancer 2024, 15, 2116–2127. [Google Scholar] [CrossRef]
- Kopsida, M.; Clavero, A.L.; Khaled, J.; Balgoma, D.; Luna-Marco, C.; Chowdhury, A.; Nyman, S.S.; Rorsman, F.; Barbier, C.E.; Bergsten, P.; et al. Inhibiting the endoplasmic reticulum stress response enhances the effect of doxorubicin by altering the lipid metabolism of liver cancer cells. Mol. Metab. 2023, 79, 101846. [Google Scholar] [CrossRef]
- Cook, K.L.; Soto-Pantoja, D.R.; Clarke, P.A.; Cruz, M.I.; Zwart, A.; Wärri, A.; Hilakivi-Clarke, L.; Roberts, D.D.; Clarke, R. Endoplasmic Reticulum Stress Protein GRP78 Modulates Lipid Metabolism to Control Drug Sensitivity and Antitumor Immunity in Breast Cancer. Cancer Res. 2016, 76, 5657–5670. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, X.; Yuan, M.; Lv, W.; Feng, T.; Bai, R.; Zhong, H. Activation of the PERK-ATF4 pathway promotes chemo-resistance in colon cancer cells. Sci. Rep. 2019, 9, 3210. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Panada, E.; Moiso, E.; Buondonno, I.; Provero, P.; Rubinstein, M.; Kopecka, J.; Riganti, C. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol. Cancer 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, X.-X.; Xu, Y.-M.; Zhang, J.-Z.; Rong, S.-D.; Qin, Y.-Q.; Fang, J. IRE1α-targeting downregulates ABC transporters and overcomes drug resistance of colon cancer cells. Cancer Lett. 2020, 476, 67–74. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, L.; Wu, M.; Zhu, S.; Wang, Y.; Liu, L.; Li, Y.; Zhang, T.; Wu, Y.; Chen, H.; et al. IRE1α inhibitor reduces cisplatin resistance in ovarian cancer by modulating IRE1α/XBP1 pathway. Cell. Oncol. 2024, 47, 2233–2246. [Google Scholar] [CrossRef] [PubMed]
- Dadey, D.Y.; Kapoor, V.; Khudanyan, A.; Urano, F.; Kim, A.H.; Thotala, D.; Hallahan, D.E. The ATF6 pathway of the ER stress response contributes to enhanced viability in glioblastoma. Oncotarget 2015, 7, 2080–2092. [Google Scholar] [CrossRef]
- Su, S.; Wu, L.; Huang, C.; He, C.; Wang, L.; Li, W.; Liu, W.; Liu, L. ATF6 activation promotes tumorigenesis and drug resistance in diffuse large B-cell lymphoma (DLBCL) by regulating the mTOR/S6K signaling pathway. Discov. Oncol. 2025, 16, 499. [Google Scholar] [CrossRef]
- Gifford, J.B.; Huang, W.; Zeleniak, A.E.; Hindoyan, A.; Wu, H.; Donahue, T.R.; Hill, R. Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2016, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2020, 21, 71–88. [Google Scholar] [CrossRef]
- Koritzinsky, M.; Levitin, F.; van den Beucken, T.; Rumantir, R.A.; Harding, N.J.; Chu, K.C.; Boutros, P.C.; Braakman, I.; Wouters, B.G. Two phases of disulfide bond formation have differing requirements for oxygen. J. Cell Biol. 2013, 203, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramirez, L.; Cao, H.; Nelson, D.; Hammond, E.; Lee, A.-H.; Yoshida, H.; Mori, K.; Glimcher, L.H.; Denko, N.C.; Giaccia, A.J.; et al. XBP1 Is Essential for Survival under Hypoxic Conditions and Is Required for Tumor Growth. Cancer Res. 2004, 64, 5943–5947. [Google Scholar] [CrossRef]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.; et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef]
- Koumenis, C.; Naczki, C.; Koritzinsky, M.; Rastani, S.; Diehl, A.; Sonenberg, N.; Koromilas, A.; Wouters, B.G. Regulation of Protein Synthesis by Hypoxia via Activation of the Endoplasmic Reticulum Kinase PERK and Phosphorylation of the Translation Initiation Factor eIF2α. Mol. Cell. Biol. 2002, 22, 7405–7416. [Google Scholar] [CrossRef]
- Denzel, M.S.; Antebi, A. Hexosamine pathway and (ER) protein quality control. Curr. Opin. Cell Biol. 2015, 33, 14–18. [Google Scholar] [CrossRef]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-Box Binding Protein 1 Couples the Unfolded Protein Response to Hexosamine Biosynthetic Pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, B. Decoding Tumor Angiogenesis for Therapeutic Advancements: Mechanistic Insights. Biomedicines 2024, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Auf, G.; Jabouille, A.; Guérit, S.; Pineau, R.; Delugin, M.; Bouchecareilh, M.; Magnin, N.; Favereaux, A.; Maitre, M.; Gaiser, T.; et al. Inositol-requiring enzyme 1α is a key regulator of angiogenesis and invasion in malignant glioma. Proc. Natl. Acad. Sci. 2010, 107, 15553–15558. [Google Scholar] [CrossRef]
- Wang, Y.; Alam, G.N.; Ning, Y.; Visioli, F.; Dong, Z.; Nör, J.E.; Polverini, P.J. The Unfolded Protein Response Induces the Angiogenic Switch in Human Tumor Cells through the PERK/ATF4 Pathway. Cancer Res. 2012, 72, 5396–5406. [Google Scholar] [CrossRef]
- McGrath, E.P.; Logue, S.E.; Mnich, K.; Deegan, S.; Jäger, R.; Gorman, A.M.; Samali, A. The Unfolded Protein Response in Breast Cancer. Cancers 2018, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huo, Y.; Zhou, S.; Guo, J.; Ma, X.; Li, T.; Fan, C.; Wang, L. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022, 34, 2018–2035.e8. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Anufreichik, V.; Rodvold, J.J.; Chiu, K.T.; Sepulveda, H.; Zanetti, M. Cell-Extrinsic Effects of Tumor ER Stress Imprint Myeloid Dendritic Cells and Impair CD8+ T Cell Priming. PLoS ONE 2012, 7, e51845. [Google Scholar] [CrossRef]
- Orth, M.F.; Surdez, D.; Faehling, T.; Ehlers, A.C.; Marchetto, A.; Grossetête, S.; Volckmann, R.; Zwijnenburg, D.A.; Gerke, J.S.; Zaidi, S.; et al. Systematic multi-omics cell line profiling uncovers principles of Ewing sarcoma fusion oncogene-mediated gene regulation. Cell Rep. 2022, 41, 111761. [Google Scholar] [CrossRef]
- Hsieh, J.; Danis, E.P.; Owens, C.R.; Parrish, J.K.; Nowling, N.L.; Wolin, A.R.; Purdy, S.C.; Rosenbaum, S.R.; Ivancevic, A.M.; Chuong, E.B.; et al. Dependence of PAX3-FOXO1 chromatin occupancy on ETS1 at important disease-promoting genes exposes new targetable vulnerability in Fusion-Positive Rhabdomyosarcoma. Oncogene 2024, 44, 19–29. [Google Scholar] [CrossRef]
- Lee, A.T.J.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- de Azevedo, J.W.V.; Fernandes, T.A.A.D.M.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araujo, J.M.G. Biology and pathogenesis of human osteosarcoma. Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef]
- Conyers, R.; Young, S.; Thomas, D.M. Liposarcoma: Molecular Genetics and Therapeutics. Sarcoma 2010, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Falcao, R.M.; de Souza, J.E.S.; Gonzalez-Molina, J.; Mathieson, W.; Carlson, J.W.; Petta, T.B. Deep multi-omics integration approach reveals new molecular features of uterine leiomyosarcoma. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2024, 1871, 167632. [Google Scholar] [CrossRef]
- Xian, S.; Dosset, M.; Almanza, G.; Searles, S.; Sahani, P.; Waller, T.C.; Jepsen, K.; Carter, H.; Zanetti, M. The unfolded protein response links tumor aneuploidy to local immune dysregulation. Embo Rep. 2021, 22, e52509. [Google Scholar] [CrossRef] [PubMed]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Prim. 2022, 8, 77. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Cheng, D.; Dang, J.; Mi, Z.; Shi, Y.; Wang, L.; Fan, H. Unfolded Protein Response–Related Signature Associates With the Immune Microenvironment and Prognostic Prediction in Osteosarcoma. Front. Genet. 2022, 13, 911346. [Google Scholar] [CrossRef] [PubMed]
- Bugter, J.M.; Fenderico, N.; Maurice, M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 2020, 21, 5–21. [Google Scholar] [CrossRef]
- Ge, R.; Huang, G.M. Targeting transforming growth factor beta signaling in metastatic osteosarcoma. J. Bone Oncol. 2023, 43, 100513. [Google Scholar] [CrossRef]
- Luo, J.; Xia, Y.; Luo, J.; Li, J.; Zhang, C.; Zhang, H.; Ma, T.; Yang, L.; Kong, L. GRP78 inhibition enhances ATF4-induced cell death by the deubiquitination and stabilization of CHOP in human osteosarcoma. Cancer Lett. 2017, 410, 112–123. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, D.; Zhou, S.; Zhu, B.; Hu, T.; Yang, Q. Overexpression of X-Box Binding Protein 1 (XBP1) Correlates to Poor Prognosis and Up-Regulation of PI3K/mTOR in Human Osteosarcoma. Int. J. Mol. Sci. 2015, 16, 28635–28646. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Wu, X.; Tian, K.; Wang, Y. The oncogenic role of GNL3 in the progression and metastasis of osteosarcoma. Cancer Manag. Res. 2019, 11, 2179–2188. [Google Scholar] [CrossRef]
- Chen, W.; Liao, Y.; Sun, P.; Tu, J.; Zou, Y.; Fang, J.; Chen, Z.; Li, H.; Chen, J.; Peng, Y.; et al. Construction of an ER stress-related prognostic signature for predicting prognosis and screening the effective anti-tumor drug in osteosarcoma. J. Transl. Med. 2024, 22, 66. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, S.; Han, J.; Li, N.; Ji, R.; Li, X.; Han, C.; Zhao, W.; Zhang, L. LINC00629 protects osteosarcoma cell from ER stress-induced apoptosis and facilitates tumour progression by elevating KLF4 stability. J. Exp. Clin. Cancer Res. 2022, 41, 354. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, X.; Xu, R.; Zhang, X.; Shen, J.; Xu, W. GADD45GIP1 promotes osteosarcoma progression by modulating RPL35 ubiquitination and alleviating endoplasmic reticulum stress via the PERK/eIF2α pathway. Cancer Cell Int. 2025, 25, 242. [Google Scholar] [CrossRef]
- Hu, Q.; Mao, Y.; Liu, M.; Luo, R.; Jiang, R.; Guo, F. The active nuclear form of SREBP1 amplifies ER stress and autophagy via regulation of PERK. FEBS J. 2019, 287, 2348–2366. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Yoshida, A.; Chitnis, N.; Altman, B.J.; Tameire, F.; Oran, A.; Gennaro, V.; Armeson, K.E.; McMahon, S.B.; Wertheim, G.B.; et al. A PERK–miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat. Cell Biol. 2017, 20, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhang, W.; Liu, Z.; Yang, Z.; Tu, C.; Li, Z. A Novel Glutamine Metabolism-Related Gene Signature in Prognostic Prediction of Osteosarcoma. Int. J. Gen. Med. 2022, 15, 997–1011. [Google Scholar] [CrossRef]
- Bi, Y.; Meng, D.; Wan, M.; Xu, N.; Xu, Y.; Yuan, K.; Liu, P.; Fang, H.; Hu, H.; Lan, S. m6A-Related lncRNAs Predict Overall Survival of Patients and Regulate the Tumor Immune Microenvironment in Osteosarcoma. Comput. Intell. Neurosci. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Ardakani, A.H.G.; Woollard, A.; Ware, H.; Gikas, P. Soft tissue sarcoma: Recognizing a rare disease. Clevel. Clin. J. Med. 2022, 89, 73–80. [Google Scholar] [CrossRef]
- Aghaei, M.; Nasimian, A.; Rahmati, M.; Kawalec, P.; Machaj, F.; Rosik, J.; Bhushan, B.; Bathaie, S.Z.; Azarpira, N.; Łos, M.J.; et al. The Role of BiP and the IRE1α–XBP1 Axis in Rhabdomyosarcoma Pathology. Cancers 2021, 13, 4927. [Google Scholar] [CrossRef]
- Johnston, B.P.; McCormick, C. Herpesviruses and the Unfolded Protein Response. Viruses 2019, 12, 17. [Google Scholar] [CrossRef]
- De Raedt, T.; Walton, Z.; Yecies, J.L.; Li, D.; Chen, Y.; Malone, C.F.; Maertens, O.; Jeong, S.M.; Bronson, R.T.; Lebleu, V.; et al. Exploiting Cancer Cell Vulnerabilities to Develop a Combination Therapy for Ras-Driven Tumors. Cancer Cell 2011, 20, 400–413. [Google Scholar] [CrossRef]
- Galoian, K.; Dahl, V.; Perez, A.; Denny, C.; Becker, B.; Sedani, A.; Moran, A.; Martinez, D.; Hoyt, A.; Brown, J. PRP-1, a toll-like receptor ligand, upregulates the unfolded protein response in human chondrosarcoma cells. Cancer Treat. Res. Commun. 2022, 33, 100644. [Google Scholar] [CrossRef] [PubMed]
- Haney, S.L.; Feng, D.; Kollala, S.S.; Chhonker, Y.S.; Varney, M.L.; Williams, J.T.; Ford, J.B.; Murry, D.J.; Holstein, S.A. Investigation of the activity of a novel tropolone in osteosarcoma. Drug Dev. Res. 2023, 85, e22129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tu, H. Psoralen inhibits the proliferation and promotes apoptosis through endoplasmic reticulum stress in human osteosarcoma cells. Folia Histochem. et Cytobiol. 2022, 60, 101–109. [Google Scholar] [CrossRef]
- Buondonno, I.; Gazzano, E.; Tavanti, E.; Chegaev, K.; Kopecka, J.; Fanelli, M.; Rolando, B.; Fruttero, R.; Gasco, A.; Hattinger, C.; et al. Endoplasmic reticulum-targeting doxorubicin: A new tool effective against doxorubicin-resistant osteosarcoma. Cell. Mol. Life Sci. 2018, 76, 609–625. [Google Scholar] [CrossRef]
- Obu, S.; Niture, S.; Hoang, H.; Gadi, S.; Vandana; He, Y.; Kumar, D. Clemastine and hyperthermia enhance sensitization of osteosarcoma cells for apoptosis. Mol. Cell. Oncol. 2024, 11, 2351622. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, M.; Zhang, X.; Jin, Q.; Wang, Y.; Zou, C.; Huang, G.; Yin, J.; Xie, X.; Shen, J. CB-5083, an inhibitor of P97, suppresses osteosarcoma growth and stem cell properties by altering protein homeostasis. Am. J. Transl. Res. 2020, 12, 2956–2967. [Google Scholar]
- Xue, X.-C.; Zhou, Y.-Y.; Xu, L.-Y.; Wei, L.-Y.; Hu, Y.-J.; Yang, J.; Zhang, X.-Q.; Wang, M.-Y.; Han, Y.-L.; Chen, J.-J. Tongguanteng injection exerts anti-osteosarcoma effects through the ER stress-associated IRE1/CHOP pathway. BMC Complement. Med. Ther. 2024, 24, 400. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, Y.; Pang, H.; Sun, J.; Wang, Z.; Li, J. Melittin protein inhibits the proliferation of MG63 cells by activating inositol-requiring protein-1α and X-box binding protein 1-mediated apoptosis. Mol. Med. Rep. 2014, 9, 1365–1370. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, T.; Ma, X.; Jiao, M.; Zhao, W.; Yu, L.; Hua, Y.; Cai, Z.; Li, J.; et al. Eurycomanone inhibits osteosarcoma growth and metastasis by suppressing GRP78 expression. J. Ethnopharmacol. 2024, 335, 118709. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Du, X.; Zhao, H.; Sun, P.; Yang, J. Propofol Regulates ER Stress to Inhibit Tumour Growth and Sensitize Osteosarcoma to Doxorubicin. Int. J. Clin. Pract. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Q.; Li, C.; Chen, M.; Wang, C.; Wang, Z.; Xia, T.; Yi, C.; Shi, S. Ion interference induced by Ca-Mn nanoplatform enhances ferroptosis and promotes immune response for osteosarcoma treatment. J. Adv. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Grochala, J.; Bloniarz, D.; Zielinska, K.; Lewinska, A.; Wnuk, M. DNMT2/TRDMT1 gene knockout compromises doxorubicin-induced unfolded protein response and sensitizes cancer cells to ER stress-induced apoptosis. Apoptosis 2022, 28, 166–185. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, Z.; Zhou, Y.; Li, X.; Li, X.; Ma, B.; Zhang, Q. β-Elemonic acid inhibits the growth of human Osteosarcoma through endoplasmic reticulum (ER) stress-mediated PERK/eIF2α/ATF4/CHOP activation and Wnt/β-catenin signal suppression. Phytomedicine 2020, 69, 153183. [Google Scholar] [CrossRef]
- Haney, S.L.; Feng, D.; Chhonker, Y.S.; Varney, M.L.; Williams, J.T.; Smith, L.M.; Ford, J.B.; Murry, D.J.; Holstein, S.A. Evaluation of geranylgeranyl diphosphate synthase inhibition as a novel strategy for the treatment of osteosarcoma and Ewing sarcoma. Drug Dev. Res. 2022, 84, 62–74. [Google Scholar] [CrossRef]
- Iuliano, L.; Dalla, E.; Picco, R.; Mallavarapu, S.; Minisini, M.; Malavasi, E.; Brancolini, C. Proteotoxic stress-induced apoptosis in cancer cells: Understanding the susceptibility and enhancing the potency. Cell Death Discov. 2022, 8, 407. [Google Scholar] [CrossRef]
- Ludwig, M.P.; Galbraith, M.D.; Eduthan, N.P.; Hill, A.A.; Clay, M.R.; Tellez, C.M.; Wilky, B.A.; Elias, A.; Espinosa, J.M.; Sullivan, K.D. Proteasome Inhibition Sensitizes Liposarcoma to MDM2 Inhibition with Nutlin-3 by Activating the ATF4/CHOP Stress Response Pathway. Cancer Res. 2023, 83, 2543–2556. [Google Scholar] [CrossRef]
- Guan, M.; Fousek, K.; Jiang, C.; Guo, S.; Synold, T.; Xi, B.; Shih, C.-C.; Chow, W.A. Nelfinavir Induces Liposarcoma Apoptosis through Inhibition of Regulated Intramembrane Proteolysis of SREBP-1 and ATF6. Clin. Cancer Res. 2011, 17, 1796–1806. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Hurwitz, B.S.; Bolyard, C.; Yu, J.-G.; Zhang, J.; Selvendiran, K.; Rath, K.S.; He, S.; Bailey, Z.; Eaves, D.; et al. Bortezomib-Induced Unfolded Protein Response Increases Oncolytic HSV-1 Replication Resulting in Synergistic Antitumor Effects. Clin. Cancer Res. 2014, 20, 3787–3798. [Google Scholar] [CrossRef]
- Kwong, K.; Pan, Y.; Morales, J.; Watson, M.; Allegakoen, D.V.; Lee, A.G.; Bivona, T.G.; Wipf, P.; Guerriero, C.J.; Brodsky, J.L.; et al. In vivo manipulation of the protein homeostasis network in rhabdomyosarcoma. Oncotarget 2025, 16, 681–696. [Google Scholar] [CrossRef]
- Kiang, K.M.-Y.; Tang, W.; Song, Q.; Liu, J.; Li, N.; Lam, T.-L.; Shum, H.C.; Zhu, Z.; Leung, G.K.-K. Targeting unfolded protein response using albumin-encapsulated nanoparticles attenuates temozolomide resistance in glioblastoma. Br. J. Cancer 2023, 128, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Jiang, X.; Gan, J.; Tian, X.; Xing, Z.; Yan, Y.; Chen, J.; Zhang, J.; Wang, C.; et al. Denatured corona proteins mediate the intracellular bioactivities of nanoparticles via the unfolded protein response. Biomaterials 2021, 265, 120452. [Google Scholar] [CrossRef]
- Pelizzari-Raymundo, D.; Doultsinos, D.; Pineau, R.; Sauzay, C.; Koutsandreas, T.; Langlais, T.; Carlesso, A.; Gkotsi, E.; Negroni, L.; Avril, T.; et al. A novel IRE1 kinase inhibitor for adjuvant glioblastoma treatment. iScience 2023, 26, 106687. [Google Scholar] [CrossRef]
- Abbasi, S.; Rivand, H.; Eshaghi, F.; Moosavi, M.A.; Amanpour, S.; McDermott, M.F.; Rahmati, M. Inhibition of IRE1 RNase activity modulates tumor cell progression and enhances the response to chemotherapy in colorectal cancer. Med Oncol. 2023, 40, 247. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Denko, N.C.; Olson, M.; Van Melckebeke, H.; Lust, S.; Tam, A.; Solow-Cordero, D.E.; Bouley, D.M.; Offner, F.; Niwa, M.; et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 2011, 117, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Le Reste, P.J.; Pineau, R.; Voutetakis, K.; Samal, J.; Jégou, G.; Lhomond, S.; Gorman, A.M.; Samali, A.; Patterson, J.B.; Zeng, Q.; et al. Local intracerebral inhibition of IRE1 by MKC8866 sensitizes glioblastoma to irradiation/chemotherapy in vivo. Cancer Lett. 2020, 494, 73–83. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Y.; Bi, Y.; Zheng, S.; Wu, Y.; Wu, Y.; Xu, Y.; Chen, J. Suppression of PERK/eIF2α/CHOP pathway enhances oridonin-induced apoptosis by inhibiting autophagy in Small-Cell lung cancer cells. Biomed. Pharmacother. 2024, 175, 116684. [Google Scholar] [CrossRef]
- Li, X.; Lu, F.; Zhou, J.; Li, X.; Li, Y.; Ye, W.; Li, J.; Yang, L.; Tang, S.; Zhou, Y.; et al. IFNγ augments TKI efficacy by alleviating protein unfolding stress to promote GSDME-mediated pyroptosis in hepatocellular carcinoma. Cell Death Dis. 2025, 16, 512. [Google Scholar] [CrossRef]
- Hurst, K.E.; Lawrence, K.A.; Essman, M.T.; Walton, Z.J.; Leddy, L.R.; Thaxton, J.E. Endoplasmic Reticulum Stress Contributes to Mitochondrial Exhaustion of CD8+ T Cells. Cancer Immunol. Res. 2019, 7, 476–486. [Google Scholar] [CrossRef]
- Vandewynckel, Y.-P.; Laukens, D.; Bogaerts, E.; Paridaens, A.; Bussche, A.V.D.; Verhelst, X.; Van Steenkiste, C.; Descamps, B.; Vanhove, C.; Libbrecht, L.; et al. Modulation of the unfolded protein response impedes tumor cell adaptation to proteotoxic stress: A PERK for hepatocellular carcinoma therapy. Hepatol. Int. 2014, 9, 93–104. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Guo, J.; Wei, Y.; Ma, S.; Mai, Y.; Gou, G.; Zuo, W.; Yang, J. Synergistic anti-tumor effects of mRNA vaccine and PERK inhibitor combination in melanoma treatment. Colloids Surfaces B Biointerfaces 2025, 254, 114808. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.E.; Calvo, V.; Fujisawa, S.; Dudgeon, C.; Huang, S.; Ballal, N.; Shen, L.; Gasparek, J.; Betzenhauser, M.; Taylor, S.J.; et al. PERK Inhibition by HC-5404 Sensitizes Renal Cell Carcinoma Tumor Models to Antiangiogenic Tyrosine Kinase Inhibitors. Clin. Cancer Res. 2023, 29, 4870–4882. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Bakewell, S.; Bendell, J.C.; Infante, J.; Jones, S.F.; Spigel, D.R.; Weiss, G.J.; Ramanathan, R.K.; Ogden, A.; Von Hoff, D. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 2016, 1, e000154. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bo, Y.; Zeng, Q.; Diao, L.; Greene, S.; Patterson, J.; Liu, L.; Yang, F. Population pharmacokinetic model for oral ORIN1001 in Chinese patients with advanced solid tumors. Front. Pharmacol. 2024, 15, 1322557. [Google Scholar] [CrossRef]
| Drug | Combination Drug Therapy | Target | Tumor | Clinical Trial ID | Clinical Trial Phase |
|---|---|---|---|---|---|
| BOLD-100 | FOLFOX Chemotherapy | BiP | Gastric, Pancreatic, Colorectal Cancer and Cholangiocarcinoma | NCT04421820 | Phase 1 & 2 |
| BOLD-100 | DOX | BiP | Soft tissue sarcomas | NCT07027423 | Phase 1 |
| NKP-1339 | None | BiP | Solid tumors | NCT01415297 | Phase 1 |
| HC 5404-FU | None | PERK | Renal cell carcinoma, gastric cancer, metastatic breast cancer, small cell lung cancer, and other solid tumors (e.g., non-small cell lung cancer, colorectal cancer, carcinoma of unknown primary) | NCT04834778 | Phase 1 |
| NMS-03597812 | Dexamethasone | PERK | Multiple Myeloma | NCT05027594 | Phase 1 |
| NMS-03597812 | None | PERK | Acute Myeloid Leukemia | NCT06549790 | Phase 1 |
| ORIN 1001 (MKC8866) | Abraxane | IRE1 RNase | Breast cancer and other solid tumors | NCT03950570 | Phase 1 & 2 |
| ORIN 1001 (MKC8866) | Standard of Care | IRE1 RNase | Solid tumors | NCT05154201 | Phase 1 & 2 |
| IXAZOMIB | None | Proteasome | Nonhematologic Malignancies including Soft Tissue Sarcomas | NCT00830869 | Phase 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, E.; Avnet, S.; Baldini, N.; Cortini, M. The Unfolded Protein Response in Sarcomas: From Proteostasis to Therapy Resistance. Cancers 2025, 17, 3489. https://doi.org/10.3390/cancers17213489
Ilieva E, Avnet S, Baldini N, Cortini M. The Unfolded Protein Response in Sarcomas: From Proteostasis to Therapy Resistance. Cancers. 2025; 17(21):3489. https://doi.org/10.3390/cancers17213489
Chicago/Turabian StyleIlieva, Elizabeta, Sofia Avnet, Nicola Baldini, and Margherita Cortini. 2025. "The Unfolded Protein Response in Sarcomas: From Proteostasis to Therapy Resistance" Cancers 17, no. 21: 3489. https://doi.org/10.3390/cancers17213489
APA StyleIlieva, E., Avnet, S., Baldini, N., & Cortini, M. (2025). The Unfolded Protein Response in Sarcomas: From Proteostasis to Therapy Resistance. Cancers, 17(21), 3489. https://doi.org/10.3390/cancers17213489









