Propensity Score-Matched Analysis of Neoadjuvant vs. Adjuvant Therapy in Renal Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Population
2.2. Data
2.3. Statistical Analysis
3. Results
3.1. Descriptive Analysis
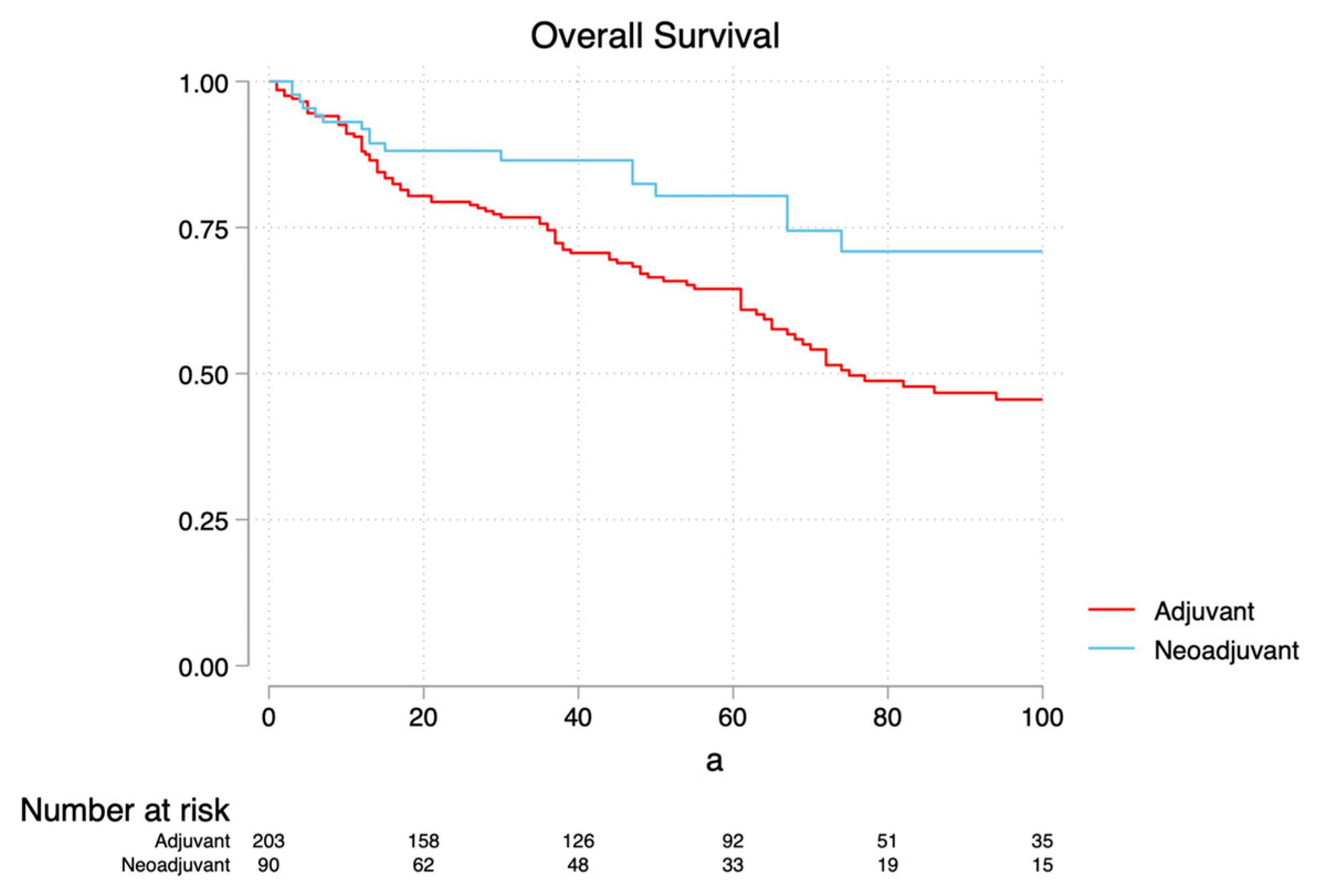
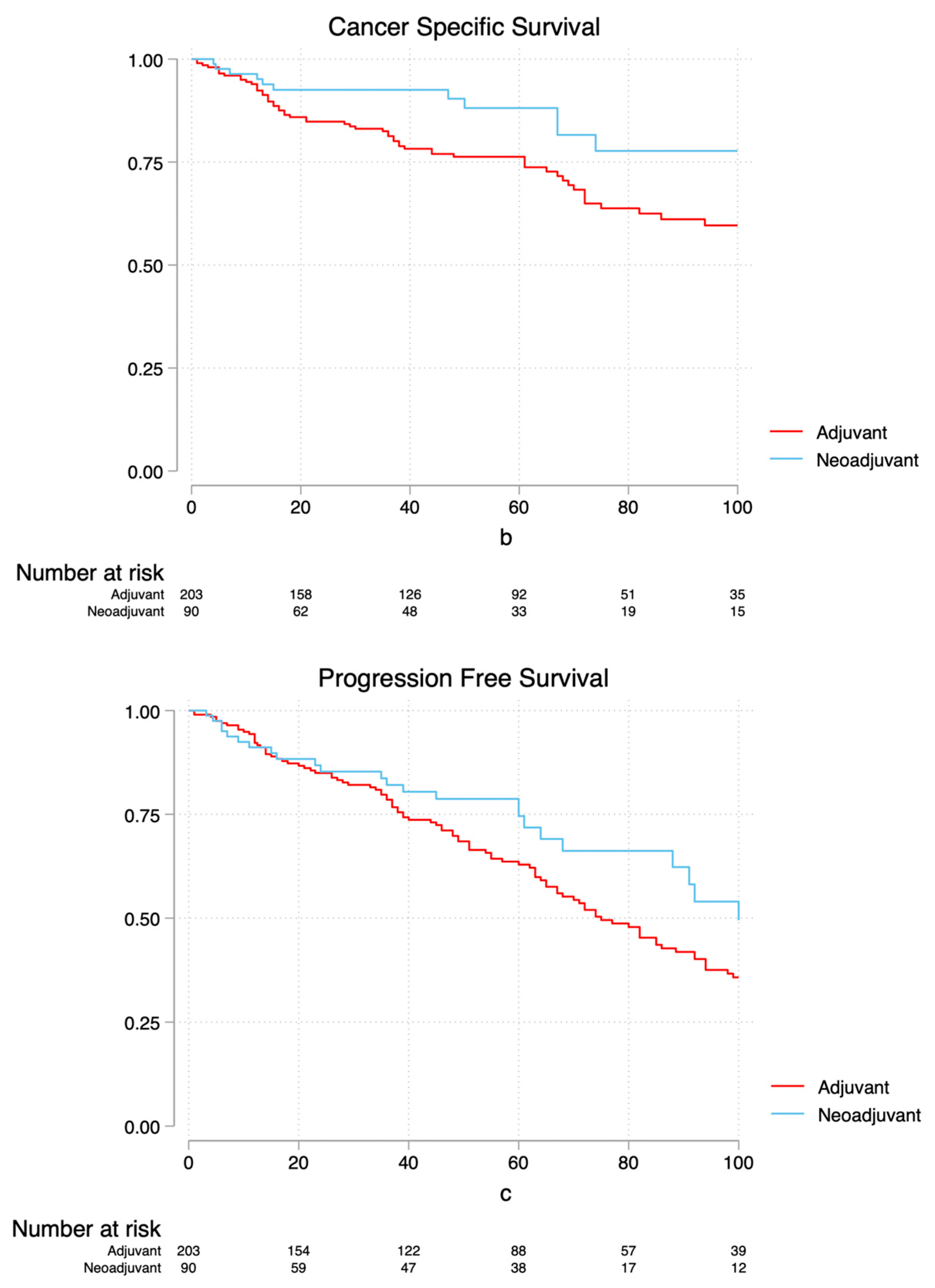
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Kane, C.J.; Mallin, K.; Ritchey, J.; Cooperberg, M.R.; Carroll, P.R. Renal Cell Cancer Stage Migration. Cancer 2008, 113, 78–83. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; Motzer, R.J.; Pandha, H.S.; George, D.J.; Pantuck, A.J.; Patel, A.; Chang, Y.-H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib In High-Risk Renal-Cell Carcinoma After Nephrectomy. N. Engl. J. Med. 2016, 375, 2246–2254. [Google Scholar] [CrossRef]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Hajek, J.; Gurney, H.; Chang, Y.-H.; Lee, J.L.; Sarwar, N.; et al. Pembrolizumab Versus Placebo As Post-Nephrectomy Adjuvant Therapy for Clear Cell Renal Cell Carcinoma (Keynote-564): 30-Month Follow-Up Analysis of A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Berquist, S.W.; Yim, K.; Ryan, S.T.; Patel, S.H.; Eldefrawy, A.; Cotta, B.H.; Bradshaw, A.W.; Meagher, M.F.; Bindayi, A.; Mckay, R.R.; et al. Systemic Therapy In The Management of Localized and Locally Advanced Renal Cell Carcinoma: Current State and Future Perspectives. Int. J. Urol. 2019, 26, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, K.; Campbell, S.C.; Nguyen, M.V.; Rathi, N.; Wang, L.; Meagher, M.F.; Rini, B.I.; Ornstein, M.; Mckay, R.R.; Derweesh, I.H. Padres: A Phase 2 Clinical Trial of Neoadjuvant Axitinib for Complex Partial Nephrectomy. BJU Int. 2024, 133, 425–431. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, Nccn Clinical Practice Guidelines In Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; Kwon, T.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.; Byun, S.-S.; Lee, J.; Master, V.; Jin, J.; et al. Axitinib Versus Placebo As An Adjuvant Treatment of Renal Cell Carcinoma: Results from The Phase Iii, Randomized Atlas Trial. Ann. Oncol. 2018, 29, 2371–2378. [Google Scholar] [CrossRef]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; Jewett, M.; Dutcher, J.P.; Atkins, M.B.; Pins, M.; et al. Adjuvant Sunitinib or Sorafenib for High-Risk, Non-Metastatic Renal-Cell Carcinoma (Ecog-Acrin E2805): A Double-Blind, Placebo-Controlled, Randomised, Phase 3 Trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase Iii Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy In Patients with Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef]
- Field, C.A.; Cotta, B.H.; Jimenez, J.; Lane, B.R.; Yim, K.; Lee, H.J.; Ryan, S.T.; Hamilton, Z.A.; Patel, S.; Wang, S.; et al. Neoadjuvant Sunitinib Decreases Inferior Vena Caval Thrombus Size and Is Associated with Improved Oncologic Outcomes: A Multicenter Comparative Analysis. Clin. Genitourin. Cancer 2019, 17, E505–E512. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Uzzo, R.G.; Karam, J.A.; Chang, S.S.; Clark, P.E.; Souter, L. Renal Mass And Localized Renal Cancer: Evaluation, Management, and Follow-Up: Aua Guideline: Part Ii. J. Urol. 2021, 206, 209–218. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; Mackenzie, C.R. A New Method of Classifying Prognostic Comorbidity In Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Artibani, W.; Graefen, M.; Remzi, M.; Rouprêt, M.; Truss, M. Reporting And Grading of Complications After Urologic Surgical Procedures: An Ad Hoc Eau Guidelines Panel Assessment And Recommendations. Eur. Urol. 2012, 61, 341–349. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition Ajcc Cancer Staging Manual: Continuing To Build A Bridge From A Population-Based To A More “Personalized” Approach To Cancer Staging. Ca A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 Who Classification of Tumours of The Urinary System And Male Genital Organs—Part A: Renal, Penile, And Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival Results from The Phase 3 Keynote-564 Study of Adjuvant Pembrolizumab Versus Placebo for The Treatment of Clear Cell Renal Cell Carcinoma (Ccrcc). J. Clin. Oncol. 2024, 42, Lba359. [Google Scholar] [CrossRef]
- Vartolomei, L.; Schmidinger, M.; Vartolomei, M.D.; Shariat, S.F. Psychological Distress In Patients Treated For Renal Cell Carcinoma: A Systematic Literature Review. J. Clin. Med. 2022, 11, 6383. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant Nivolumab Plus Ipilimumab Versus Placebo For Localised Renal Cell Carcinoma After Nephrectomy (Checkmate 914): A Double-Blind, Randomised, Phase 3 Trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant Atezolizumab Versus Placebo for Patients With Renal Cell Carcinoma At Increased Risk of Recurrence Following Resection (Immotion010): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, G.; Hohenfellner, M.; Arslan, A.; Hadaschik, B.; Teber, D.; Radtke, J.P.; Hallscheidt, P.; Tolstov, Y.; Roth, W.; Grüllich, C.; et al. Effective Downsizing But Enhanced Intratumoral Heterogeneity Following Neoadjuvant Sorafenib In Patients with Non-Metastatic Renal Cell Carcinoma. Langenbeck’s Arch. Surg. 2017, 402, 637–644. [Google Scholar] [CrossRef]
- Lane, B.R.; Derweesh, I.H.; Kim, H.L.; O’Malley, R.; Klink, J.; Ercole, C.E.; Palazzi, K.L.; Thomas, A.A.; Rini, B.I.; Campbell, S.C. Presurgical Sunitinib Reduces Tumor Size and May Facilitate Partial Nephrectomy In Patients with Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 112.E15–112.E21. [Google Scholar] [CrossRef]
- Ghali, F.; Patel, S.H.; Derweesh, I.H. Current Status of Immunotherapy for Localized and Locally Advanced Renal Cell Carcinoma. J. Oncol. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gorin, M.A.; Patel, H.D.; Rowe, S.P.; Hahn, N.M.; Hammers, H.J.; Pons, A.; Trock, B.J.; Pierorazio, P.M.; Nirschl, T.R.; Salles, D.C.; et al. Neoadjuvant Nivolumab In Patients With High-Risk Nonmetastatic Renal Cell Carcinoma. Eur. Urol. Oncol. 2022, 5, 113–117. [Google Scholar] [CrossRef]
- Hakimi, K.; Saidian, A.; Panian, J.; Barata, P.; Berg, S.; Chang, S.L.; Saliby, R.M.; Dzimitrowicz, H.; Emamekhoo, H.; Gross, E.; et al. Outcomes of Consolidative Nephrectomy Following Primary Immunotherapy In Advanced Renal Cell Carcinoma: A Multicenter Analysis. Clin. Genitourin. Cancer 2023, 21, 694–702. [Google Scholar] [CrossRef]
- Bex, A.; Abu-Ghanem, Y.; Van Thienen, J.V.; Graafland, N.; Lagerveld, B.; Zondervan, P.; Beerlage, H.; Van Moorselaar, J.; Kockx, M.; Van Dam, P.-J.; et al. Efficacy, Safety, And Biomarker Analysis Of Neoadjuvant Avelumab/Axitinib In Patients (Pts) with Localized Renal Cell Carcinoma (Rcc) Who Are At High Risk of Relapse After Nephrectomy (Neoavax). J. Clin. Oncol. 2022, 40, 289. [Google Scholar] [CrossRef]
- Allaf, M.E.; Kim, S.-E.; Master, V.; Mcdermott, D.F.; Harshman, L.C.; Cole, S.M.; Drake, C.G.; Signoretti, S.; Akgul, M.; Baniak, N.; et al. Perioperative Nivolumab Versus Observation In Patients With Renal Cell Carcinoma Undergoing Nephrectomy (Prosper Ecog-Acrin Ea8143): An Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 2024, 25, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Jewett, M.; Lewis, B.; Abel, E.J.; Albiges, L.; Berg, S.A.; Bratslavsky, G.; Braun, D.; Brugarolas, J.; Choueiri, T.K.; et al. A Call For A Neoadjuvant Kidney Cancer Consortium: Lessons Learned from Other Cancer Types. Eur. Urol. 2025, 87, 385–389. [Google Scholar] [CrossRef]
| (a) | |||||||||
| Before PSM | After PSM | ||||||||
| Neoadjuvant Breakdown | n | % | Cumulative | Neoadjuvant Breakdown | n | % | Cumulative | ||
| Axitinib | 29 | 32.22 | 32.22 | Axitinib | 29 | 32.22 | 32.22 | ||
| Nivolumab | 29 | 32.22 | 64.44 | Nivolumab | 29 | 32.22 | 64.44 | ||
| Sunitinib | 32 | 35.56 | 100 | Sunitinib | 32 | 35.56 | 100 | ||
| Total | 90 | 100 | Total | 90 | 100 | ||||
| Before PSM | After PSM | ||||||||
| Adjuvant Breakdown | n | % | Cumulative | Adjuvant Breakdown | n | % | Cumulative | ||
| Atezolizumab | 4 | 1.56 | 1.56 | Atezolizumab | 4 | 1.81 | 1.81 | ||
| Axitinib | 13 | 5.06 | 6.61 | Axitinib | 12 | 5.43 | 7.24 | ||
| Pazopanib | 35 | 13.62 | 20.23 | Pazopanib | 35 | 15.84 | 23.08 | ||
| Pembrolizumab | 121 | 47.08 | 67.32 | Pembrolizumab | 90 | 40.72 | 63.8 | ||
| Sunitinib | 84 | 32.68 | 100 | Sunitinib | 80 | 36.2 | 100 | ||
| Total | 257 | 100 | Total | 221 | 100 | ||||
| (b) | |||||||||
| Before Propensity Score Matching | After Propensity Score Matching | ||||||||
| Neoadjuvant | Adjuvant | p-Value | Neoadjuvant | Adjuvant | p-Value | ||||
| n = 90 | n = 257 | n = 90 | n = 221 | ||||||
| Age median (IQR) | 65.5 (56–70) | 60 (53–69) | 0.007 § | 65.5 (56–70) | 60 (53–69) | 0.037 § | |||
| Sex n (%) | Female | 31 (34.4%) | 76 (29.6%) | 0.39 * | 31 (34.4%) | 56 (25.3%) | 0.10 * | ||
| Male | 59 (65.6%) | 181 (70.4%) | 59 (65.6%) | 165 (74.7%) | |||||
| Ethnicity n (%) | White | 44 (48.9%) | 173 (67.3%) | <0.001 * | 44 (48.9%) | 145 (65.6%) | <0.001 * | ||
| Latin | 11 (12.2%) | 3 (1.2%) | 11 (12.2%) | 2 (0.9%) | |||||
| Asian | 29 (32.2%) | 37 (14.4%) | 29 (32.2%) | 37 (16.7%) | |||||
| Afro-American | 3 (3.3%) | 38 (14.8%) | 3 (3.3%) | 32 (14.5%) | |||||
| Others | 3 (3.3%) | 6 (2.3%) | 3 (3.3%) | 5 (2.3%) | |||||
| BMI median (IQR) | 25.9 (23.3–30.8) | 27.9 (24–32.5) | 0.085 § | 25.9 (23.3–30.8) | 27.6 (24–32.3) | 0.088 § | |||
| Hypertension n (%) | No | 49 (54.4%) | 111 (43.2%) | 0.065 * | 49 (54.4%) | 102 (46.2%) | 0.18 * | ||
| Yes | 41 (45.6%) | 146 (56.8%) | 41 (45.6%) | 119 (53.8%) | |||||
| Diabetes n (%) | No | 70 (77.8%) | 181 (70.4%) | 0.18 * | 70 (77.8%) | 162 (73.3%) | 0.41 * | ||
| Yes | 20 (22.2%) | 76 (29.6%) | 20 (22.2%) | 59 (26.7%) | |||||
| CCI n (%) | <5 | 36 (40.0%) | 77 (30.0%) | 0.001 * | 36 (40.0%) | 76 (34.4%) | 0.11 * | ||
| 5–8 | 39 (43.3%) | 83 (32.3%) | 39 (43.3%) | 83 (37.6%) | |||||
| ≥9 | 15 (16.7%) | 97 (37.7%) | 15 (16.7%) | 62 (28.1%) | |||||
| Preoperative eGFR median (IQR) | 63.9 (52.7–71.7) | 68 (56–82) | 0.10 § | 63.9 (52.7–71.7) | 68 (56–82) | 0.14 § | |||
| CKD-S3a n (%) | No | 75 (83.3%) | 230 (89.5%) | 0.12 * | 75 (83.3%) | 198 (89.6%) | 0.13 * | ||
| Yes | 15 (16.7%) | 27 (10.5%) | 15 (16.7%) | 23 (10.4%) | |||||
| Systemic therapy received n (%) | TMT | 61 (67.8%) | 132 (51.4%) | 0.007 * | 61 (67.8%) | 127 (57.5%) | 0.092 * | ||
| IO | 29 (32.2%) | 125 (48.6%) | 29 (32.2%) | 94 (42.5%) | |||||
| Systemic therapy breakdown | Atezolizumab | 0 (0.0%) | 4 (1.6%) | <0.001 | 0 (0.0%) | 4 (1.8%) | <0.001 | ||
| Axitinib | 29 (32.2%) | 13 (5.1%) | 29 (32.2%) | 12 (5.4%) | |||||
| Nivolumab | 29 (32.2%) | 0 (0.0%) | 29 (32.2%) | 0 (0.0%) | |||||
| Pazopanib | 0 (0.0%) | 35 (13.6%) | 0 (0.0%) | 35 (15.8%) | |||||
| Pembrolizumab | 0 (0.0%) | 121 (47.1%) | 0 (0.0%) | 90 (40.7%) | |||||
| Sunitinib | 32 (35.6%) | 84 (32.7%) | 32 (35.6%) | 80 (36.2%) | |||||
| Tumor size, median cm (IQR) | 6.05 (4.7–8.5) | 6.8 (5.1–9.7) | 0.069 § | 6.05 (4.7–8.5) | 6.7 (5.1–9.2) | 0.16 § | |||
| Surgery type n (%) | PN | 19 (21.1%) | 31 (12.1%) | 0.035 * | 19 (21.1%) | 29 (13.1%) | 0.077 * | ||
| RN | 71 (78.9%) | 226 (87.9%) | 71 (78.9%) | 192 (86.9%) | |||||
| Histology n (%) | non-ccRCC | 29 (32.2%) | 62 (24.1%) | 0.13 * | 29 (32.2%) | 50 (22.6%) | 0.078 * | ||
| ccRCC | 61 (67.8%) | 195 (75.9%) | 61 (67.8%) | 171 (77.4%) | |||||
| Tumor necrosis n (%) | No | 54 (60.0%) | 122 (47.5%) | 0.041 * | 54 (60.0%) | 109 (49.3%) | 0.087 * | ||
| Yes | 36 (40.0%) | 135 (52.5%) | 36 (40.0%) | 112 (50.7%) | |||||
| Tumor grade n (%) | Low grade | 21 (23.3%) | 90 (35.0%) | 0.041 * | 21 (23.3%) | 66 (29.9%) | 0.24 * | ||
| High grade | 69 (76.7%) | 167 (65.0%) | 69 (76.7%) | 155 (70.1%) | |||||
| Stage AJCC n (%) | Stage I | 28 (31.1%) | 55 (21.4%) | 0.088 * | 28 (31.1%) | 51 (23.1%) | 0.22 * | ||
| Stage II | 8 (8.9%) | 14 (5.4%) | 8 (8.9%) | 13 (5.9%) | |||||
| Stage III | 49 (54.4%) | 178 (69.3%) | 49 (54.4%) | 148 (67.0%) | |||||
| Stage IV | 5 (5.6%) | 10 (3.9%) | 5 (5.6%) | 9 (4.1%) | |||||
| Surgical margin n (%) | Negative | 81 (90.0%) | 240 (93.4%) | 0.29 * | 81 (90.0%) | 205 (92.8%) | 0.42 * | ||
| Positive | 9 (10.0%) | 17 (6.6%) | 9 (10.0%) | 16 (7.2%) | |||||
| Complication n (%) | No | 70 (77.8%) | 195 (75.9%) | 0.71 * | 70 (77.8%) | 173 (78.3%) | 0.92 * | ||
| Yes | 20 (22.2%) | 62 (24.1%) | 20 (22.2%) | 48 (21.7%) | |||||
| Clavien grade ≥ III n (%) | No | 82 (91.1%) | 257 (100.0%) | <0.001 * | 82 (91.1%) | 221 (100.0%) | <0.001 * | ||
| Yes | 8 (8.9%) | 0 (0.0%) | 8 (8.9%) | 0 (0.0%) | |||||
| Status at last follow-up | Alive | 70 (77.8%) | 133 (51.8%) | <0.001 * | 70 (77.8%) | 115 (52.0%) | <0.001 * | ||
| ACM | 20 (22.2%) | 124 (48.2%) | 20 (22.2%) | 106 (48.0%) | |||||
| Cause of death | Alive | 70 (77.8%) | 133 (51.8%) | <0.001 * | 70 (77.8%) | 115 (52.0%) | <0.001 * | ||
| CSM | 12 (13.3%) | 84 (32.7%) | 12 (13.3%) | 72 (32.6%) | |||||
| OCM | 8 (8.9%) | 40 (15.6%) | 8 (8.9%) | 34 (15.4%) | |||||
| Recurrence | No | 61 (67.8%) | 96 (37.4%) | <0.001 * | 61 (67.8%) | 84 (38.0%) | <0.001 * | ||
| Yes | 29 (32.2%) | 161 (62.6%) | 29 (32.2%) | 137 (62.0%) | |||||
| Length of follow-up, median months (IQR) | 40 (17–73) | 44 (22–76) | 0.33 § | 40 (17–73) | 46 (26–74) | 0.27 § | |||
| (a) | ||||
| Covariates | HR | 95% CI | p-Value | |
| Increasing Age | 1.03 | 1.01 | 1.04 | 0.01 |
| CKD ≥ 3a | 0.72 | 0.44 | 1.17 | 0.179 |
| Increasing BMI | 1.02 | 0.99 | 1.04 | 0.198 |
| HTN (Yes vs. No) | 1.52 | 1.01 | 2.27 | 0.044 |
| Increasing Tumor Size | 1.02 | 0.96 | 1.08 | 0.51 |
| RN vs. PN | 1.17 | 0.66 | 2.09 | 0.593 |
| Stages III–IV vs. I–II | 1.38 | 0.86 | 2.21 | 0.18 |
| Necrosis (Yes vs. No) | 1.29 | 0.88 | 1.88 | 0.19 |
| Positive Surgical Margin (Yes vs. No) | 1.94 | 1.01 | 3.72 | 0.046 |
| High Grade vs. Low Grade | 0.94 | 0.61 | 1.45 | 0.789 |
| Adjuvant vs. Neoadjuvant | 1.97 | 1.20 | 3.24 | 0.007 |
| IO vs. TKI | 0.60 | 0.40 | 0.89 | 0.011 |
| (b) | ||||
| Covariates | HR | 95% CI | p-Value | |
| Increasing Age | 1.03 | 1.00 | 1.05 | 0.018 |
| HTN (Yes vs. No) | 1.88 | 1.15 | 3.05 | 0.011 |
| RN vs. PN | 1.79 | 0.82 | 3.90 | 0.144 |
| Stages III–IV vs. I–II | 1.76 | 1.01 | 3.06 | 0.047 |
| Positive Surgical Margin (Yes vs. No) | 2.00 | 0.94 | 4.29 | 0.074 |
| High Grade vs. Low Grade | 1.57 | 0.90 | 2.75 | 0.111 |
| Adjuvant vs. Neoadjuvant | 2.37 | 1.27 | 4.43 | 0.007 |
| IO vs. TKI | 0.59 | 0.36 | 0.95 | 0.029 |
| (c) | ||||
| Covariates | HR | 95% CI | p-Value | |
| Increasing Age | 1.03 | 1.01 | 1.05 | <0.001 |
| Increasing BMI | 1.02 | 0.99 | 1.04 | 0.133 |
| HTN (Yes vs. No) | 1.14 | 0.80 | 1.62 | 0.458 |
| Increasing Tumor Size | 1.06 | 1.01 | 1.11 | 0.016 |
| RN vs. PN | 1.50 | 0.90 | 2.50 | 0.119 |
| Stages III–IV vs. I–II | 1.17 | 0.80 | 1.72 | 0.423 |
| Necrosis (Yes vs. No) | 1.09 | 0.78 | 1.53 | 0.619 |
| Positive Surgical Margin (Yes vs. No) | 3.10 | 1.77 | 5.43 | <0.001 |
| High Grade vs. Low Grade | 1.10 | 0.75 | 1.61 | 0.632 |
| Adjuvant vs. Neoadjuvant | 1.64 | 1.08 | 2.50 | 0.02 |
| IO vs. TKI | 1.04 | 0.74 | 1.44 | 0.834 |
| (a) | ||||
| Covariates | HR | 95% CI | p-Value | |
| Increasing Age | 1.03 | 1.01 | 1.04 | 0.01 |
| CKD ≥ 3a | 0.72 | 0.44 | 1.17 | 0.18 |
| Increasing BMI | 1.02 | 0.99 | 1.04 | 0.2 |
| HTN (Yes vs. No) | 1.51 | 1.01 | 2.27 | 0.047 |
| Increasing Tumor Size | 1.02 | 0.96 | 1.08 | 0.499 |
| RN vs. PN | 1.16 | 0.65 | 2.09 | 0.608 |
| Stages III–IV vs. I–II | 1.38 | 0.86 | 2.22 | 0.179 |
| Necrosis (Yes vs. No) | 1.29 | 0.88 | 1.88 | 0.191 |
| Positive Surgical Margin (Yes vs. No) | 1.94 | 1.01 | 3.72 | 0.045 |
| High Grade vs. Low Grade | 0.94 | 0.61 | 1.45 | 0.786 |
| Adjuvant vs. Neoadjuvant IO vs. TKI | Adjuvant TKI (Reference) | |||
| Neoadjuvant TKI | 0.49 | 0.28 | 0.88 | 0.016 |
| Neoadjuvant IO | 0.32 | 0.13 | 0.81 | 0.016 |
| Adjuvant IO | 0.59 | 0.38 | 0.90 | 0.015 |
| (b) | ||||
| Covariates | HR | 95% CI | p-Value | |
| Increasing Age | 1.03 | 1.00 | 1.05 | 0.018 |
| HTN (Yes vs. No) | 1.90 | 1.17 | 3.10 | 0.01 |
| RN vs. PN | 1.82 | 0.83 | 3.98 | 0.133 |
| Stages III–IV vs. I–II | 1.74 | 1.00 | 3.04 | 0.049 |
| Positive Surgical Margin (Yes vs. No) | 2.00 | 0.93 | 4.27 | 0.074 |
| High Grade vs. Low Grade | 1.59 | 0.91 | 2.77 | 0.105 |
| Adjuvant vs. Neoadjuvant IO vs. TKI | Adjuvant TKI (Reference) | |||
| Neoadjuvant TKI | 0.47 | 0.24 | 0.95 | 0.036 |
| Neoadjuvant IO | 0.18 | 0.04 | 0.73 | 0.017 |
| Adjuvant IO | 0.62 | 0.37 | 1.03 | 0.065 |
| (c) | ||||
| Covariates | HR | 95% CI | p-Value | |
| Increasing Age | 1.03 | 1.01 | 1.05 | 0.001 |
| Increasing BMI | 1.02 | 0.99 | 1.04 | 0.134 |
| HTN (Yes vs. No) | 1.14 | 0.80 | 1.63 | 0.454 |
| Increasing Tumor Size | 1.06 | 1.01 | 1.11 | 0.017 |
| RN vs. PN | 1.50 | 0.90 | 2.51 | 0.119 |
| Stages III–IV vs. I–II | 1.17 | 0.80 | 1.72 | 0.423 |
| Necrosis (Yes vs. No) | 1.09 | 0.78 | 1.53 | 0.62 |
| Positive Surgical Margin (Yes vs. No) | 3.09 | 1.76 | 5.44 | <0.001 |
| High Grade vs. Low Grade | 1.10 | 0.75 | 1.61 | 0.633 |
| Adjuvant vs. Neoadjuvant IO vs. TKI | Adjuvant TKI (Reference) | |||
| Neoadjuvant TKI | 0.62 | 0.37 | 1.04 | 0.068 |
| Neoadjuvant IO | 0.62 | 0.30 | 1.26 | 0.184 |
| Adjuvant IO | 1.04 | 0.73 | 1.50 | 0.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitta, C.; Musso, G.; Garofano, G.; Tanaka, H.; Patil, D.; Meagher, M.F.; Vourganti, S.; Cherullo, E.; Liss, M.; Paciotti, M.; et al. Propensity Score-Matched Analysis of Neoadjuvant vs. Adjuvant Therapy in Renal Cell Carcinoma. Cancers 2025, 17, 3481. https://doi.org/10.3390/cancers17213481
Saitta C, Musso G, Garofano G, Tanaka H, Patil D, Meagher MF, Vourganti S, Cherullo E, Liss M, Paciotti M, et al. Propensity Score-Matched Analysis of Neoadjuvant vs. Adjuvant Therapy in Renal Cell Carcinoma. Cancers. 2025; 17(21):3481. https://doi.org/10.3390/cancers17213481
Chicago/Turabian StyleSaitta, Cesare, Giacomo Musso, Giuseppe Garofano, Hajime Tanaka, Dattatraya Patil, Margaret F. Meagher, Srinivas Vourganti, Edward Cherullo, Michael Liss, Marco Paciotti, and et al. 2025. "Propensity Score-Matched Analysis of Neoadjuvant vs. Adjuvant Therapy in Renal Cell Carcinoma" Cancers 17, no. 21: 3481. https://doi.org/10.3390/cancers17213481
APA StyleSaitta, C., Musso, G., Garofano, G., Tanaka, H., Patil, D., Meagher, M. F., Vourganti, S., Cherullo, E., Liss, M., Paciotti, M., Lughezzani, G., Buffi, N. M., Master, V., Fujii, Y., McKay, R. R., & Derweesh, I. H. (2025). Propensity Score-Matched Analysis of Neoadjuvant vs. Adjuvant Therapy in Renal Cell Carcinoma. Cancers, 17(21), 3481. https://doi.org/10.3390/cancers17213481








