1. Introduction
Plitidepsin plus dexamethasone (DXM) showed activity in a phase II clinical trial conducted in relapsed/refractory multiple myeloma (r/r MM) patients [
1] and was later assessed versus DXM alone in the randomized phase III ADMYRE trial [
2]. ADMYRE met its primary endpoint and showed prolongation of progression-free survival (PFS) with plitidepsin plus DXM: median PFS without disease progression (PD) confirmed by an Independent Review Committee (IRC) assessment was 2.6 months (plitidepsin plus DXM) and 1.7 months (DXM) (HR = 0.650;
p = 0.0054). Median overall survival [OS, intention-to-treat (ITT) analysis] was 11.6 months (plitidepsin plus DXM) and 8.9 months (DXM) (HR = 0.797;
p = 0.1261). The safety profile was tolerable and did not overlap with the toxicity observed with other agents used in MM [
2]. Based on these findings, the plitidepsin plus DXM combination was approved in Australia in 2018 for the treatment of patients with r/r MM.
According to the
European Medicines Agency (EMA) Guideline on the investigation of subgroups in confirmatory clinical trials (
Section 4.3.a, EMA/CHMP/539146/2013), heterogeneity relates to the extent of differences within a clinical trial population in factors that are prognostic for outcome or predictive of treatment effects: the more heterogeneous the population, the more important the investigation of treatment effects in well-defined subgroups. Age-related heterogeneity in efficacy outcomes (PFS and overall survival, OS) has been found in several clinical trials evaluating new therapies in r/r MM [
3,
4,
5]. Overall, these findings highlight the critical importance of reporting subgroup analyses by age and support the importance of evaluating subgroups for the determination of clinical benefit [
6].
Seventy-five years has been used as the age cutoff point in previous trials in MM for reporting data [
4,
5] and has been found to be clinically relevant [
7], as two thirds of patients with newly diagnosed MM are aged <75 years [
8], and represents a distinct cohort with less advanced disease at diagnosis, as reflected by lower International Staging System stage, and with a lower incidence of renal failure and anemia. Results for plitidepsin plus DXM in the pre-planned subgroup of patients aged <75 years of the ADMYRE trial are shown here.
2. Materials and Methods
The multicenter, multinational ADMYRE study (clinicaltrials.gov identifier: NCT01102426) received protocol assistance by the Committee for Medicinal Products for Human Use, was approved by the Health authorities and the Independent Local Ethics Committee of each participating center (the study was conducted at 61 investigational sites from 17 countries including Australia, Austria, Belgium, Czech Republic, France, Germany, Great Britain, Greece, Italy, The Netherlands, New Zealand, Poland, Portugal, Spain, South Korea, Taiwan and the USA) and was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulations on clinical trials. Signed informed consent was obtained from all patients prior to any study-specific procedure.
The ADMYRE trial received Scientific Advice from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) prior to starting the study. Scientific Advice involved the proposed study population; the planned dose and schedule; the control arm comparator (DXM); the primary endpoint; and the statistical analyses. In this Scientific Advice, it was recognized that DXM was an active compound that had been widely used as a single agent as well as a part of combination regimens for the treatment of MM patients. A low-dose DXM regimen was chosen because, based on data from the last available publications at the time of protocol writing, DXM administered at reduced doses was associated with a better safety profile while reporting better survival [
9].
The ADMYRE study design and main results have been described elsewhere [
2]. Briefly, eligible subjects were patients ≥ 18 years old with r/r MM after at least three, but not more than six, prior therapeutic regimens, including at least bortezomib and lenalidomide or thalidomide; measurable disease; Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; life expectancy ≥ 3 months; and adequate major organ function. Patients were stratified according to their ECOG PS score (0 and 1 vs. 2) and Durie-Salmon stage (I/II vs. III) and were randomly assigned (2:1) to receive plitidepsin 5 mg/m
2 Day (D)1,15 plus low-dose DXM 40 mg D1,8,15,22 (Arm A,
n = 171) or low-dose DXM 40 mg D1,8,15,22 (Arm B,
n = 84) every four weeks (q4wk). Patients in the control arm (DXM, Arm B) with documented disease progression after a minimum of eight weeks from randomization could cross over to the combination arm (Arm A).
The primary efficacy endpoint was PFS assessed according to an IRC per the International Myeloma Working Group (IMWG) criteria current at the time of study protocol design (i.e., for the evaluation of PFS, a first PD event was assigned, but two consecutive evaluations for confirming PD were not required) [
10]. OS was a secondary endpoint.
Safety was evaluated in all patients who received at least one dose of the study treatment by assessment of adverse events (Aes), clinical laboratory test results, physical examinations, and vital signs.
Kaplan–Meier estimates, the unstratified log rank test, and Cox regression were used to compare the time-to-event endpoints (e.g., PFS/OS) and to calculate the risk reduction. The study was powered for the evaluation of the main endpoint (PFS) and to ascertain if a trend in OS was observed in favor of the experimental arm. Analysis of efficacy and safety in subgroups by age was preplanned in the ADMYRE Statistical Analysis Plan to characterize a potential benefit.
3. Results
3.1. Patient Characteristics
In ADMYRE, a total of 255 patients were randomized (2:1) between June 2010 and May 2015: 171 in Arm A and 84 in Arm B. The subgroup of patients aged <75 years includes most of the patients evaluated in the ADMYRE study: 145 out of the 171 patients (84.8%) in the plitidepsin plus DXM arm and 71 out of the 84 (84.5%) patients in the DXM alone arm. The baseline characteristics of patients aged <75 years were well balanced between treatment arms despite this subgroup having a non-randomized basis (
Table 1).
3.2. Efficacy in Patients Aged <75 Years
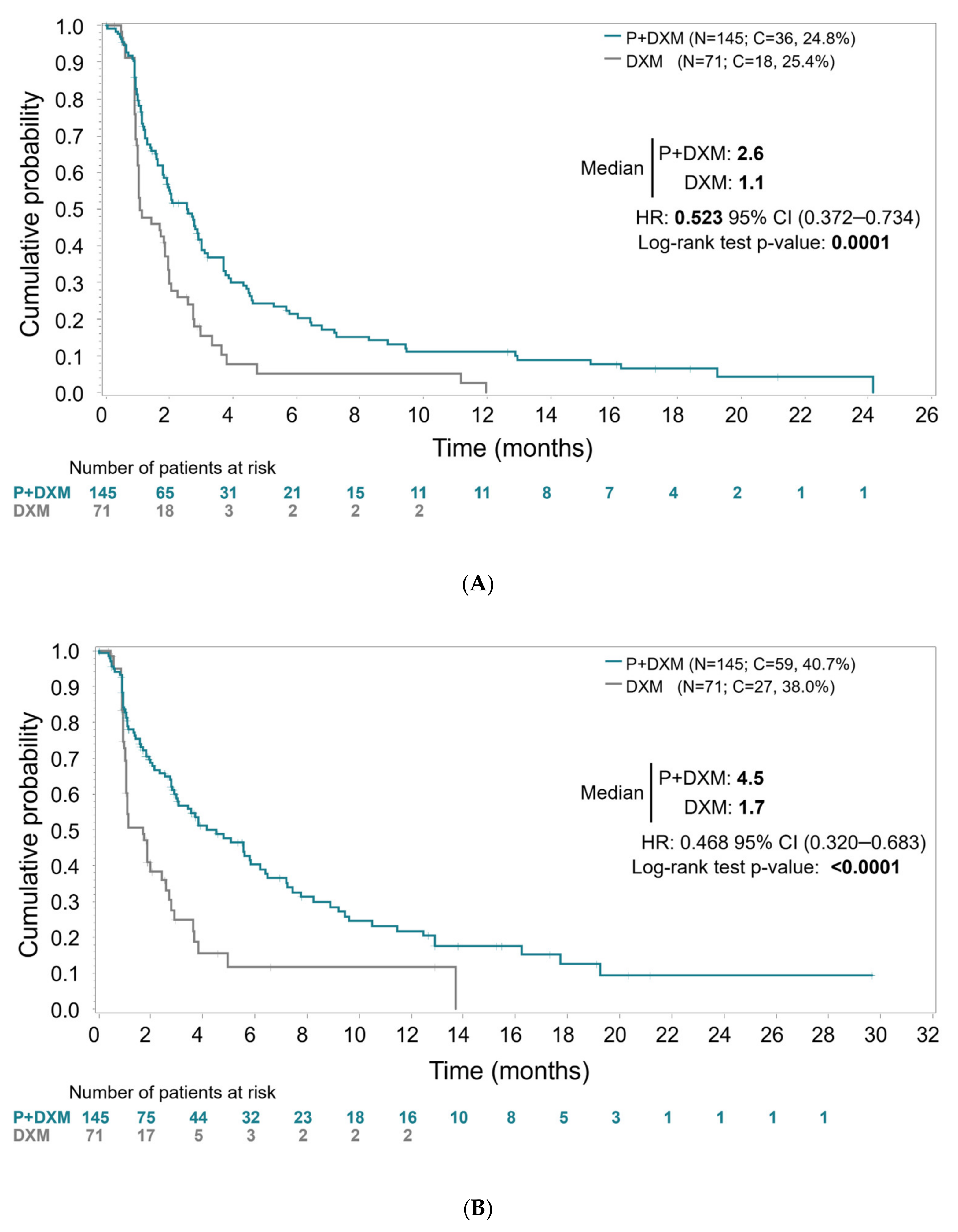
The primary efficacy analysis, with blinded IRC assessment of all randomized patients performed without PD confirmation, showed statistically significant longer PFS for patients aged <75 years treated with plitidepsin plus DXM. Median PFS was 2.6 months (95% CI, 1.8–3.0 months) in Arm A (plitidepsin plus DXM) and 1.1 months (95% CI, 1.0–1.9 months) in Arm B (DXM) (log-rank
p < 0.0001) (
Figure 1). The relative risk of progression or death was reduced by 47.7% in patients treated with plitidepsin plus DXM (HR = 0.523; 95%CI, 0.372–0.734;
p < 0.0002).
PFS analysis requiring PD confirmation by investigator’s assessment (a planned sensitivity analysis) showed median PFS of 4.5 months (95% CI, 3.0–5.8 months) in Arm A (plitidepsin plus DXM) and 1.7 months (95% CI, 1.1–2.4 months) in Arm B (DXM) (log-rank p < 0.0001), with a relative risk of progression or death reduced by 53.2% in patients treated with plitidepsin plus DXM (HR = 0.468, 95%CI, 0.320–0.683; p < 0.0001).
The median (range) cycle where the first response was obtained was Cycle 1 (Cycle 1-Cycle 11) in Arm A (plitidepsin plus DXM) and Cycle 2 (only one response was observed) in Arm B. The median (range) time on treatment of patients with response was 33.1 weeks (7.3–157.9 weeks) in Arm A (plitidepsin plus DXM) and 24.0 weeks in Arm B.
Mature final intention-to-treat OS analysis was based on 162 death events (75.0% of the 216 randomized patients aged <75 years). Median OS was 13.0 months (95% CI, 9.9–17.3 months) in Arm A (plitidepsin plus DXM) and 8.1 months (95% CI, 5.7–16.0 months) in Arm B (DXM) (log-rank
p = 0.0340) (
Figure 2). Despite the crossover in the control arm to the experimental arm of 31 patients (43.7%), the relative risk of death was reduced by 28.9% in patients treated with plitidepsin plus DXM (HR = 0.711, 95%CI, 0.517–0.976;
p = 0.0350).
Two-stage OS analysis, which mitigates the effect of crossover, showed a more statistically significant difference in favor of Arm A (plitidepsin plus DXM). Median OS remained as 13.0 months (95% CI, 9.9–17.3 months) in Arm A (plitidepsin/DXM) and was estimated as 6.4 months (95% CI, 5.0–8.6 months) in Arm B (DXM) (log-rank p < 0.0001). Relative risk of death was reduced by 46.6% in patients treated with plitidepsin plus DXM (HR = 0.534, 95%CI, 0.386–0.738; p < 0.0001).
3.3. Safety in Patients Aged <75 Years
All treated patients (
n = 143 in Arm A, plitidepsin plus DXM, and
n = 70 in Arm B, DXM) were evaluable for safety. The median (range) of cycles received was 3 (1–39) in Arm A (plitidepsin plus DXM) and 2 (1–27) in Arm B (DXM). The median (range) time on treatment was 12.3 weeks (1.3–162.3 weeks) in Arm A (plitidepsin plus DXM) and 8.4 weeks (1.4–56.0 weeks) in Arm B. In Arm A (plitidepsin plus DXM), the most common AEs (all grades) related to the study treatment (or with unknown causality) in patients aged <75 years were nausea (39.2% of patients), fatigue (39.2%), vomiting (19.6%), diarrhea (14.7%) and myalgia (14.0%). The most common grade 3/4 treatment-related (or with unknown causality) AEs were fatigue (11.2%), myalgia (4.2%) and nausea (3.5%) (
Table 2). The most common grade 3/4 hematological abnormalities were anemia (30.7%), lymphopenia (21.2%), thrombocytopenia (20.4%) and neutropenia (13.9%). Most of these hematological abnormalities were already present at baseline, as expected for patients with r/r MM. The most common grade 3/4 biochemical abnormalities were increases in creatine phosphokinase (18.0%) and alanine aminotransferase (14.7%).
In Arm B (DXM), the most common AEs (all grades) related to the study treatment (or with unknown causality) in patients aged <75 years were nausea (12.9%), insomnia (10.0%) and fatigue (8.6%). All grade 3/4 AEs occurred in one patient each (1.2%) (
Table 2). The most common grade 3/4 hematological abnormalities were anemia (35.8%), thrombocytopenia (31.3%), lymphopenia (16.7%), and neutropenia (4.5%). The most common grade 3/4 biochemical abnormality was increased creatinine (6.0%).
Tolerance to treatment may also be evaluated by the time to performance status deterioration measured by ECOG PS or death, indicative of the patients’ general status. The time until ECOG PS deterioration or death was 4.7 months (95%CI, 3.0–8.4 months) for the plitidepsin plus DXM arm and 2.1 months (95%CI, 1.4–3.8 months) for the DXM arm (HR = 0.662; 95%CI, 0.472–0.928; p = 0.0154).
4. Discussion
The ADMYRE study met its primary endpoint, PFS, demonstrating benefit for the combination of plitidepsin plus DXM compared to DXM alone in r/r MM patients pretreated with at least three regimens, including bortezomib, and either lenalidomide or thalidomide [
2]. The present results show a larger effect in PFS and OS of plitidepsin plus DXM compared to DXM alone in the ADMYRE trial in patients aged <75 years.
The reduction in the relative risk of progression or death in patients aged <75 years treated in the plitidepsin plus DXM arm compared to DXM alone using the most conservative method (i.e., by IRC without PD confirmation) was 47.7% (HR = 0.523;
p < 0.0001), which is higher than that observed previously in the overall intention-to-treat population treated in ADMYRE (35.0%; HR = 0.650;
p = 0.0054) [
2]. The reduction in the relative risk of progression or death by investigator assessment with confirmation of PD (53.2%; HR = 0.468;
p < 0.0001) is also higher than that observed in the overall population (38.9%; HR = 0.611;
p = 0.0040) [
2].
Median OS in patients aged <75 years treated with plitidepsin plus DXM was 13.0 months vs. 8.1 months with DXM alone, with a statistically significant reduction in the relative risk of death of 28.9% compared to DXM alone (HR = 0.711;
p = 0.0350). These values were higher than the median of 11.6 months and the risk reduction in death of 20.3% (HR = 0.797;
p = 0.1261) found in the overall population of randomized patients treated with plitidepsin plus DXM in ADMYRE [
2]. The two-stage OS analysis, which mitigates the effect of crossover, also showed a higher reduction in the relative risk of death (46.6% vs. 33.3%; HR = 0.534 vs. HR = 0.667) compared to the overall ADMYRE population.
A new therapy with a novel mechanism of action that shows a PFS benefit along with a likelihood of survival benefit in a largely treatment-refractory population is considered a clinically meaningful contribution to the r/r MM therapeutic armamentarium. eEF1A2, a supposed oncogene that is overexpressed in MM, was identified as the primary target for plitidepsin [
11,
12]. Of note, all recently introduced new anti-MM drugs have mechanisms of activity not targeting eEF1A2.
The safety profile of plitidepsin plus DXM in patients aged <75 years was similar to that observed in the overall population of patients treated in the ADMYRE study, mainly consisting of transient laboratory abnormalities controlled by dose adjustment [
2]. In this subgroup of patients aged <75 years, plitidepsin plus DXM also showed a low incidence of toxicities common with available agents used in the treatment of r/r MM (e.g., neurotoxicity, neutropenia and infections, thrombocytopenia and bleeding, or cardiac events).
5. Conclusions
In summary, compared to the overall population treated in the ADMYRE study, the efficacy of the combination of plitidepsin and DXM in the patient population subgroup aged <75 years was larger and characterized by a higher reduction in the risk of progression or death at the primary endpoint, PFS by IRC (47.7% vs. 35.0%); a higher reduction in the risk of progression or death at the secondary endpoint, PFS by IA with PD confirmation (53.2% vs. 38.9%); and a higher reduction in the risk of death at the secondary endpoint OS (28.9% vs. 20.3%). A difference in the median OS of about 5 months (
p = 0.0350) is considered clinically meaningful in this heavily pretreated patient population. The combination of plitidepsin and DXM has shown anti-myeloma activity, with prolonged PFS and OS. Its distinct safety profile, in addition to its novel mechanism of action, likely provides plitidepsin with a specific place among the available options for haemato-oncologists treating r/r MM and could provide a new therapeutic option for patients aged <75 years with r/r MM. Although innovative therapies such as BCMA-directed CAR-T cells and bispecific antibodies have changed the management of relapsed disease [
13], MM remains an incurable disease in which most patients continue to experience successive relapses. In this setting, drugs such as melflufen and selinexor have been approved for use in later-line r/r MM; however, some patients may not be candidates for these treatments due to their safety profile or because they have developed resistance to the main drug classes. In this context, plitidepsin plus DXM, although its efficacy appears to be more modest compared with more recent therapeutic innovations, could represent an available therapeutic alternative. Its distinct mechanism of action, targeting eEF1A2, suggests its potential for activity in patients refractory to immunomodulatory agents, proteasome inhibitors, or monoclonal antibodies, thus offering a potential option in settings with unmet medical needs.
Author Contributions
Conceptualization, M.V.M., E.T., S.M., C.K., J.J., J.G., V.A. and H.L.; Formal analysis, S.M., C.K., S.E., J.G. and V.A.; Investigation, M.V.M., S.M., C.K., J.J., J.G. and V.A.; Writing—original draft, M.V.M., S.M., C.K. and V.A.; Writing—review and editing, M.V.M., E.T., S.M., C.K., J.J., S.E., J.G., V.A. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by PharmaMar S.A (Study APL-C-001-09, ADMYRE).
Institutional Review Board Statement
This multicenter, multinational study was conducted at 61 investigational sites from 17 countries including Australia, Austria, Belgium, Czech Republic, France, Germany, Great Britain, Greece, Italy, The Netherlands, New Zealand, Poland, Portugal, Spain, South Korea, Taiwan and the USA according to the Helsinki Declaration Guidelines and was approved by respective Health Authorities (protocol code: APL-C-001-09) and the Independent Ethics Committee of each investigational site. It was registered within ClinicalTrials.gov Trials Register (NCT01102426). The first ethic committee approval date was 30 April 2017 (United Kingdom) and the last ethic committee approval date was 7 August 2017 (Spain). This study followed the ICH Good Clinical Practice (GCP) guidelines, and medicine was produced in accordance with Good Manufacturing Practice (GMP).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study have been included in the article; further inquiries can be directly addressed to the corresponding author.
Acknowledgments
The authors wish to thank the patients, their families, and the clinical research teams for their time and trust, and for enabling this clinical trial and associated research to take place.
Conflicts of Interest
M.V.M. received honoraria derived from lectures and participation in advisory boards from Johnson & Johnson, BMS, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides, and Kite. E.T. was present in advisory boards for Amgen, AstraZeneca, EUSA Pharma, BMS, GSK, J&J, Menarini/Stemline, Pfizer, Sanofi, and Takeda; received honoraria from Amgen, Antengene, AstraZeneca, EUSA Pharma, BMS, Forus, GSK, J&J, Menarini/Stemline, Novartis, Pfizer, Sanofi, Swixx, and Takeda; received research funding from Amgen, GSK, J&J, Sanofi, and Takeda; and received travel expenses from Takeda. S.M., C.K., J.J., S.E., J.G. and V.A. are employees of PharmaMar. S.A., C.K., J.J., J.G. and V.A. have stock ownerships in PharmaMar. S.A. and H.L received research support by AMGEN and Sanofi, and was speaker or participated in advisory boards for Janssen, BMS, Pfizer, Oncopetides, Menarini/Stemline, and GSK.
Abbreviations
The following abbreviations are used in this manuscript:
| AEs | Adverse Events |
| Day | D |
| DXM | Dexamethasone |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| EMA | European Medicines Agency |
| IMWG | International Myeloma Working Group |
| IRC | Independent Review Committee |
| OS | Overall Survival |
| PD | Disease Progression |
| PFS | Progression-Free Survival |
| r/r MM | Relapsed/Refractory Multiple Myeloma |
References
- Mateos, M.V.; Cibeira, M.T.; Richardson, P.G.; Prosper, F.; Oriol, A.; de la Rubia, J.; Lahuerta, J.J.; Garcia-Sanz, R.; Extremera, S.; Szyldergemajn, S.; et al. Phase II clinical and pharmacokinetic study of plitidepsin 3-h infusion every two weeks alone or with dexamethasone in relapsed and refractory multiple myeloma. Clin. Cancer Res. 2010, 16, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Spicka, I.; Ocio, E.M.; Oakervee, H.E.; Greil, R.; Banh, R.H.; Huang, S.Y.; D’Rozario, J.M.; Dimopoulos, M.A.; Martinez, S.; Extremera, S.; et al. Randomized phase III study (ADMYRE) of plitidepsin in combination with dexamethasone vs. dexamethasone alone in patients with relapsed/refractory multiple myeloma. Ann. Hematol. 2019, 98, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Schjesvold, F.H.; Dimopoulos, M.A.; Delimpasi, S.; Robak, P.; Coriu, D.; Legiec, W.; Pour, L.; Spicka, I.; Masszi, T.; Doronin, V.; et al. Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): A randomised, head-to-head, open-label, phase 3 study. Lancet Haematol. 2022, 9, e98–e110. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, J.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Schjesvold, F.H.; Cairns, D.A.; Wei, L.J.; Davies, F.; Nadeem, O.; Abdulhaq, H.; Mateos, M.V.; Laubach, J.; Weisel, K.; et al. Progression-free survival as a surrogate endpoint in myeloma clinical trials: An evolving paradigm. Blood Cancer J. 2024, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, N.; Kumar, S.K. Up-Front Treatment of Elderly (Age ≥ 75 Years) and Frail Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2024, 22, e247039. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Myeloma. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 18 August 2021).
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R.; et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Kyle, R.A.; Rajkumar, S.V.; Stewart, A.K.; Weber, D.; Richardson, P.; ASH/FDA Panel on Clinical Endpoints in Multiple Myeloma. Clinically relevant end points and new drug approvals for myeloma. Leukemia 2008, 22, 231–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Losada, A.; Munoz-Alonso, M.J.; Garcia, C.; Sanchez-Murcia, P.A.; Martinez-Leal, J.F.; Dominguez, J.M.; Lillo, M.P.; Gago, F.; Galmarini, C.M. Translation Elongation Factor eEF1A2 is a Novel Anticancer Target for the Marine Natural Product Plitidepsin. Sci. Rep. 2016, 6, 35100. [Google Scholar] [CrossRef] [PubMed]
- Leisch, M.; Egle, A.; Greil, R. Plitidepsin: A potential new treatment for relapsed/refractory multiple myeloma. Future Oncol. 2019, 15, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.S.; Tiger, Y.K.R.; Jamouss, K.T.; Hassani, J.; Bou Zerdan, M.; Raza, S. Updates on Therapeutic Strategies in the Treatment of Relapsed/Refractory Multiple Myeloma. Cancers 2024, 16, 2931. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).