Radiomics for Dynamic Lung Cancer Risk Prediction in USPSTF-Ineligible Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
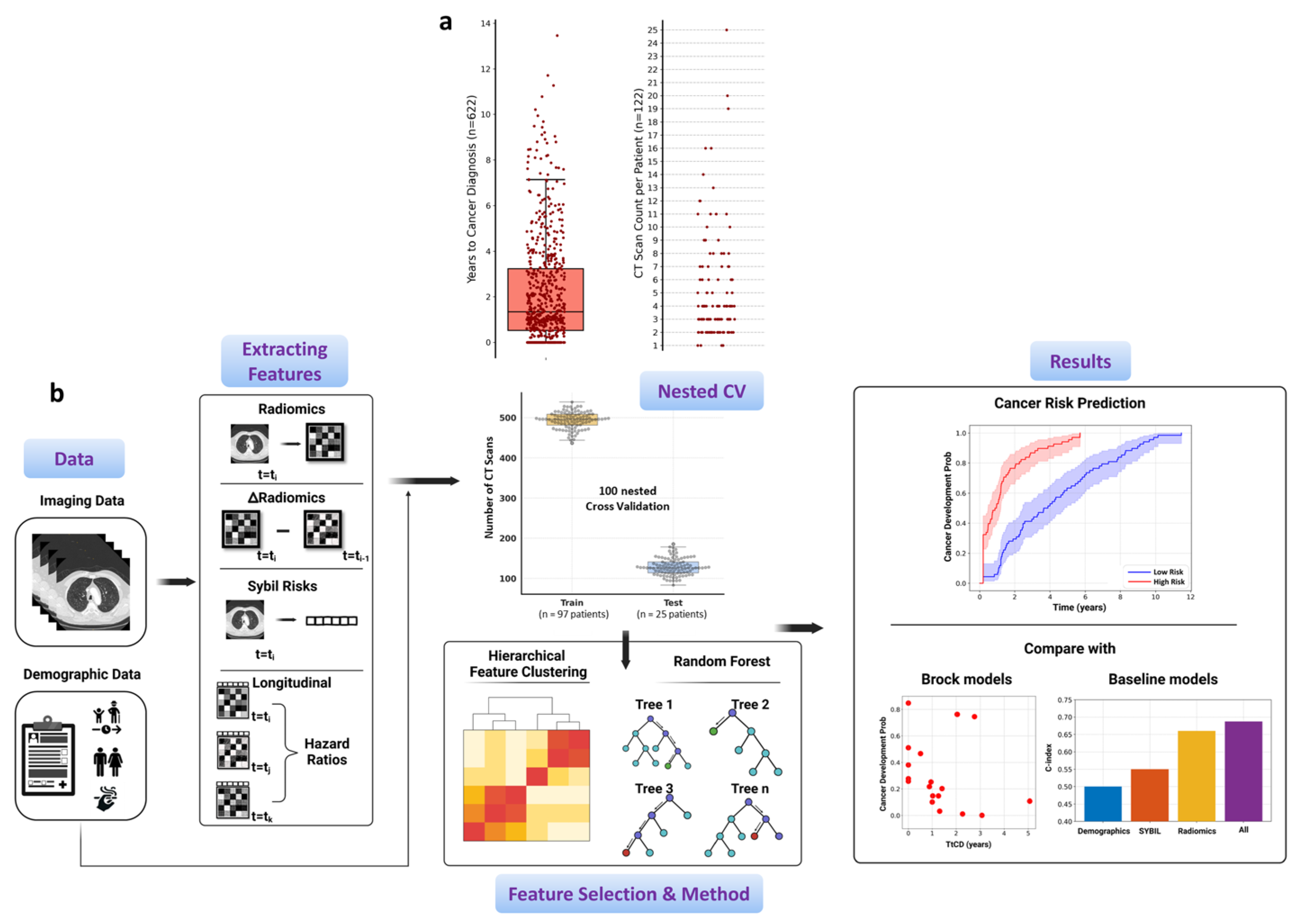
2.1. Longitudinal Patient Data Curation
2.2. Study Design
2.3. Details of Extracted Multi-Modal Features
2.4. Dynamic Lung Cancer Risk Modeling
2.5. Statistical Analysis
3. Results
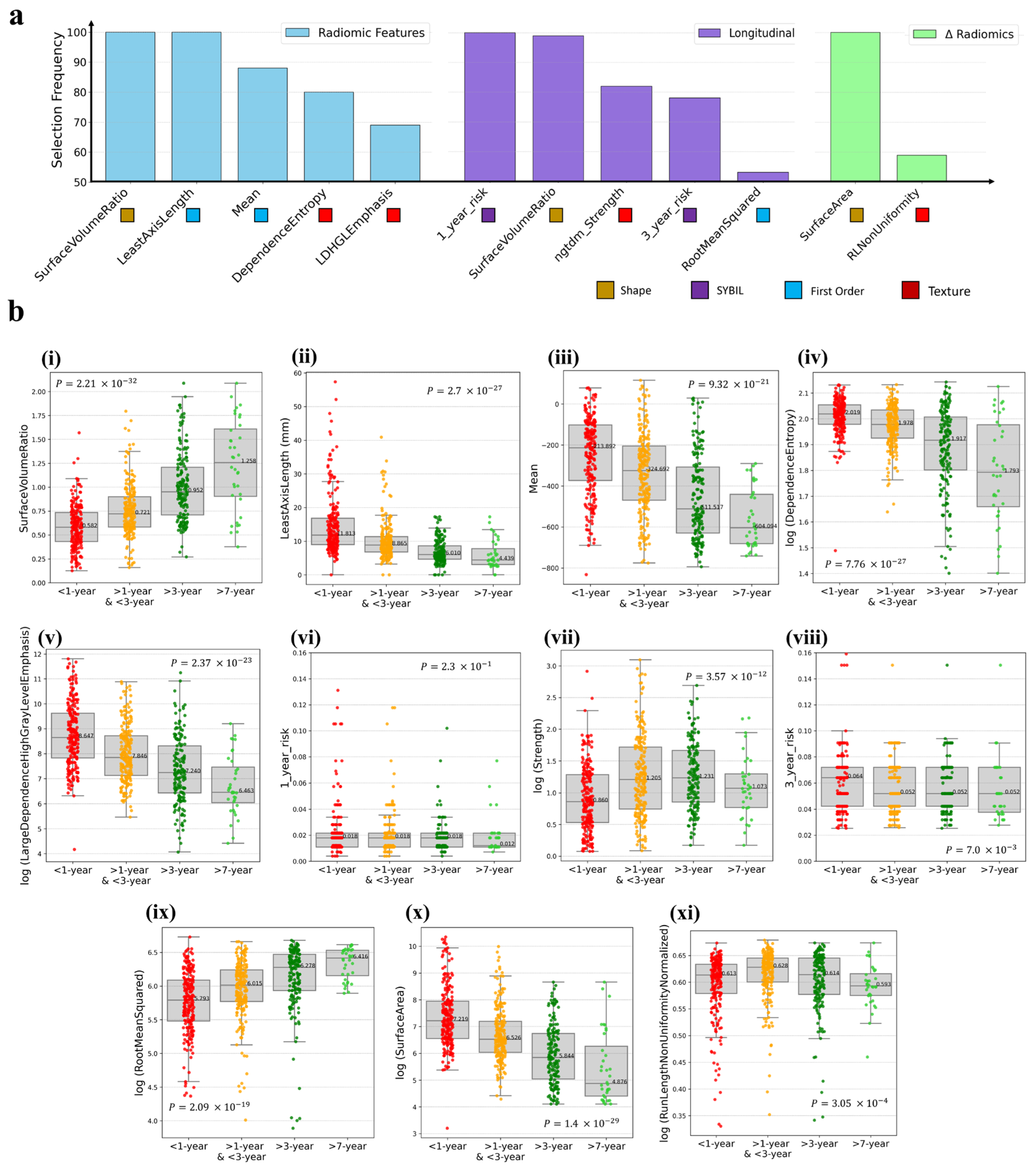
3.1. Radiomics Feature Preprocessing
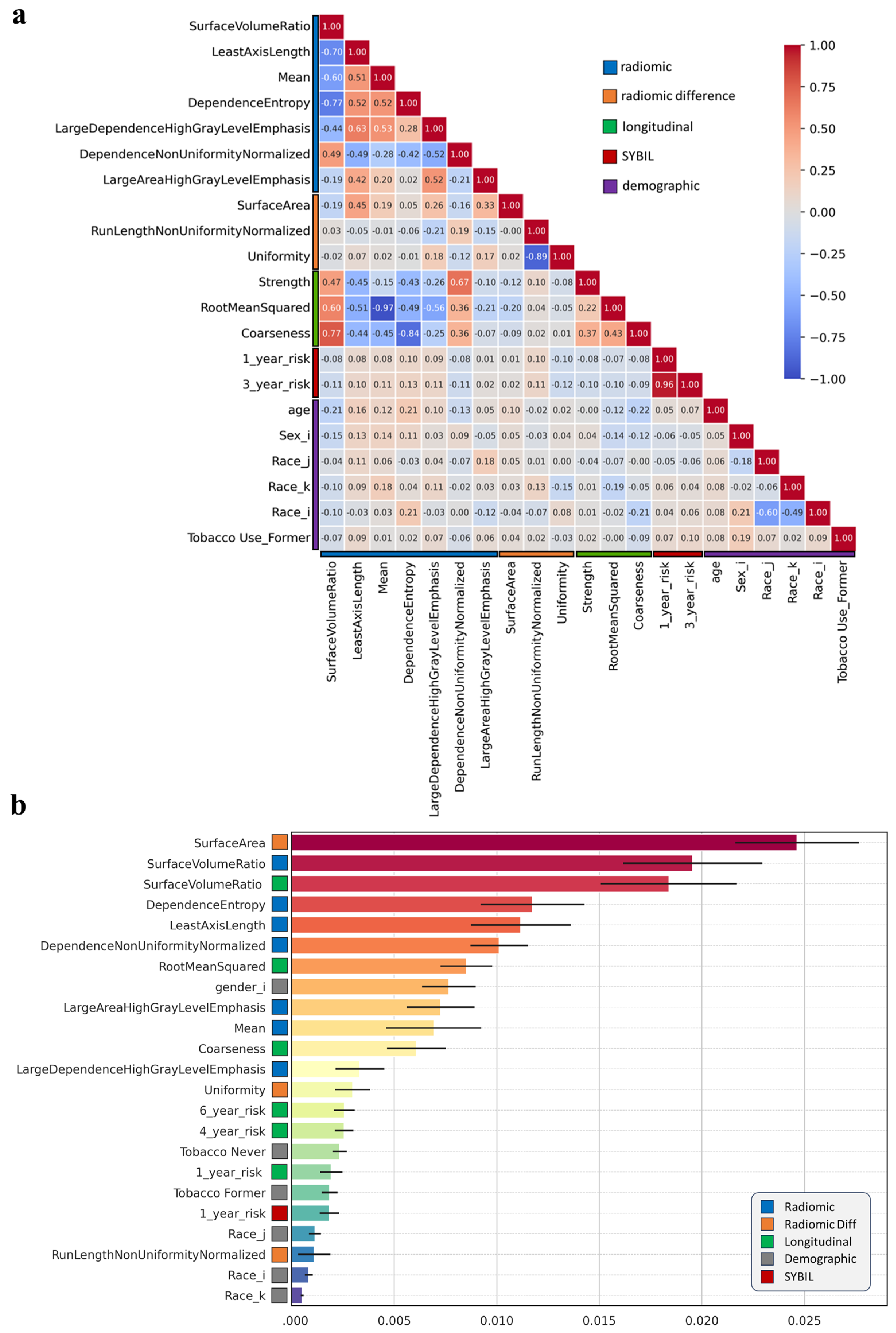
3.2. Individual Radiomics Features Closely Associated with Lung Cancer Risk
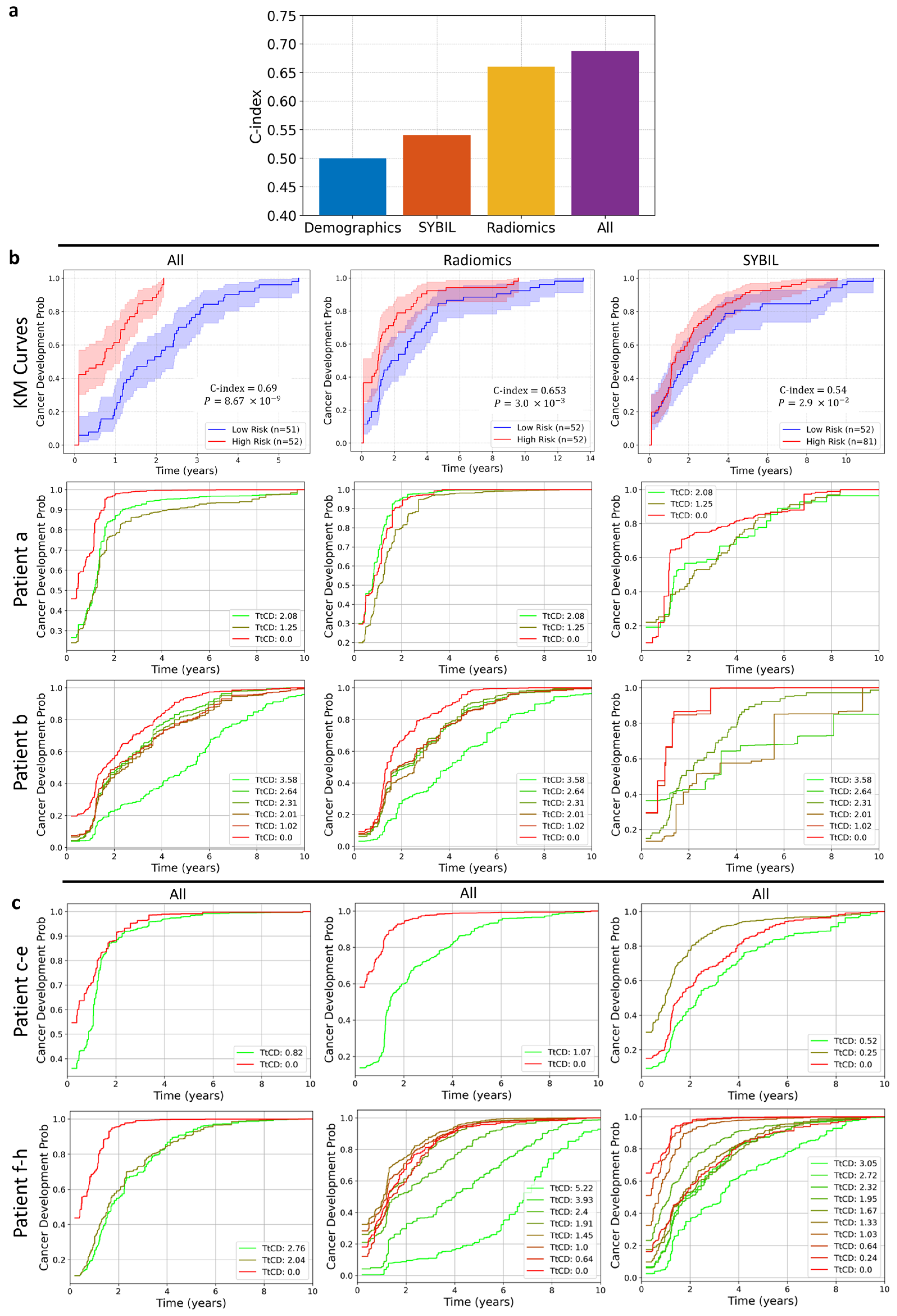
3.3. Composite Risk Model Achieves Optimal Dynamic Lung Cancer Risk Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C-index | Concordance index |
| CT | Computed Tomography |
| CV | Cross Validation |
| GLCM | Gray Level Co-occurrence Matrix |
| GLDM | Gray Level Dependence Matrix |
| GLRLM | Gray Level Run Length Matrix |
| GLSZM | Gray Level Size Zone Matrix |
| HR | Hazard Ratio |
| KM | Kaplan–Meier |
| LDCT | Low dose Computed Tomography |
| NGTDM | Neighboring Gray Tone Difference Matrix |
| NLST | National Lung Screening Trial |
| RSF | Random Survival Forest |
| TtCD | Time to Cancer Development |
References
- Dubin, S.; Griffin, D. Lung cancer in non-smokers. Mo. Med. 2020, 117, 375. [Google Scholar] [CrossRef]
- LoPiccolo, J.; Gusev, A.; Christiani, D.C.; Jänne, P.A. Lung cancer in patients who have never smoked—An emerging disease. Nat. Rev. Clin. Oncol. 2024, 21, 121–146. [Google Scholar] [CrossRef]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M. Probability of cancer in pulmonary nodules detected on first screening ct. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, P.G.; Wohlwend, J.; Yala, A.; Karstens, L.; Xiang, J.; Takigami, A.K.; Bourgouin, P.P.; Chan, P.; Mrah, S.; Amayri, W. Sybil: A validated deep learning model to predict future lung cancer risk from a single low-dose chest computed tomography. J. Clin. Oncol. 2023, 41, 2191–2200. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, Y.; Wang, S.; Qi, L.; Zhang, D.; Hou, H.; Li, H.; Zhao, S. Artificial intelligence in lung cancer screening: Detection, classification, prediction, and prognosis. Cancer Med. 2024, 13, e7140. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Cherezov, D.; Hawkins, S.H.; Goldgof, D.B.; Hall, L.O.; Liu, Y.; Li, Q.; Balagurunathan, Y.; Gillies, R.J.; Schabath, M.B. Delta radiomic features improve prediction for lung cancer incidence: A nested case–control analysis of the national lung screening trial. Cancer Med. 2018, 7, 6340–6356. [Google Scholar] [CrossRef] [PubMed]
- Davidson-Pilon, C. Time Varying Survival Regression. 2024. Available online: https://github.com/CamDavidsonPilon/lifelines/blob/master/docs/Time%20varying%20survival%20regression.rst (accessed on 16 April 2024).
- Thomas, L.; Reyes, E.M. Tutorial: Survival estimation for cox regression models with time-varying coefficients using sas and r. J. Stat. Softw. 2014, 61, 1–23. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Maldonado, S.G.; Delorme, S.; Hüsing, A.; Motsch, E.; Kauczor, H.-U.; Heussel, C.-P.; Kaaks, R. Evaluation of prediction models for identifying malignancy in pulmonary nodules detected via low-dose computed tomography. JAMA Netw. Open 2020, 3, e1921221. [Google Scholar] [CrossRef]
- de Koning, H.J.; van Der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N. Reduced lung-cancer mortality with volume ct screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Team, N.L.S.T.R. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M. Screening for lung cancer: Us preventive services task force recommendation statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Pu, C.Y.; Lusk, C.M.; Neslund-Dudas, C.; Gadgeel, S.; Soubani, A.O.; Schwartz, A.G. Comparison between the 2021 uspstf lung cancer screening criteria and other lung cancer screening criteria for racial disparity in eligibility. JAMA Oncol. 2022, 8, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Midthun, D.E.; Wampfler, J.A.; Deng, B.; Stoddard, S.M.; Zhang, S.; Yang, P. Trends in the proportion of patients with lung cancer meeting screening criteria. JAMA 2015, 313, 853–855. [Google Scholar] [CrossRef]
- Callister, M.E.; de Koning, H.J. Lung cancer screening in never-smokers: A balancing act. Lancet Respir. Med. 2024, 12, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Oudkerk, M.; Liu, S.; Heuvelmans, M.A.; Walter, J.E.; Field, J.K. Lung cancer ldct screening and mortality reduction—Evidence, pitfalls and future perspectives. Nat. Rev. Clin. Oncol. 2020, 18, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-C.; Chiu, C.-H.; Yu, C.-J.; Chang, Y.-C.; Chang, Y.-H.; Hsu, K.-H.; Wu, Y.-C.; Chen, C.-Y.; Hsu, H.-H.; Wu, M.-T. Low-dose ct screening among never-smokers with or without a family history of lung cancer in taiwan: A prospective cohort study. Lancet Respir. Med. 2024, 12, 141–152. [Google Scholar] [CrossRef]
- McCarty, R.D.; Barnard, M.E.; Lawson-Michod, K.A.; Owens, M.; Green, S.E.; Derzon, S.; Karabegovic, L.; Akerley, W.L.; Watt, M.H.; Doherty, J.A. Pathways to lung cancer diagnosis among individuals who did not receive lung cancer screening: A qualitative study. BMC Prim. Care 2023, 24, 203. [Google Scholar] [CrossRef]
- Chang, A.E.; Potter, A.L.; Yang, C.-F.J.; Sequist, L.V. Early detection and interception of lung cancer. Hematol. Oncol. Clin. North Am. 2024, 38, 755–770. [Google Scholar] [CrossRef]
- Zhu, E.; Muneer, A.; Zhang, J.; Xia, Y.; Li, X.; Zhou, C.; Heymach, J.V.; Wu, J.; Le, X. Progress and challenges of artificial intelligence in lung cancer clinical translation. npj Precis. Oncol. 2025, 9, 210. [Google Scholar] [CrossRef]
- Zhang, J.; Salehjahromi, M.; Godoy, M.; Antonoff, M.; Ostrin, E.; Le, X.; Gay, C.; Negrao, M.; Byers, L.; Lu, C. Ma03. 10 the interim analysis of can-prevent-lung trial: Canakinumab for the prevention of lung cancer. J. Thorac. Oncol. 2023, 18, S108–S109. [Google Scholar] [CrossRef]
- Gillies, R.J.; Schabath, M.B. Radiomics improves cancer screening and early detection. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2556–2567. [Google Scholar] [CrossRef] [PubMed]
- Thawani, R.; McLane, M.; Beig, N.; Ghose, S.; Prasanna, P.; Velcheti, V.; Madabhushi, A. Radiomics and radiogenomics in lung cancer: A review for the clinician. Lung Cancer 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef]
- Kastner, J.; Hossain, R.; Jeudy, J.; Dako, F.; Mehta, V.; Dalal, S.; Dharaiya, E.; White, C. Lung-rads version 1.0 versus lung-rads version 1.1: Comparison of categories using nodules from the national lung screening trial. Radiology 2021, 300, 199–206. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P. Biomarkers in lung cancer screening: Achievements, promises, and challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Patients (n = 122) | CTs (n = 622) |
|---|---|---|
| Sex | ||
| Female | 75 (61%) | 397 (64%) |
| Male | 47 (39%) | 225 (36%) |
| Race | ||
| White | 102 (84%) | 512 (82%) |
| Black | 9 (7%) | 45 (7%) |
| Asian | 6 (5%) | 34 (5%) |
| Other | 5 (4%) | 31 (5%) |
| Tobacco Use | ||
| Former | 84 (69%) | 375 (60%) |
| --Former (<20 pack-year history) | 39 (32%) | |
| --Former (quit >15 years ago) | 45 (37%) | |
| Never (<100 lifetime cigarettes) | 36 (30%) | 209 (34%) |
| Current | 2 (2%) | 38 (6%) |
| Imaging Type | ||
| Contrast CT | 280 (45%) | |
| Non-Contrast CT | 342 (55%) | |
| Prior Cancer History | ||
| Breast Cancer | 10 (8%) | |
| Skin Cancer | 9 (7%) | |
| Blood Cancer | 8 (7%) | |
| Head and Neck | 7 (6%) | |
| Other Cancer | 33 (27%) | |
| No Cancer History | 55 (45%) | |
| Time to Cancer Development (TtDC) | Med: 1.4 Min: 0.0 Max: 13.5 | |
| Age for patients (n = 122), represented at the time of cancer diagnosis | Med: 72.62 Min: 42.79 Max: 89.19 | Med: 70.67 Min: 34.05 Max: 89.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehjahromi, M.; Li, H.; Showkatian, E.; Saad, M.B.; Qayati, M.; Ismail, S.M.; Sujit, S.J.; Muneer, A.; Aminu, M.; Hong, L.; et al. Radiomics for Dynamic Lung Cancer Risk Prediction in USPSTF-Ineligible Patients. Cancers 2025, 17, 3406. https://doi.org/10.3390/cancers17213406
Salehjahromi M, Li H, Showkatian E, Saad MB, Qayati M, Ismail SM, Sujit SJ, Muneer A, Aminu M, Hong L, et al. Radiomics for Dynamic Lung Cancer Risk Prediction in USPSTF-Ineligible Patients. Cancers. 2025; 17(21):3406. https://doi.org/10.3390/cancers17213406
Chicago/Turabian StyleSalehjahromi, Morteza, Hui Li, Eman Showkatian, Maliazurina B. Saad, Mohamed Qayati, Sherif M. Ismail, Sheeba J. Sujit, Amgad Muneer, Muhammad Aminu, Lingzhi Hong, and et al. 2025. "Radiomics for Dynamic Lung Cancer Risk Prediction in USPSTF-Ineligible Patients" Cancers 17, no. 21: 3406. https://doi.org/10.3390/cancers17213406
APA StyleSalehjahromi, M., Li, H., Showkatian, E., Saad, M. B., Qayati, M., Ismail, S. M., Sujit, S. J., Muneer, A., Aminu, M., Hong, L., Han, X., Heeke, S., Cascone, T., Le, X., Vokes, N., Gibbons, D. L., Toumazis, I., Ostrin, E. J., Antonoff, M. B., ... Wu, J. (2025). Radiomics for Dynamic Lung Cancer Risk Prediction in USPSTF-Ineligible Patients. Cancers, 17(21), 3406. https://doi.org/10.3390/cancers17213406








