Treatment Outcomes and Significance of Multimodal Treatment in Esophageal Squamous Cell Carcinoma with Synchronous Oligometastasis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Oligometastasis
2.3. Treatment Selection
2.4. Chemotherapy and Immunotherapy
2.5. Definitive Chemoradiotherapy and Radiotherapy
2.6. Preoperative Chemotherapy, Immunotherapy, and Chemoradiotherapy
2.7. Surgical Treatment
2.8. Statistical Analysis
3. Results
3.1. Details of Oligometastases and Treatments
3.2. OS for All Cases and According to the Type of Treatment
3.3. OS According to Type of Distant Metastasis, Number of Metastatic Organs, Number of Metastatic Lesions, and Maximum Long Diameter of Distant Metastasis
3.4. Univariate and Multivariate Analyses of OS
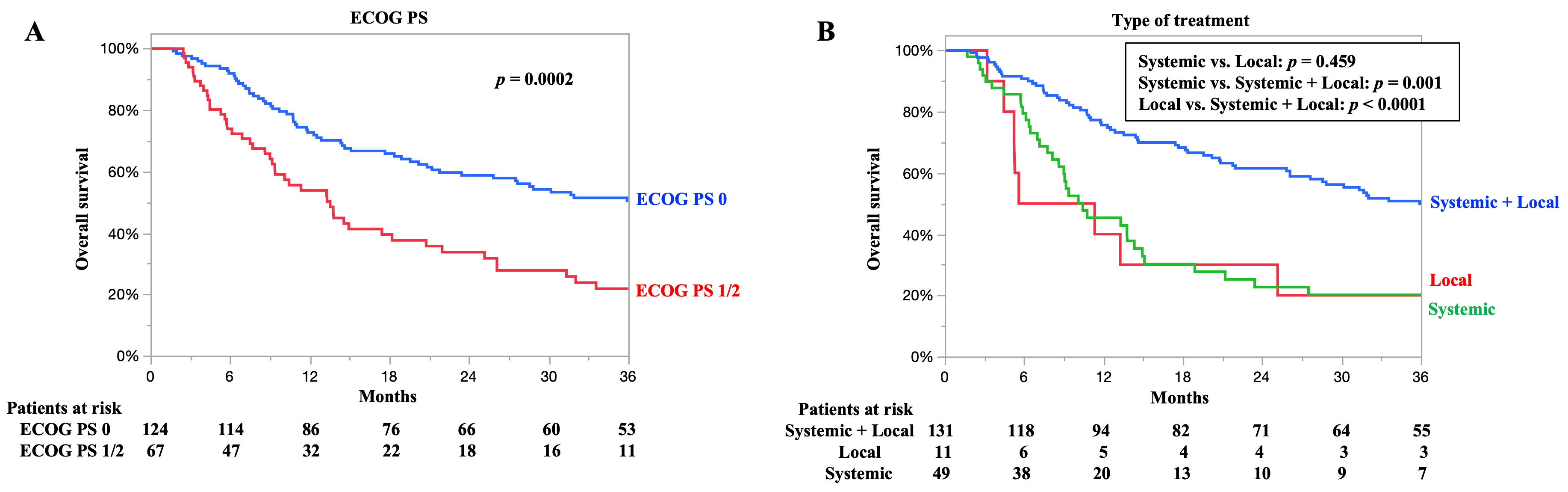
3.5. OS According to ECOG PS and Type of Treatment
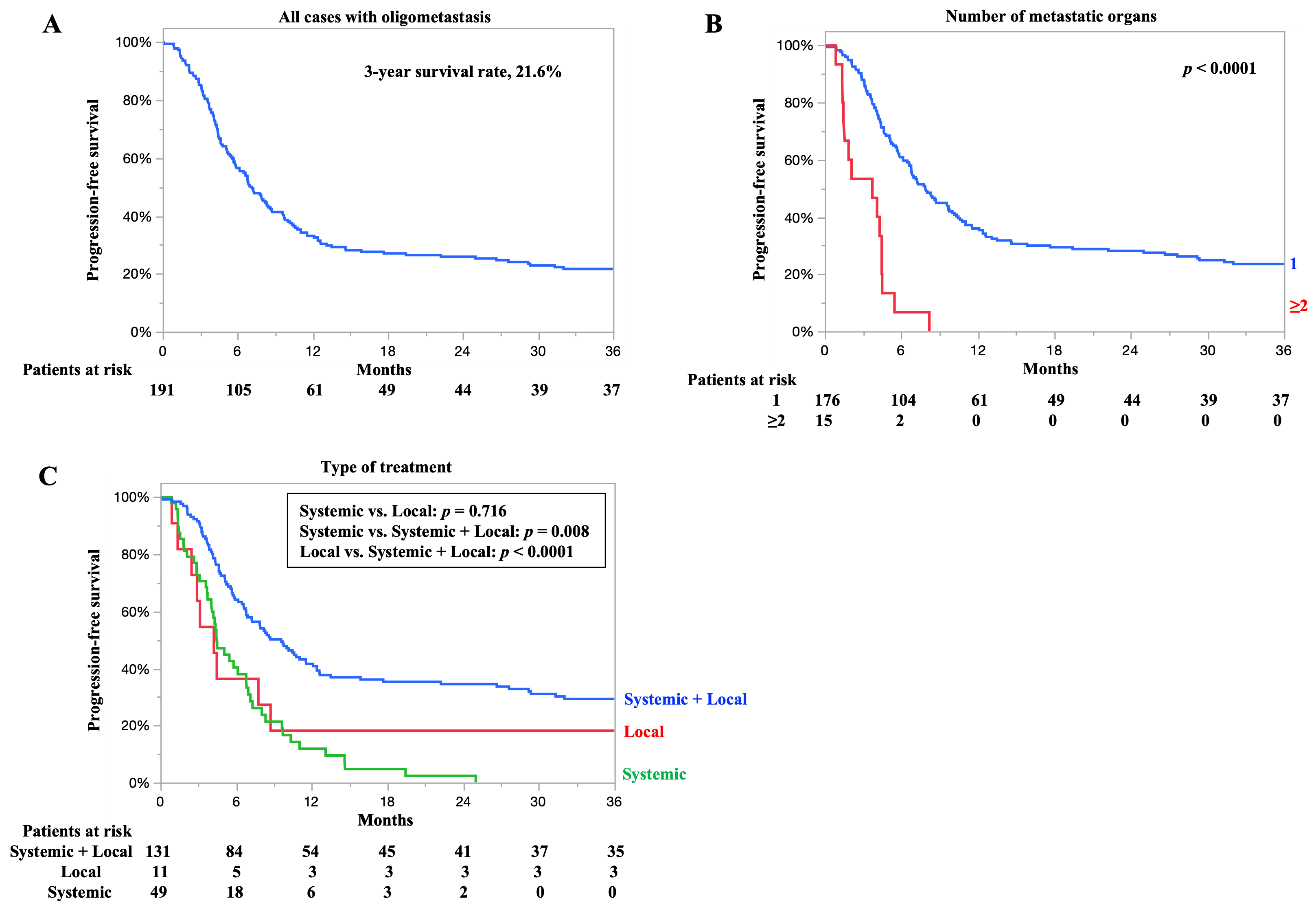
3.6. PFS for All Cases and According to Number of Metastatic Organs and Type of Treatment
3.7. Relationship Between Number of Metastatic Organs and Clinical Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | cisplatin plus 5-fluorouracil |
| CI | confidence interval |
| CT | computed tomography |
| ECOG PS | Eastern Cooperative Oncology Group performance status |
| ESCC | esophageal squamous cell carcinoma |
| HR | hazard ratio |
| OMEC | OligoMetastatic Esophagogastric Cancer |
| OS | overall survival |
| PFS | progression-free survival |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) International Union Against Cancer (UICC): TNM Classification of Malignant Tumors, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Dubecz, A.; Gall, I.; Solymosi, N.; Schweigert, M.; Peters, J.H.; Feith, M.; Stein, H.J. Temporal trends in long-term survival and cure rates in esophageal cancer: A SEER database analysis. J. Thorac. Oncol. 2012, 7, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C.; on behalf of the ESMO Guidelines Committee. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Depypere, L.; Lerut, T.; Moons, J.; Coosemans, W.; Decker, G.; Van Veer, H.; De Leyn, P.; Nafteux, P. Isolated local recurrence or solitary solid organ metastasis after esophagectomy for cancer is not the end of the road. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Ghaly, G.; Harrison, S.; Kamel, M.K.; Rahouma, M.; Nasar, A.; Port, J.L.; Stiles, B.M.; Altorki, N.K. Predictors of survival after treatment of oligometastases after esophagectomy. Ann. Thorac. Surg. 2018, 105, 357–362. [Google Scholar] [CrossRef]
- Nobel, T.B.; Sihag, S.; Xing, X.; Eljalby, M.; Hsu, M.; Tan, K.S.; Sewell, D.B.; Bains, M.S.; Janjigian, Y.; Wu, A.; et al. Oligometastases after curative esophagectomy are not one size fits all. Ann. Thorac. Surg. 2021, 112, 1775–1781. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Z.; Chen, Y.; Deng, J.; Ai, D.; Liu, Q.; Wang, S.; Wu, S.; Chen, J.; Zhao, K. Phase 2 study of stereotactic body radiation therapy for patients with oligometastatic esophageal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 707–715. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Nakajima, J.; Mun, M.; Shintani, Y.; Kuroda, H.; Iwata, T.; Endo, M.; Azuma, Y.; Chida, M.; Sakao, Y.; et al. Survival after lung metastasectomy from esophageal cancer: Results from a multi-institutional database. Cancers 2023, 15, 1472. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Matsuda, S.; Tsushima, T.; Kato, K.; Hsu, C.H.; Lee, J.M.; Wong, I.Y.H.; Wang, H.C.; Kang, C.H.; Guo, X.; Yamamoto, S.; et al. Defining conversion therapy for esophageal squamous cell carcinoma. Ann. Gastroenterol. Surg. 2023, 7, 7–9. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Machida, R.; Ito, Y.; Daiko, H.; Ozawa, S.; Ogata, T.; Hara, H.; Kojima, T.; Abe, T.; Bamba, T.; et al. Doublet chemotherapy, triplet chemotherapy, or doublet chemotherapy combined with radiotherapy as neoadjuvant treatment for locally advanced oesophageal cancer (JCOG1109 NExT): A randomised, controlled, open-label, phase 3 trial. Lancet 2024, 404, 55–66. [Google Scholar] [CrossRef]
- Kawahara, D.; Murakami, Y.; Awane, S.; Emoto, Y.; Iwashita, K.; Kubota, H.; Sasaki, R.; Nagata, Y. Radiomics and dosiomics for predicting complete response to definitive chemoradiotherapy patients with oesophageal squamous cell cancer using the hybrid institution model. Eur. Radiol. 2024, 34, 1200–1209. [Google Scholar] [CrossRef]
- Hamai, Y.; Hihara, J.; Emi, M.; Murakami, Y.; Kenjo, M.; Nagata, Y.; Okada, M. Results of neoadjuvant chemoradiotherapy with docetaxel and 5-fluorouracil followed by esophagectomy to treat locally advanced esophageal cancer. Ann. Thorac. Surg. 2015, 99, 1887–1893. [Google Scholar] [CrossRef]
- Murakami, Y.; Hamai, Y.; Emi, M.; Hihara, J.; Imano, N.; Takeuchi, Y.; Takahashi, I.; Nishibuchi, I.; Kimura, T.; Okada, M.; et al. Long-term results of neoadjuvant chemoradiotherapy using cisplatin and 5-fluorouracil followed by esophagectomy for resectable, locally advanced esophageal squamous cell carcinoma. J. Radiat. Res. 2018, 59, 616–624. [Google Scholar] [CrossRef]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Kroese, T.E.; Bronzwaer, S.; van Rossum, P.S.N.; Schoppman, S.F.; Deseyne, P.R.A.J.; van Cutsem, E.; Haustermans, K.; Nafteux, P.; Thomas, M.; Obermannova, R.; et al. European clinical practice guidelines for the definition, diagnosis, and treatment of oligometastatic esophagogastric cancer (OMEC-4). Eur. J. Cancer 2024, 204, 114062. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, X.; Ruan, C.; Wei, G.; Li, J.; Qiu, H.; Gao, L.; Cai, G.; Zhangcai, Y.; Li, B.; et al. Evaluation of concurrent chemoradiotherapy for survival outcomes in patients with synchronous oligometastatic esophageal squamous cell carcinoma. JAMA Netw. Open 2022, 5, e2244619. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, X.; Song, H.; Wu, A.J.; Ku, G.Y.; Lee, P.; Slingerland, M.; Koyanagi, K.; Ke, S.; Qiu, H.; et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for esophageal squamous cell cancer patients presenting with oligometastases. J. Thorac. Dis. 2019, 11, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, K.; Tanaka, K.; Sugase, T.; Momose, K.; Kanemura, T.; Yamashita, K.; Makino, T.; Shiraishi, O.; Motoori, M.; Yamasaki, M.; et al. Clinical impact of conversion surgery after induction therapy for esophageal cancer with synchronous distant metastasis: A multi-institutional retrospective study. Ann. Surg. Oncol. 2024, 31, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Matsuda, S.; Sato, Y.; Tanaka, K.; Sasaki, K.; Watanabe, M.; Hamai, Y.; Nasu, M.; Saze, Z.; Nakashima, Y.; et al. Safety and efficacy of conversion therapy after systemic chemotherapy in advanced esophageal cancer with distant metastases: A multicenter retrospective observational study. Ann. Surg. Oncol. 2025, 32, 274–283. [Google Scholar] [CrossRef]
- Igaue, S.; Nozaki, R.; Utsunomiya, D.; Kubo, Y.; Kubo, K.; Kurita, D.; Yamamoto, S.; Ishiyama, K.; Oguma, J.; Kato, K.; et al. Significance of surgery for resectable M1 lymph node metastases without organ metastasis in esophageal carcinoma in the era of neoadjuvant treatment. Ann. Surg. Oncol. 2024, 31, 1525–1535. [Google Scholar] [CrossRef]
- Norén, N.; Rouvelas, I.; Lundell, L.; Nilsson, M.; Sunde, B.; Szabo, E.; Edholm, D.; Hedberg, J.; Smedh, U.; Hermansson, M.; et al. Curative treatment for oligometastatic gastroesophageal cancer– results of a prospective multicenter study. Langenbecks Arch. Surg. 2024, 410, 10. [Google Scholar] [CrossRef]
- van Hootegem, S.J.M.; de Pasqual, C.A.; Giacopuzzi, S.; Van Daele, E.; Vanommeslaeghe, H.; Moons, J.; Nafteux, P.; van der Sluis, P.C.; Lagarde, S.M.; Wijnhoven, B.P.L. Outcomes after surgical treatment of oesophagogastric cancer with synchronous liver metastases: A multicentre retrospective cohort study. Cancers 2024, 16, 797. [Google Scholar] [CrossRef]


| Parameters | N = 191 |
|---|---|
| Age (mean ± SD, y) | 68.2 ± 10.1 |
| Sex | |
| Male | 169 (88.5%) |
| Female | 22 (11.5%) |
| ECOG PS | |
| 0 | 124 (64.9%) |
| 1 | 62 (32.5%) |
| 2 | 5 (2.6%) |
| Tumor markers | |
| SCC (mean ± SD, ng/mL) | 2.2 ± 1.9 |
| CEA (mean ± SD, ng/mL) | 3.9 ± 3.9 |
| Primary tumor location | |
| Upper | 64 (33.5%) |
| Middle | 82 (42.9%) |
| Lower | 42 (22.0%) |
| Abdominal | 3 (1.6%) |
| Histology (biopsy specimens) | |
| Well-differentiated | 13 (6.8%) |
| Moderately differentiated | 30 (15.7%) |
| Poorly differentiated | 38 (19.9%) |
| Squamous cell carcinoma (not assessable) | 110 (57.6%) |
| Clinical T a | |
| cT1 | 7 (3.7%) |
| cT2 | 15 (7.9%) |
| cT3 | 116 (60.7%) |
| cT4 | 53 (27.7%) |
| Clinical N a | |
| cN0 | 30 (15.7%) |
| cN1 | 81 (42.4%) |
| cN2 | 74 (38.8%) |
| cN3 | 6 (3.1%) |
| Details of distant metastasis b | |
| Distant lymph node | 175 (92.1%) |
| Liver | 14 (7.3%) |
| Bone | 8 (4.2%) |
| Lung | 6 (3.1%) |
| Adrenal gland | 2 (1.1%) |
| Skeletal muscle | 1 (0.5%) |
| Type of distant metastasis c | |
| Organ | 24 (12.6%) |
| Lymph node | 167 (87.4%) |
| Number of metastatic organs d | |
| 1 | 176 (92.1%) |
| 2 | 13 (6.8%) |
| 3 | 2 (1.1%) |
| Number of metastatic lesions | |
| 1 | 120 (62.8%) |
| 2 | 43 (22.5%) |
| 3 | 19 (10.0%) |
| 4 | 8 (4.2%) |
| 5 | 1 (0.5%) |
| Maximum long diameter of distant metastasis | |
| (mean ± SD, cm) | 2.0 ± 0.9 |
| 1–1.9 cm | 111 (58.1%) |
| 2–2.9 cm | 51 (26.7%) |
| 3–3.9 cm | 19 (9.9%) |
| 4–4.9 cm | 7 (3.7%) |
| 5–5.9 cm | 3 (1.6%) |
| Type of treatment | |
| Systemic + local therapy | |
| Preoperative chemotherapy and surgery | 26 (13.6%) |
| Preoperative chemoradiotherapy and surgery | 29 (15.2%) |
| Chemoradiotherapy | 76 (39.8%) |
| Local therapy | |
| Surgery alone | 3 (1.6%) |
| Radiotherapy | 8 (4.2%) |
| Systemic therapy e | |
| Chemotherapy + immunotherapy | 7 (3.6%) |
| Chemotherapy | 39 (20.4%) |
| Immunotherapy | 3 (1.6%) |
| Clinical response f,g | |
| Complete response | 35 (18.6%) |
| Partial response | 94 (50.0%) |
| Stable disease | 18 (9.6%) |
| Progressive disease | 37 (19.6%) |
| No assessment | 4 (2.2%) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age, y (continuous) | 1.02 | 0.98–1.04 | 0.978 | – | – | – |
| Sex | ||||||
| Female (reference) | 1 | – | – | – | ||
| Male | 1.19 | 0.68–2.07 | 0.535 | – | – | – |
| ECOG PS | ||||||
| 0 (reference) | 1 | 1 | ||||
| 1/2 | 1.99 | 1.38–2.86 | 0.0002 | 1.52 | 1.02–2.27 | 0.036 |
| SCC a | ||||||
| <1.5 ng/mL | 1 | 1 | ||||
| ≥1.5 ng/mL | 1.50 | 1.05–2.14 | 0.024 | 1.39 | 0.95–2.02 | 0.084 |
| CEA a | ||||||
| <5 ng/mL | 1 | – | – | – | ||
| ≥5 ng/mL | 1.50 | 0.96–2.33 | 0.071 | – | – | – |
| Primary tumor location | ||||||
| Lower, abdominal (reference) | 1 | – | – | – | ||
| Upper, middle | 1.01 | 0.66–1.54 | 0.956 | – | – | – |
| Histology (biopsy specimens) | ||||||
| Others (reference) | 1 | – | – | – | ||
| Poorly differentiated | 1.04 | 0.56–1.59 | 0.856 | – | – | – |
| Clinical T b | ||||||
| T1/2 (reference) | 1 | – | – | – | ||
| T3/4 | 1.42 | 0.82–2.44 | 0.199 | – | – | – |
| Clinical N b | ||||||
| N0/1 (reference) | 1 | – | – | – | ||
| N2/3 | 1.21 | 0.84–1.73 | 0.305 | – | – | – |
| Type of distant metastasis c | ||||||
| Lymph node (reference) | 1 | |||||
| Organ | 2.27 | 1.39–3.68 | 0.001 | 1.14 | 0.57–2.24 | 0.702 |
| Number of metastatic organs d | ||||||
| 1 (reference) | 1 | 1 | ||||
| ≥2 | 4.23 | 2.35–7.60 | <0.0001 | 2.72 | 1.09–6.78 | 0.031 |
| Number of metastatic lesions | ||||||
| 1 (reference) | 1 | 1 | ||||
| 2 | 1.06 | 0.69–1.64 | 0.768 | 1.05 | 0.66–1.68 | 0.815 |
| ≥3 | 2.09 | 1.29–3.38 | 0.003 | 1.19 | 0.61–2.33 | 0.599 |
| Maximum long diameter of distant metastasis | ||||||
| 1–1.9 cm (reference) | 1 | – | – | – | ||
| 2–2.9 cm | 1.41 | 0.95–2.11 | 0.087 | – | – | – |
| ≥3 cm | 1.05 | 0.63–1.74 | 0.844 | – | – | – |
| Type of treatment e | ||||||
| Systemic therapy (reference) | 1 | 1 | ||||
| Local therapy | 1.30 | 0.64–2.63 | 0.459 | 1.26 | 0.59–2.73 | 0.541 |
| Systemic + local therapy | 0.43 | 0.29–0.65 | <0.0001 | 0.56 | 0.34–0.93 | 0.026 |
| Treatment period | ||||||
| 2006–2017 | 1 | – | – | – | ||
| 2018–2022 | 1.16 | 0.79–1.71 | 0.433 | – | – | – |
| Clinical Response a,b | N = 191 | Number of Metastatic Organs c | p-Value | ||
|---|---|---|---|---|---|
| 1 (n = 173) | 2 (n = 13) | 3 (n = 2) | |||
| Complete response | 35 (18.6%) | 35 (20.2%) | 0 | 0 | 0.0001 |
| Partial response | 94 (50.0%) | 91 (52.6%) | 3 (23.1%) | 0 | |
| Stable disease | 18 (9.6%) | 17 (9.9%) | 1 (7.7%) | 0 | |
| Progressive disease | 37 (19.6%) | 26 (15.0%) | 9 (69.2%) | 2 (100%) | |
| No assessment | 4 (2.2%) | 4 (2.3%) | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohsawa, M.; Hamai, Y.; Ibuki, Y.; Kurokawa, T.; Kitasaki, N.; Okada, M. Treatment Outcomes and Significance of Multimodal Treatment in Esophageal Squamous Cell Carcinoma with Synchronous Oligometastasis. Cancers 2025, 17, 3407. https://doi.org/10.3390/cancers17213407
Ohsawa M, Hamai Y, Ibuki Y, Kurokawa T, Kitasaki N, Okada M. Treatment Outcomes and Significance of Multimodal Treatment in Esophageal Squamous Cell Carcinoma with Synchronous Oligometastasis. Cancers. 2025; 17(21):3407. https://doi.org/10.3390/cancers17213407
Chicago/Turabian StyleOhsawa, Manato, Yoichi Hamai, Yuta Ibuki, Tomoaki Kurokawa, Nao Kitasaki, and Morihito Okada. 2025. "Treatment Outcomes and Significance of Multimodal Treatment in Esophageal Squamous Cell Carcinoma with Synchronous Oligometastasis" Cancers 17, no. 21: 3407. https://doi.org/10.3390/cancers17213407
APA StyleOhsawa, M., Hamai, Y., Ibuki, Y., Kurokawa, T., Kitasaki, N., & Okada, M. (2025). Treatment Outcomes and Significance of Multimodal Treatment in Esophageal Squamous Cell Carcinoma with Synchronous Oligometastasis. Cancers, 17(21), 3407. https://doi.org/10.3390/cancers17213407





