Oncological Outcomes of De-Escalation of Axillary Surgery in Breast Cancer Patients at a Referral Cancer Center in Colombia
Simple Summary
Abstract
1. Introduction
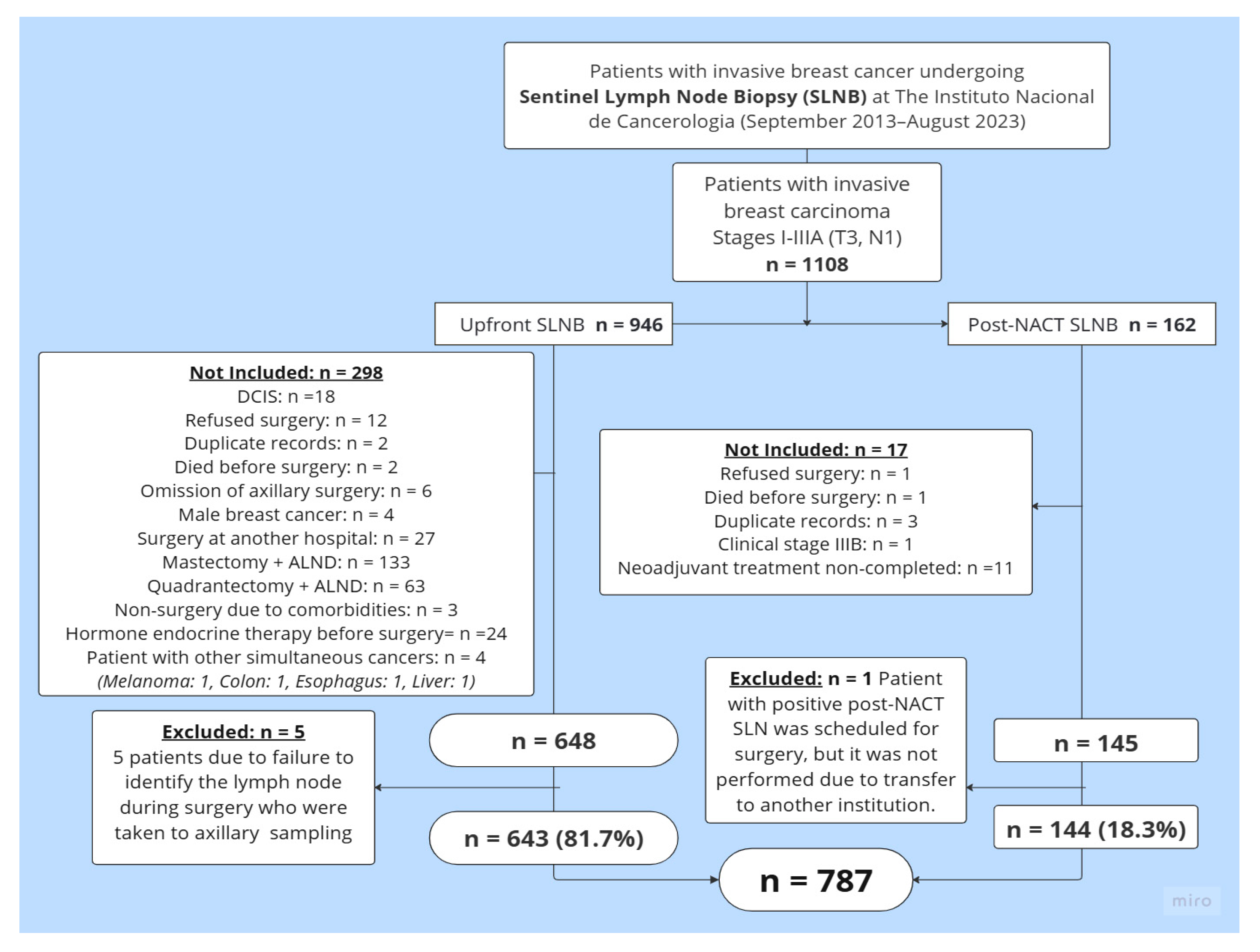
2. Materials and Methods
2.1. Study Design and Patient Eligibility
2.2. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
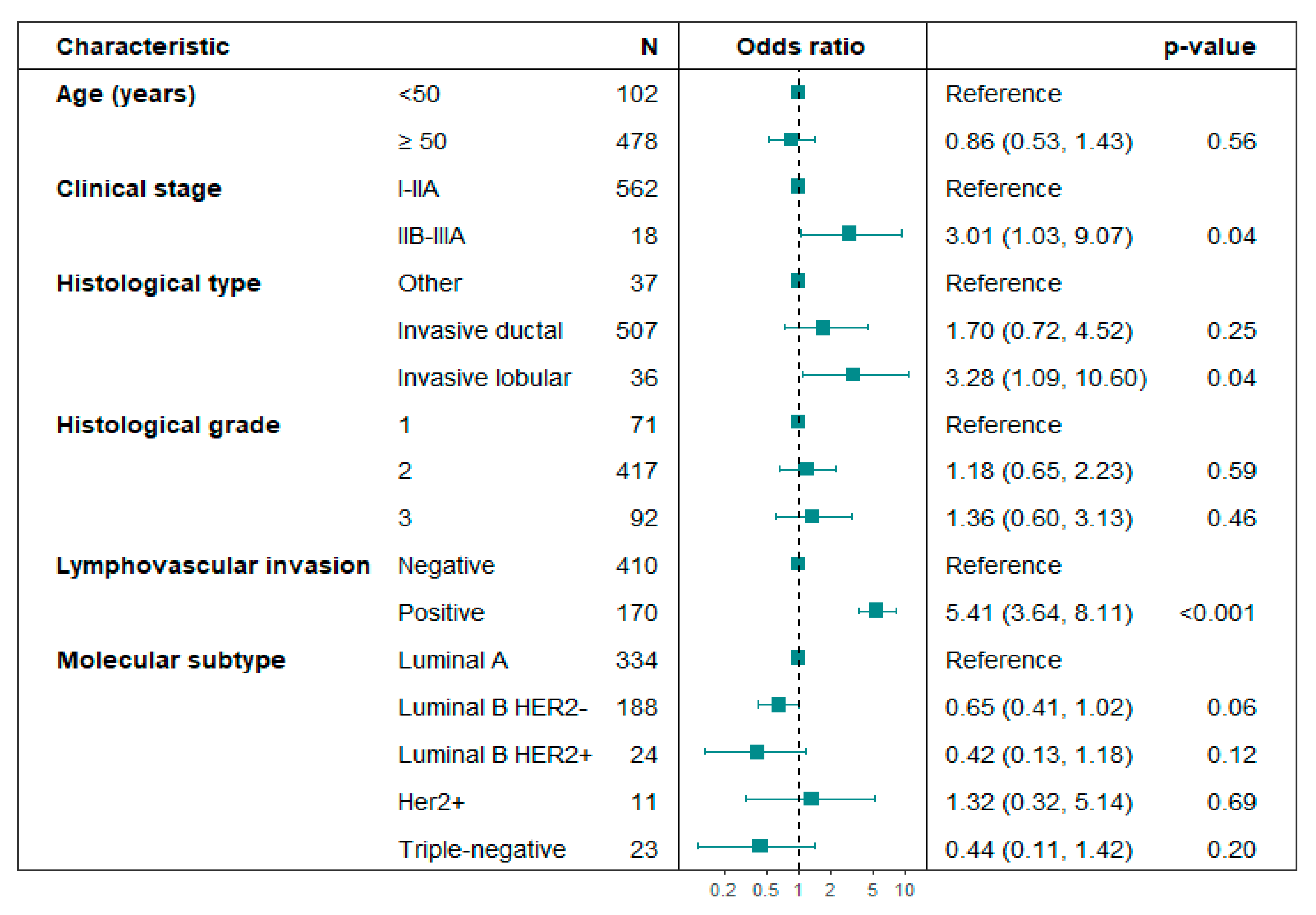
3.2. Sentinel Lymph Node Outcome
3.3. Treatment Types
3.4. Recurrence Location and Treatment
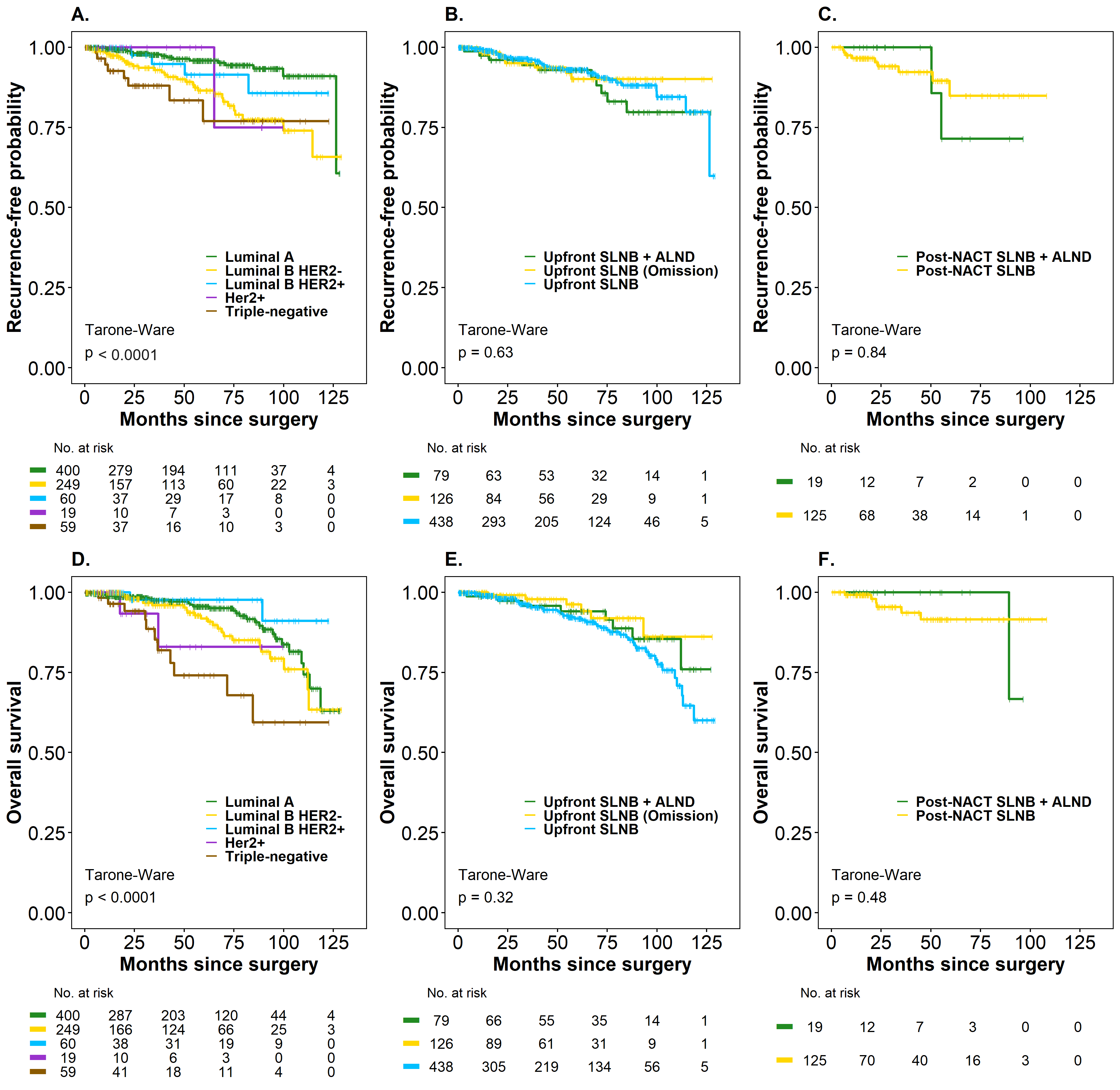
3.5. Time to Recurrence (TR) and Overall Survival (OS)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 99mTc | Technetium-99m (radioisotope commonly used in nuclear medicine for diagnostic imaging) |
| AC | Anthracycline, cyclophosphamide |
| AI | Aromatase inhibitor |
| ALND | Axillary lymph node dissection |
| AP | Anatomic pathology |
| BCS | Breast-conserving surgery |
| CI | Confidence interval |
| DCIS | Ductal carcinoma in situ |
| DFS | Disease-free survival |
| ECE | Extracapsular extension |
| ET | Endocrine therapy |
| ETDs | Extranodal tumor deposits |
| FNR | False negative rate |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hazard ratio |
| HT | Hormone therapy |
| iCDK4/6 | Inhibitor of cyclin-dependent kinase 4 and cyclin-dependent kinase 6 |
| ICH-GCP | International Conference on Harmonization of Good Clinical Practices |
| IQR | Interquartile range |
| INC | Instituto Nacional de Cancerología |
| LVI | Lymphovascular invasion |
| NACT | Neoadjuvant chemotherapy |
| NOS | Not Otherwise Specified |
| OR | Odds ratio |
| OS | Overall survival |
| pCR | Pathological complete response |
| Post-NACT SLNB | Sentinel lymph node biopsy after neoadjuvant chemotherapy |
| RCB | Residual Cancer Burden |
| RFS | Recurrence-free survival |
| RT | Radiotherapy |
| SD | Standard deviation |
| SLN | Sentinel lymph node |
| SLNB | Sentinel lymph node biopsy |
| T | Taxanes |
| TAD | Targeted axillary dissection |
| TC | Taxane and cyclophosphamide |
| TH | Taxanes and trastuzumab |
| TNBC | Triple Negative Breast Cancer |
| TR | Time to recurrence |
| UFM-INC | Functional Unit for Breast and Soft Tissue Tumors, Instituto Nacional de Cancerología |
| Upfront SLNB | Sentinel lymph node biopsy at the time of the initial surgery, before the administration of any systemic therapy (such as neoadjuvant chemotherapy) |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today—Colombia. World Health Organization: Lyon, France, 2022. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/170-colombia-fact-sheet.pdf (accessed on 6 July 2025).
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; McCall, L.M.; Morrow, M. Axillary dissection vs. no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA 2011, 305, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mansel, R.E.; Fallowfield, L.; Kissin, M.; Goyal, A.; Newcombe, R.G.; Dixon, J.M.; Yiangou, C.; Horgan, K.; Bundred, N.; Monypenny, I.; et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J. Natl. Cancer Inst. 2006, 98, 599–609. [Google Scholar] [CrossRef]
- Krag, D.N.; Anderson, S.J.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs. no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rodríguez, R.; Briceño-Morales, X.; Quintero-Ortíz, M.A.; López-Correa, P.; Guzman-AbiSaab, L.; Cervera-Bonilla, S.; Angel-Aristizabal, J.; Lehmann-Mosquera, C.; García-Mora, M.; Duarte-Torres, C.; et al. Omission of axillary lymph node dissection in early-stage breast cancer with positive sentinel lymph node biopsy: Update of evidence and therapeutic approach at the National Cancer Institute of Colombia. Rev. Colomb. Cancerol. 2021, 25 (Suppl. 1), 123–129. [Google Scholar] [CrossRef]
- Mamtani, A.; Barrio, A.V.; Goldman, D.A.; Wen, H.Y.; Vincent, A.; Morrow, M. Extranodal tumor deposits in the axillary fat indicate the need for axillary dissection among T1–T2 cN0 patients with positive sentinel nodes. Ann. Surg. Oncol. 2020, 27, 3585–3592. [Google Scholar] [CrossRef]
- Galimberti, V.; Cole, B.F.; Zurrida, S.; Viale, G.; Luini, A.; Veronesi, P.; Baratella, P.; Chifu, C.; Sargenti, M.; Intra, M.; et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): A phase 3 randomised controlled trial. Lancet Oncol. 2013, 14, 297–305. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Sávolt, Á.; Péley, G.; Polgár, C.; Udvarhelyi, N.; Rubovszky, G.; Kovács, E.; Győrffy, B.; Kásler, M.; Mátrai, Z. Eight-year follow-up result of the OTOASOR trial: The Optimal Treatment of the Axilla—Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer. A randomized, single-centre, phase III, non-inferiority trial. Eur. J. Surg. Oncol. 2017, 43, 672–679. [Google Scholar] [CrossRef]
- de Boniface, J.; Tvedskov, T.F.; Rydén, L.; Szulkin, R.; Reimer, T.; Kühn, T.; Kontos, M.; Gentilini, O.D.; Olofsson Bagge, R.; Sund, M.; et al. Omitting axillary dissection in breast cancer with sentinel-node metastases. N. Engl. J. Med. 2024, 390, 1163–1175. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.M.; Loaec, C.; Gimbergues, P.; Alran, S.; Tunon de Lara, C.; Dupre, P.F.; Rouzier, R.; Faure, C.; Paillocher, N.; Chauvet, M.P.; et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: The GANEA 2 study. Breast Cancer Res. Treat. 2019, 173, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Puerto-Horta, L.J.; Lopez-Correa, P.; Cervera-Bonilla, S.; Guzman-Abisaab, L.; Garcia-Mora, M.; Lehmann-Mosquera, C.; Angel-Aristizabal, J.; Duarte-Torres, C.; Baez-Guzman, A.Z.; Gonzalez-Rodriguez, E.I.; et al. Post-neoadjuvant chemotherapy sentinel node in breast cancer: Therapeutic approach at the National Cancer Institute of Colombia. Rev. Colomb. Cancerol. 2021, 25 (Suppl. 1), 152–159. [Google Scholar] [CrossRef]
- Dixon, J.R. The International Conference on Harmonization Good Clinical Practice Guideline. Qual. Assur. (Good Pract. Regul. Law) 1999, 6, 65–74. [Google Scholar] [CrossRef]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.A.W.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef]
- MD Anderson Cancer Center. Breast Cancer Nomogram: Javascript Conversion Calculator. MD Anderson: Houston, TX, USA. Available online: https://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 (accessed on 6 July 2025).
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef]
- Di Micco, R.; Hartmann, S.; Banys-Paluchowski, M.; de Boniface, J.; Schmidt, E.; Ditsch, N.; Stickeler, E.; Schroth, J.; Karadeniz Cakmak, G.; Hahn, M.; et al. Diagnostic performance of axillary ultrasound after neoadjuvant chemotherapy in initially node-positive breast cancer patients—Results from the prospective AXSANA registry trial. Eur. J. Cancer 2025, 226, 115607. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference; et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Breast 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Viale, G.; Zurrida, S.; Maiorano, E.; Mazzarol, G.; Pruneri, G.; Paganelli, G.; Maisonneuve, P.; Veronesi, U. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 2005, 103, 492–500. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Yi, S.; Gong, M.; Lu, C.; Cai, Y.; Tang, X.; Zou, L. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 2863–2873. [Google Scholar] [CrossRef]
- Gegúndez Gómez, C.; Casariego Vales, E.; Couselo Villanueva, J.M.; Cao Pena, J.; Torres García, M.I.; Álvarez Gutiérrez, A.; Conde Vales, J.; Blasco Alonso, J.L.; Maseda Díaz, O.; Guillán Millán, R.; et al. Conventional prognostic parameters for invasive breast carcinoma. Rev. De Senol. Y Patol. Mamar.–J. Senol. Breast Dis. 2002, 15, 64–70. [Google Scholar]
- Vandorpe, T.; Smeets, A.; Van Calster, B.; Van Hoorde, K.; Leunen, K.; Amant, F.; Moerman, P.H.; Deraedt, K.; Brouckaert, O.; Van Huffel, S.; et al. Lobular and non-lobular breast cancers differ regarding axillary lymph node metastasis: A cross-sectional study on 4,292 consecutive patients. Breast Cancer Res. Treat. 2011, 128, 429–435. [Google Scholar] [CrossRef]
- Güven, H.E.; Kültüroğlu, M.O.; Gülçelik, M.A.; Özaslan, C. Sentinel lymph node metastasis in invasive lobular carcinoma of the breast. Eur. J. Breast Health 2018, 14, 117–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bevilacqua, J.L.B.; Kattan, M.W.; Fey, J.V.; Cody, H.S., III; Borgen, P.I.; Van Zee, K.J. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J. Clin. Oncol. 2007, 25, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A.; Ayers, G.D.; Hwang, R.F.; Hunt, K.K.; Ross, M.I.; Kuerer, H.M.; Singletary, S.E.; Babiera, G.V.; Ames, F.C.; Feig, B.; et al. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann. Surg. Oncol. 2006, 13, 310–320. [Google Scholar] [CrossRef]
- Montagna, G.; Mrdutt, M.M.; Sun, S.X.; Hlavin, C.; Diego, E.J.; Wong, S.M.; Barrio, A.V.; Botty van den Bruele, A.; Cabioglu, N.; Sevilimedu, V.; et al. Omission of axillary dissection following nodal downstaging with neoadjuvant chemotherapy. JAMA Oncol. 2024, 10, 793–798. [Google Scholar] [CrossRef]
- Gentilini, O.D.; Botteri, E.; Sangalli, C.; Galimberti, V.; Porpiglia, M.; Agresti, R.; Luini, A.; Viale, G.; Cassano, E.; Peradze, N.; et al. Sentinel lymph node biopsy vs. no axillary surgery in patients with small breast cancer and negative results on ultrasonography of axillary lymph nodes: The SOUND randomized clinical trial. JAMA Oncol. 2023, 9, 1557–1564. [Google Scholar] [CrossRef]
- Reimer, T.; Stachs, A.; Veselinovic, K.; Kühn, T.; Heil, J.; Polata, S.; Marmé, F.; Müller, T.; Hildebrandt, G.; Krug, D.; et al. Axillary surgery in breast cancer—primary results of the INSEMA trial. N. Engl. J. Med. 2025, 392, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Park, K.U.; Somerfield, M.R.; Anne, N.; Brackstone, M.; Conlin, A.K.; Couto, H.L.; Dengel, L.T.; Eisen, A.; Harvey, B.E.; Hawley, J.; et al. Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2025, 43, 1720–1741. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Gentile, D.; Gatzemeier, W.; Sagona, A.; Barbieri, E.; Testori, A.; Errico, V.; Bottini, A.; Marrazzo, E.; Dani, C.; et al. Preservation of Axillary Lymph Nodes Compared with Complete Dissection in T1-2 Breast Cancer Patients Presenting One or Two Metastatic Sentinel Lymph Nodes: The SINODAR-ONE Multicenter Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 5732–5744. [Google Scholar] [CrossRef]
- Heidinger, M.; Weber, W.P. Axillary Surgery for Breast Cancer in 2024. Cancers 2024, 16, 1623. [Google Scholar] [CrossRef]
- Alliance for Clinical Trials in Oncology. Comparison of Axillary Lymph Node Dissection with Axillary Radiation for Patients with Node-Positive Breast Cancer Treated with Chemotherapy. ClinicalTrials.gov Identifier: NCT01901094. Available online: https://clinicaltrials.gov/ct2/show/NCT01901094 (accessed on 13 January 2025).
- Garcia-Tejedor, A.; Ortega-Exposito, C.; Salinas, S.; Luzardo-González, A.; Falo, C.; Martinez-Pérez, E.; Pérez-Montero, H.; Soler-Monsó, M.T.; Bajen, M.-T.; Benitez, A.; et al. Axillary lymph node dissection versus radiotherapy in breast cancer with positive sentinel nodes after neoadjuvant therapy (ADARNAT trial). Front. Oncol. 2023, 13, 1184021. [Google Scholar] [CrossRef]
- Henke, G.; Knauer, M.; Ribi, K.; Hayoz, S.; Gérard, M.-A.; Ruhstaller, T.; Zwahlen, D.R.; Muenst, S.; Ackerknecht, M.; Hawle, H.; et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): Study protocol for a multicenter, randomized phase-III trial. Trials 2018, 19, 667. [Google Scholar] [CrossRef] [PubMed]

| Clinical and Histopathological Data | ||
|---|---|---|
| Age (years) | Mean ± SD | 59.9 ± 11.5 |
| Age (years), n (%) | <50 | 151 (19.2) |
| ≥50 | 636 (80.8) | |
| Tumor size (T), n (%) | cT1a-cT1b | 86 (10.9) |
| cT1c | 176 (22.4) | |
| cT2 | 515 (65.4) | |
| cT3 | 10 (1.30) | |
| Node involvement (N), n (%) | N0 | 772 (98.1) |
| N1 | 15 (1.90) | |
| Clinical stage, n (%) | I | 255 (32.4) |
| IIA | 495 (62.9) | |
| IIB | 34 (4.30) | |
| IIIA | 3 (0.40) | |
| Histological type, n (%) | Ductal (NOS) | 692 (87.9) |
| Lobular | 43 (5.50) | |
| Other | 52 (6.60) | |
| Histological grade, n (%) | 1 | 149 (18.9) |
| 2 | 540 (68.6) | |
| 3 | 92 (11.7) | |
| No data | 6 (0.80) | |
| Molecular subtype, n (%) | Luminal A | 400 (50.8) |
| Luminal B HER2- | 249 (31.6) | |
| Luminal B HER2+ | 60 (7.60) | |
| HER2+ | 19 (2.40) | |
| Triple-negative | 59 (7.50) | |
| Prognostic data of the surgical specimen | ||
| Pathological tumor size, n (%) | T0 | 77 (9.80) |
| T1a-T1b | 91 (11.6) | |
| T1c | 286 (36.3) | |
| T2 | 304 (38.6) | |
| T3 | 22 (2.80) | |
| No data | 7 (0.90) | |
| Histological grade †, n (%) | 1 | 96 (12.2) |
| 2 | 543 (69.0) | |
| 3 | 148 (18.8) | |
| Lymphovascular invasion, n (%) | Positive | 188 (23.9) |
| Negative | 529 (67.2) | |
| No data | 70 (8.89) | |
| Sentinel lymph node technique, n (%) | 99mTc | 714 (90.72) |
| Methylene Blue | 50 (6.40) | |
| Dual | 23 (2.90) | |
| Number of sentinel lymph nodes identified, n (%) | 1 | 349 (44.3) |
| 2 | 236 (30.0) | |
| ≥ 3 | 202 (25.7) | |
| Sentinel lymph node report, n (%) | Negative | 562 (71.4) |
| Positive | 225 (28.6) | |
| Sentinel lymph node result ‡ | Macrometastasis | 183 (81.3) |
| Micrometastasis | 39 (17.3) | |
| Isolated tumor cells | 3 (1.33) | |
| Number of positive lymph nodes ‡ | 1 | 137 (60.9) |
| [2,3] | 55 (24.4) | |
| ≥4 | 33 (14.7) | |
| Positive lymph node and extracapsular extension (ECE), n (%) | SLNB positive without ECE | 156 (69) |
| SLNB positive with ECE < 2 mm | 34 (15.1) | |
| SLNB positive with ECE > 2 mm | 35 (15.5) | |
| Number of lymph nodes in ALND | ||
| Upfront SLNB (n = 79) | 0 | 36 (45.6) |
| [1–3] | 27 (33.2) | |
| ≥4 | 16 (20.2) | |
| Post-NACT SLNB (n = 19) | 0 | 14 (73.7) |
| [1–3] | 5 (26.3) | |
| ≥4 | 0 (0.00) | |
| Margin involvement, n (%) | Negative | 748 (95.0) |
| Positive | 39 (5.00) | |
| Margin status ¶ | 1 margin | 30 (76.9) |
| 2 margins | 7 (17.9) | |
| 3 margins or more | 2 (5.13) | |
| Residual Cancer Burden (RCB) • | ||
| RCB class | RCB-0 | 44 (30.6) |
| RCB-1 | 10 (6.94) | |
| RCB-2 | 70 (48.6) | |
| RCB-3 | 11 (7.64) | |
| No data | 9 (6.25) | |
| Treatment Type | ||
|---|---|---|
| Initial treatment, † n (%) | NACT | 144(18.3) |
| Breast surgery | 643 (81.7) | |
| Neoadjuvant chemotherapy regimen, † n (%) | 144 (18.3) | |
| AC-T | 76 (52.8) | |
| AC-TH | 22 (15.3) | |
| TRAIN 2 | 11 (7.64) | |
| AC-TC | 6 (4.17) | |
| Other | 29 (20.1) | |
| Type of breast surgery, † n (%) | ||
| Conservative surgery * | 584 (74.2) | |
| Mastectomy | 203 (25.8) | |
| Type of axillary surgery, † n (%) | ||
| SLNB only | 689 (87.5) | |
| SLNB + ALND | 98 (12.5) | |
| Management of positive sentinel lymph node, † n (%) | 225 (28.5) | |
| Typeof management | Omission of dissection ** | 126 (56.0) |
| ALND | 98 (43.5) | |
| Patient refused ALND | 1 (0.44) | |
| Additional surgical treatments, † n (%) | 130 (16.5) | |
| Treatment type | ALND only | 90 (69.2) |
| Margin expansion only | 25 (19.2) | |
| ALND + margin expansion | 6 (4.62) | |
| Simple mastectomy | 5 (3.85) | |
| Simple mastectomy + margin expansion | 2 (1.54) | |
| ALND + simple mastectomy | 2 (0.76) | |
| Adjuvant systemic therapy agents, † n (%) | 303 (38.5) | |
| AC | 31 (10.2) | |
| AC-T | 113 (37.2) | |
| AC-TH | 17 (5.6) | |
| Capecitabine | 18 (5.9) | |
| TC | 55 (18.1) | |
| T-DM1 | 3 (0.9) | |
| Trastuzumab monotherapy | 33 (10.8) | |
| Other chemotherapy regimens | 33 (10.8) | |
| Adjuvant endocrine therapy, † n (%) | 682 (86.7) | |
| Tamoxifen | 269 (39.4) | |
| AI | 238 (34.9) | |
| Tamoxifen + AI | 164 (24.0) | |
| Other | 11 (1.61) | |
| Adjuvant radiotherapy, † n (%) | 605 (76.8) | |
| Used dose | 5 fractions 5 Gy ea. | 70 (11.6) |
| 16 fractions 2.6 Gy ea. | 303 (50.1) | |
| 25 fractions 2 Gy ea. | 143 (23.6) | |
| Other | 86 (14.2) | |
| No data | 3 (0.49) | |
| Details on radiotherapy administered to patients with positive SLN and omission of ALND, † n (%) | patients with positive SLN and omission of ALND | 126 (16) |
| Type of radiotherapy administered | Standard fractionation regimen (2 Gy×25 fractions = 50 Gy) | 55 (43.6) |
| Hypofractionated regimen (2.66 Gy×16 fractions = 42.5 Gy) | 69 (54.7) | |
| Ultra-hypofractionated regimen (5.5 Gy × 5 fractions = 26 Gy) | 2 (1.5) | |
| Radiotherapy technique | IMRT 3D-CRT IGRT VMAT IORT | 90 (73) 30 (23.8) 2 (1.5) 1 (0.7) 1 (0.7) |
| Irradiated fields | Breast—Axilla | 10 (7.9) |
| Breast—Axilla—Supraclavicular fossa | 57 (45.2) | |
| Breast—Axilla—Supraclavicular fossa—Internal mammary chain | 5 (3.9) | |
| Chest wall—Axilla | 1 (0.7) | |
| Chest wall—Axilla—Supraclavicular fossa | 44 (34.9) | |
| High tangential fields | 9 (7.1) | |
| Lymph Nodes | ||||
|---|---|---|---|---|
| Characteristic | Positive | Negative | OR [95% CI] | p-Value |
| Age (years), n (%) | ||||
| <50 | 41 (19.9) | 74 (16.9) | Ref. | |
| ≥50 | 165 (80.1) | 363 (83.1) | 0.82 [0.54, 1.26] | 0.36 |
| Clinical stage, n (%) | ||||
| I-IIA | 195 (94.7) | 428 (97.9) | Ref. | |
| IIB-IIIA | 11 (5.3) | 9 (2.1) | 2.98 [1.09, 6.75] | 0.03 |
| Histological type, n (%) | ||||
| Other | 11 (5.3) | 37 (8.5) | Ref. | |
| Invasive ductal (NOS) | 178 (86.4) | 379 (86.7) | 1.58 [0.81, 3.31] | 0.20 |
| Invasive lobular | 17 (8.3) | 21 (4.8) | 2.72 [1.09, 7.06] | 0.03 |
| Histological grade, n (%) | ||||
| 1 | 24 (11.7) | 66 (15.1) | Ref. | |
| 2 | 152 (73.8) | 301 (68.9) | 1.39 [0.85, 2.34] | 0.20 |
| 3 | 30 (14.6) | 70 (16.0) | 1.18 [0.62, 2.23] | 0.61 |
| LVI, n (%) | ||||
| Negative | 90 (43.7) | 320 (73.2) | Ref. | |
| Positive | 102 (49.5) | 68 (15.6) | 5.33 [3.64, 7.88] | <0.01 |
| Molecular subtype, n (%) | ||||
| Luminal A | 133 (64.6) | 243 (55.6) | Ref. | |
| Luminal B HER2- | 57 (27.7) | 144 (33.0) | 0.71 [0.48, 1.05] | 0.08 |
| Luminal B HER2+ | 7 (3.4) | 22 (5.0) | 0.57 [0.20, 1.42] | 0.22 |
| HER2+ | 5 (2.4) | 7 (1.6) | 1.44 [0.41, 4.90] | 0.65 |
| Triple-negative | 4 (1.9) | 21 (4.8) | 0.36 [0.10, 1.00] | 0.06 |
| Type of Recurrence † | Total | Upfront SLNB | Post-NACT SLNB |
|---|---|---|---|
| Local | 5 (8.33) | 5 (10.2) | 0 (0.00) |
| Regional | 9 (15.0) | 8 (16.3) | 1 (9.09) |
| Distant | 34 (56.7) | 26 (53.1) | 8 (72.7) |
| Mixed | 12 (20.0) | 10 (20.4) | 2 (18.2) |
| Local+ Regional | 3 (25.0) | 2 (20.0) | 1 (50.0) |
| Local + Distant | 4 (33.3) | 3 (30.0) | 1 (50.0) |
| Regional + Distant | 3 (33.0) | 3 (30.0) | 0 (0.00) |
| Local + Regional + Distant | 2 (16.7) | 2 (20.0) | 0 (0.00) |
| Localization | |||
| Regional, ‡ n (%) | 17 (22.9) | 15 (88.2) | 2 (11.7) |
| Site | |||
| Axillary | 12 (70.6) | 11 (73.3) | 1 (50.0) |
| Supraclavicular | 2 (11.7) | 2 (13.3) | 0 (0.00) |
| Infraclavicular | 1 (5.88) | 0 (0.00) | 1 (50.0) |
| Axillary + Supraclavicular | 1 (5.88) | 1 (6.67) | 0 (0.00) |
| Supra and infraclavicular | 1 (5.88) | 1 (6.67) | 0 (0.00) |
| Distant, ‡ n (%) | 43 (58.1) | 34 (79.1) | 9 (20.9) |
| Site | |||
| Bone | 8 (18.6) | 8 (23.5) | 0 (0.00) |
| Lung | 3 (6.98) | 0 (0.00) | 3 (33.3) |
| Liver | 3 (6.98) | 1 (2.94) | 2 (22.2) |
| Distant lymph nodes | 2 (4.65) | 2 (5.88) | 0 (0.00) |
| Bone + lung | 6 (13.9) | 5 (14.7) | 1 (11.1) |
| Bone + liver | 4 (9.30) | 4 (11.7) | 0 (0.00) |
| Bone + distant lymph nodes | 4 (9.30) | 3 (8.82) | 1 (11.1) |
| Bone + pleura | 2 (4.65) | 2 (5.88) | 0 (0.00) |
| Lung + brain | 1 (2.33) | 1 (2.94) | 0 (0.00) |
| Lung + pleura | 1 (2.33) | 0 (0.00) | 1 (11.1) |
| Lung + distant lymph nodes | 1 (2.33) | 1 (2.94) | 0 (0.00) |
| 3 or more ¶ | 8 (18.6) | 7 (20.6) | 1 (11.1) |
| Treatments used, † n (%) | |||
| ALND | 10 (16.7) | 9 (90.0) | 1 (10.0) |
| Chemotherapy | 14 (23.3) | 10 (71.4) | 4 (28.6) |
| Radiotherapy | 6 (10.0) | 6 (100) | 0 (0.00) |
| Anti-HER2 | 4 (6.67) | 1 (25.0) | 3 (75.0) |
| Fulvestrant | 8 (13.3) | 8 (100) | 0 (0.00) |
| AI | 11 (18.3) | 9 (81.8) | 2 (18.2) |
| Endocrine therapy | 14 (23.3) | 13 (92.8) | 1 (7.14) |
| Holobrain/bone radiation therapy | 14 (23.3) | 12 (85.7) | 2 (14.3) |
| Characteristic | TR | OS | ||
|---|---|---|---|---|
| HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| Age (years) | ||||
| <50 | Reference | Reference | ||
| ≥50 | 0.71 [0.39, 1.28] | 0.25 | 1.51 [0.75, 3.05] | 0.25 |
| Tumor size | ||||
| T0 | Reference | Reference | ||
| T1a-T1b | 0.67 [0.17, 2.71] | 0.57 | 0.42 [0.12, 1.48] | 0.17 |
| T1c | 0.78 [0.26, 2.36] | 0.66 | 0.68 [0.27, 1.69] | 0.41 |
| T2 | 1.81 [0.64, 5.11] | 0.26 | 1.09 [0.46, 2.61] | 0.84 |
| T3 | 2.16 [0.48, 9.66] | 0.31 | 1.57 [0.44, 5.57] | 0.49 |
| Histological grade | ||||
| 1 | Reference | Reference | ||
| 2 | 5.63 [1.36, 23.3] | 0.02 | 2.80 [1.11, 7.07] | 0.03 |
| 3 | 7.20 [1.64, 31.6] | <0.01 | 3.32 [1.22, 9.10] | 0.02 |
| Involved lymph nodes | ||||
| Negative | Reference | Reference | ||
| 1–3 | 0.83 [0.44, 1.55] | 0.56 | 0.66 [0.36, 1.21] | 0.18 |
| ≥4 | 2.21 [0.93, 5.21] | 0.07 | 0.89 [0.28, 2.85] | 0.84 |
| Molecular subtype | ||||
| Luminal A | Reference | Reference | ||
| Luminal B HER2- | 3.29 [1.80, 6.02] | <0.01 | 1.45 [0.84, 2.51] | 0.18 |
| Luminal B HER2+ | 1.28 [0.37, 4.40] | 0.69 | 0.42 [0.10, 1.78] | 0.24 |
| HER2+ | 1.80 [0.24, 13.6] | 0.57 | 2.55 [0.61, 10.8] | 0.20 |
| Triple-negative | 4.73 [2.02, 11.1] | <0.01 | 3.87 [1.92, 7.79] | <0.01 |
| Initial treatment | ||||
| Surgery | Reference | Reference | ||
| NACT | 1.45 [0.73, 2.90] | 0.29 | 1.04 [0.47, 2.29] | 0.93 |
| Axillary surgery | ||||
| SLNB only | Reference | Reference | ||
| SLNB + ALND | 1.22 [0.63, 2.35] | 0.55 | 0.88 [0.45, 1.73] | 0.71 |
| Breast surgery | ||||
| Breast-conserving surgery | Reference | Reference | ||
| Mastectomy | 1.71 [1.00, 2.95] | 0.05 | 2.40 [1.47, 3.91] | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Casas, S.E.; Reyes-Agudelo, A.A.; Vergara-Gamarra, O.A.; Briceño-Morales, X.; Guzmán-AbiSaab, L.; Contreras-Perez, D.; Lehmann-Mosquera, C.; Ángel-Aristizábal, J.; García-Mora, M.; Duarte-Torres, C.; et al. Oncological Outcomes of De-Escalation of Axillary Surgery in Breast Cancer Patients at a Referral Cancer Center in Colombia. Cancers 2025, 17, 3396. https://doi.org/10.3390/cancers17213396
Díaz-Casas SE, Reyes-Agudelo AA, Vergara-Gamarra OA, Briceño-Morales X, Guzmán-AbiSaab L, Contreras-Perez D, Lehmann-Mosquera C, Ángel-Aristizábal J, García-Mora M, Duarte-Torres C, et al. Oncological Outcomes of De-Escalation of Axillary Surgery in Breast Cancer Patients at a Referral Cancer Center in Colombia. Cancers. 2025; 17(21):3396. https://doi.org/10.3390/cancers17213396
Chicago/Turabian StyleDíaz-Casas, Sandra Esperanza, Andres Augusto Reyes-Agudelo, Oscar Alberto Vergara-Gamarra, Ximena Briceño-Morales, Luis Guzmán-AbiSaab, Daniel Contreras-Perez, Carlos Lehmann-Mosquera, Javier Ángel-Aristizábal, Mauricio García-Mora, Carlos Duarte-Torres, and et al. 2025. "Oncological Outcomes of De-Escalation of Axillary Surgery in Breast Cancer Patients at a Referral Cancer Center in Colombia" Cancers 17, no. 21: 3396. https://doi.org/10.3390/cancers17213396
APA StyleDíaz-Casas, S. E., Reyes-Agudelo, A. A., Vergara-Gamarra, O. A., Briceño-Morales, X., Guzmán-AbiSaab, L., Contreras-Perez, D., Lehmann-Mosquera, C., Ángel-Aristizábal, J., García-Mora, M., Duarte-Torres, C., Mariño-Lozano, I., Suárez-Rodríguez, R., & Núñez-Lemus, M. (2025). Oncological Outcomes of De-Escalation of Axillary Surgery in Breast Cancer Patients at a Referral Cancer Center in Colombia. Cancers, 17(21), 3396. https://doi.org/10.3390/cancers17213396






