Carbon Ion Radiotherapy for Retroperitoneal Sarcoma: A Single-Institution Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Follow-Up and Statistical Analysis
2.3. Carbon Ion Radiotherapy
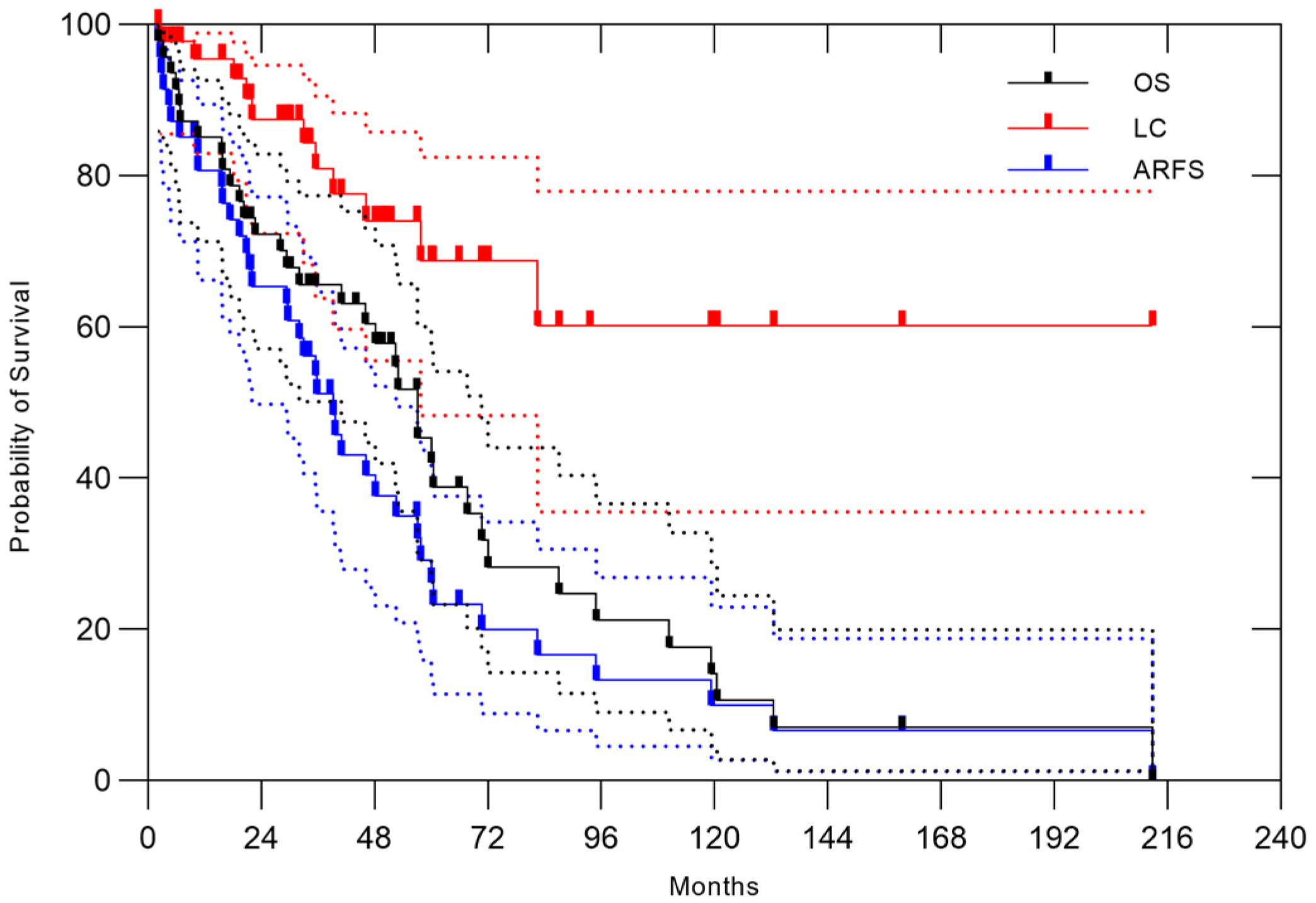
3. Results
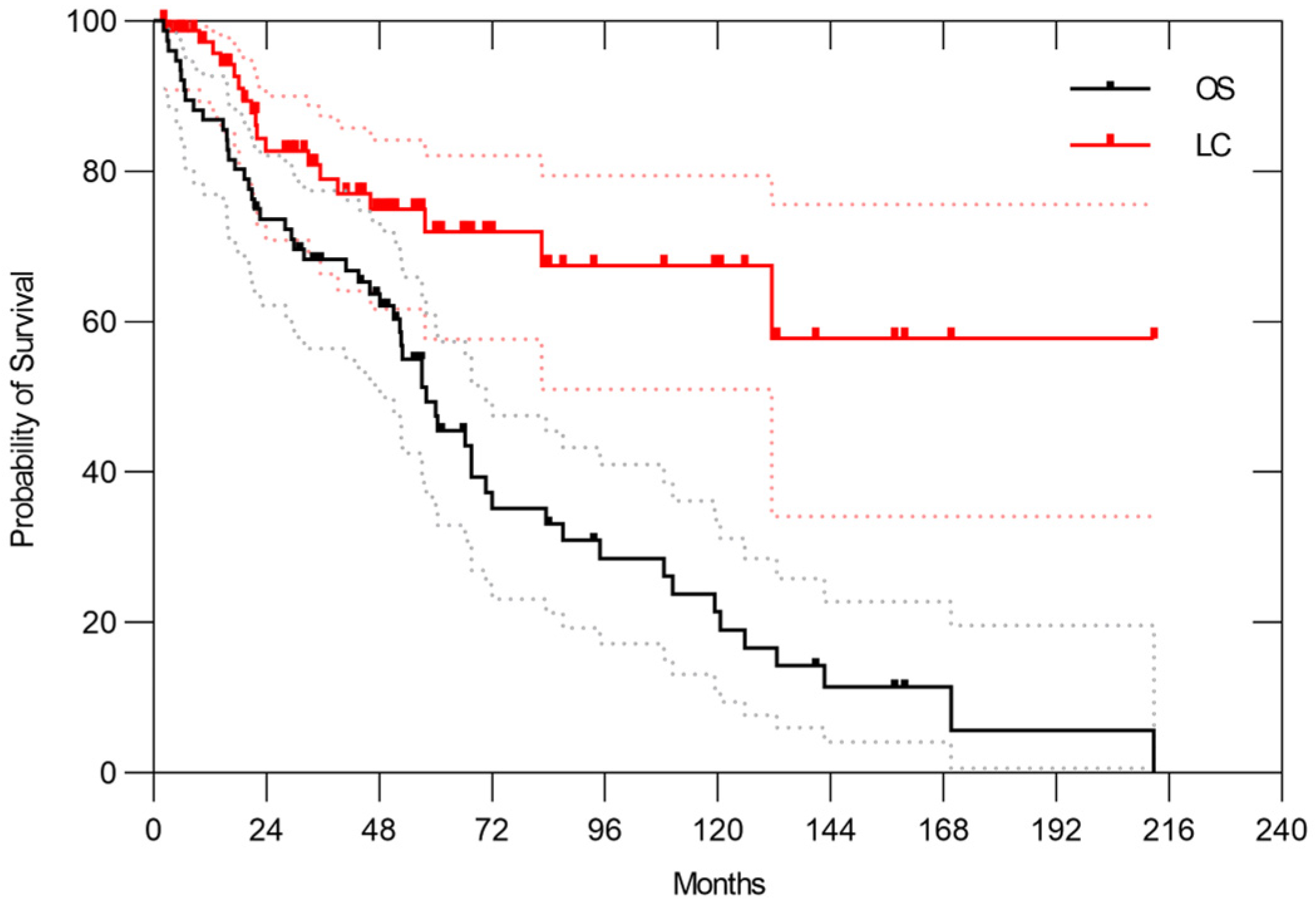
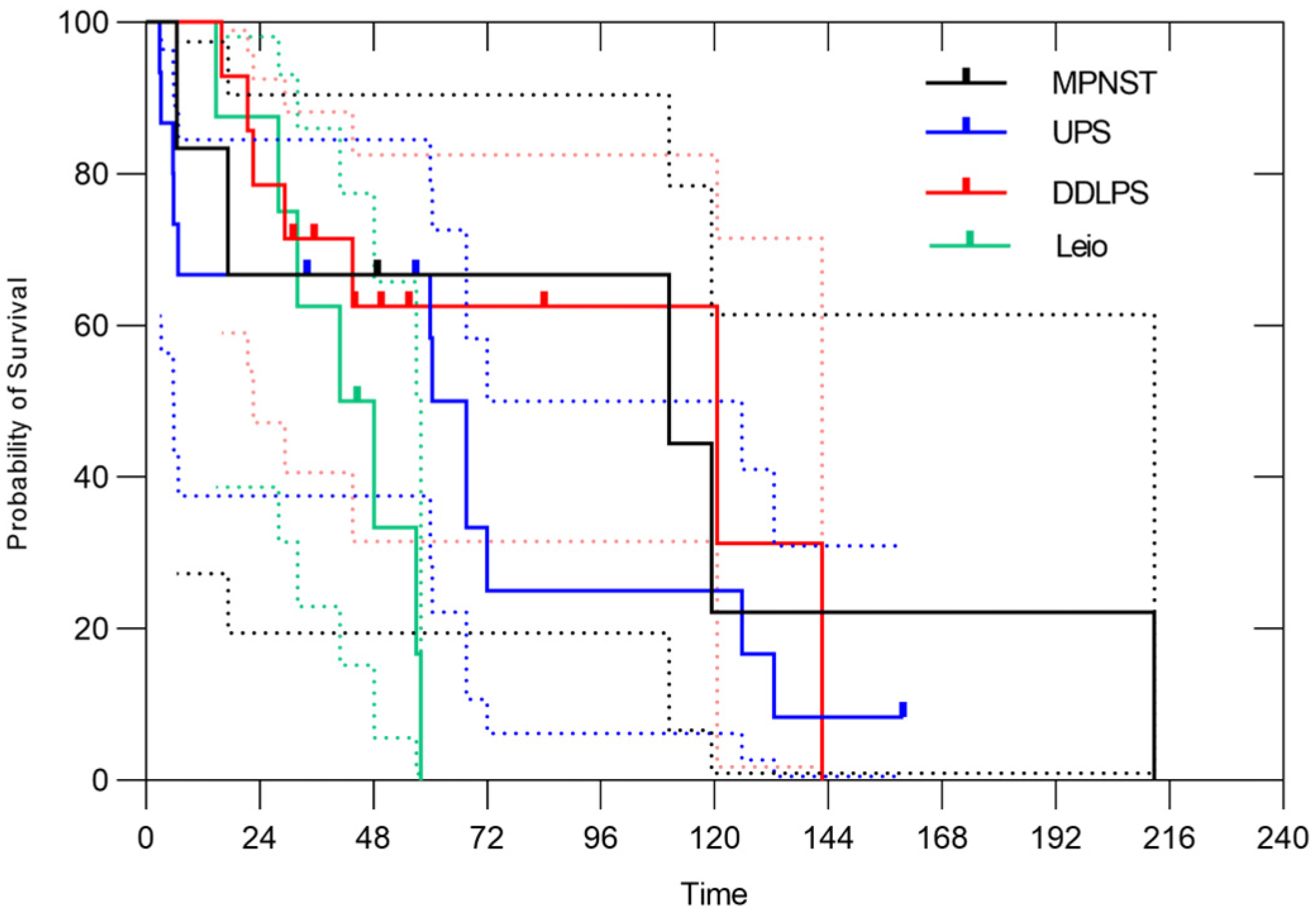
3.1. All Patients
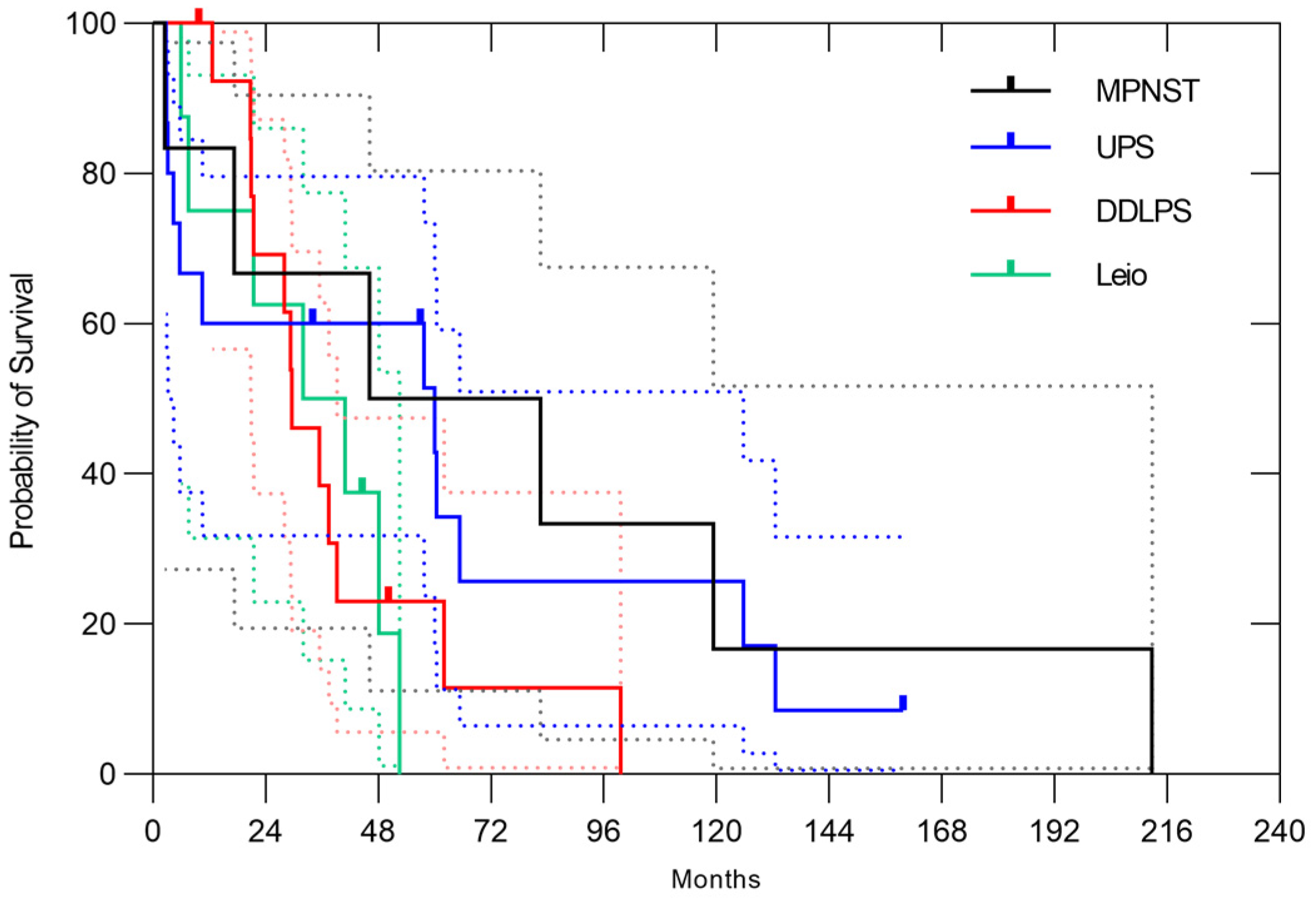
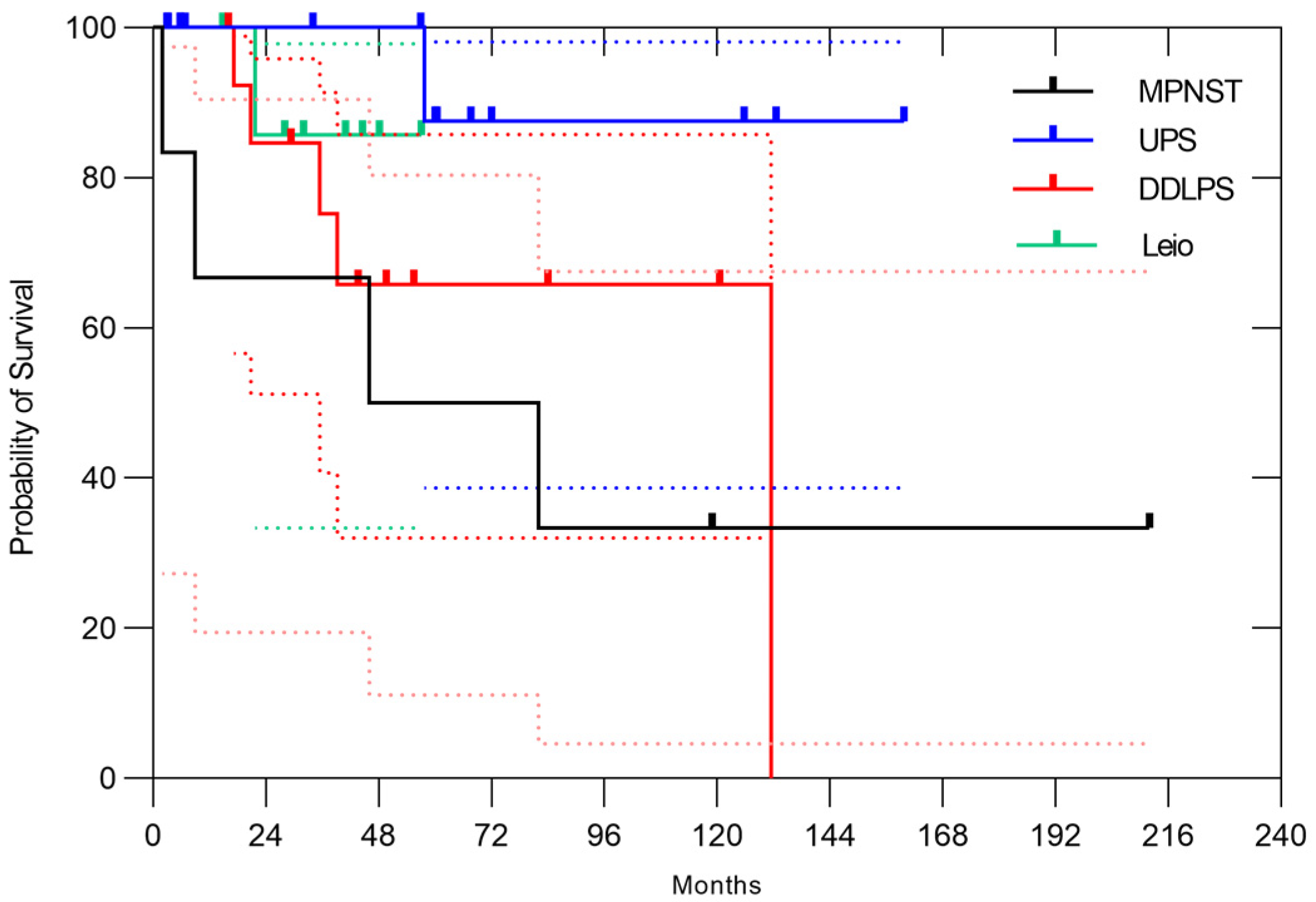
3.2. Naïve Case
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPS | retroperitoneal sarcoma |
| CIRT | carbon ion radiotherapy |
| OS | overall survival |
| LC | local control |
| ARFS | abdominal recurrence-free survival |
| LET | linear energy transfer |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| FDG | fluorodeoxyglucose |
| PET | positron emission tomography |
| TPS | treatment planning system |
| AEs | adverse events |
| CI | confidence interval; |
| OARs | organs at risk |
| GTV | gross tumor volume |
| PTV | planning target volume |
| CTV | clinical target volume |
References
- Anaya, D.A.; Lev, D.C.; Pollock, R.E. The role of surgical margin status in retroperitoneal sarcoma. J. Surg. Oncol. 2008, 98, 607–610. [Google Scholar] [CrossRef]
- Buja, A.; Rugge, M.; Barillaro, M.; Miatton, A.; Tropea, S.; Cozzolino, C.; Zorzi, M.; Vecchiato, A.; Del Fiore, P.; Brunello, A.; et al. Epidemiology, pathological characteristics and survival of retroperitoneal soft tissue sarcomas compared with non retroperitoneal soft tissue sarcomas. Oncol. Lett. 2023, 26, 301. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; Baxter, N.N.; Pisters, P.W.T. Retroperitoneal sarcoma: A population-based analysis of epidemiology, surgery, and radiotherapy. Cancer 2006, 106, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, A.; Chee, Y.; Helliwell, T.R.; Hershman, M.J.; Leinster, S.J.; Fordham, M.V.; Poston, G.J. The management of retroperitoneal soft tissue sarcoma: A single institution experience with a review of the literature. Eur. J. Surg. Oncol. 2001, 27, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Seidensaal, K.; Dostal, M.; Kudak, A.; Jaekel, C.; Meixner, E.; Liermann, J.; Weykamp, F.; Hoegen, P.; Mechtersheimer, G.; Willis, F.; et al. Preoperative dose-escalated intensity-modulated radiotherapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft-tissue sarcoma: Final results of a clinical phase I/II trial. Cancers 2023, 15, 2747. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: Strass): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef]
- Imai, R.; Kamada, T.; Araki, N.; Working Group for Carbon Ion Radiotherapy for Bone and Soft Tissue Sarcomas. Carbon ion radiotherapy for unresectable localized axial soft tissue sarcoma. Cancer Med. 2018, 7, 4308–4314. [Google Scholar] [CrossRef]
- Serizawa, I.; Kagei, K.; Kamada, T.; Imai, R.; Sugahara, S.; Okada, T.; Tsuji, H.; Ito, H.; Tsujii, H. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1105–1110. [Google Scholar] [CrossRef]
- Okonogi, N.; Fukahori, M.; Wakatsuki, M.; Ohkubo, Y.; Kato, S.; Miyasaka, Y.; Tsuji, H.; Nakano, T.; Kamada, T. Dose constraints in the rectum and bladder following carbon-ion radiotherapy for uterus carcinoma: A retrospective pooled analysis. Radiat. Oncol. 2018, 13, 119. [Google Scholar] [CrossRef]
- Aoki, S.; Koto, M.; Ikawa, H.; Imai, R.; Tokuhiko, O.; Shinoto, M.; Takiyama, H.; Yamada, S.; Tsuji, H. Long-term outcomes of high dose carbon-ion radiation therapy for unresectable upper cervical (C1−2) chordoma. Head Neck 2022, 44, 2162–2170. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Ahmed, A.R.; Matsumoto, S.; Manabe, J.; Matsushita, Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin. Orthop. Relat. Res. 2004, 419, 165–172. [Google Scholar] [CrossRef]
- Stahl, J.M.; Corso, C.D.; Park, H.S.; An, Y.; Rutter, C.E.; Han, D.; Roberts, K.B. The effect of microscopic margin status on survival in adult retroperitoneal soft tissue sarcomas. Eur. J. Surg. Oncol. 2017, 43, 168–174. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, J.; Du, X.; Huang, B. Survival outcomes of surgery for retroperitoneal sarcomas: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272044. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Casali, P.G.; Fiore, M.; Mariani, L.; Lo Vullo, S.; Bertulli, R.; Colecchia, M.; Lozza, L.; Olmi, P.; Santinami, M.; et al. Retroperitoneal soft tissue sarcomas: Patterns of recurrence in 167 patients treated at a single institution. Cancer 2004, 100, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, E.; Coindre, J.M.; Bonvalot, S.; Kantor, G.; Terrier, P.; Bonichon, F.; Nguyen Bui, B. French Federation of Cancer Centers Sarcoma Group Prognostic factors in retroperitoneal sarcoma: A multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer 2001, 92, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Catton, C.N.; O’Sullivan, B.; Kotwall, C.; Cummings, B.; Hao, Y.; Fornasier, V. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 1005–1010. [Google Scholar] [CrossRef]
- Doepker, M.P.; Hanna, K.H.; Thompson, Z.J.; Binitie, O.T.; Letson, D.G.; Gonzalez, R.J. Recurrence and survival analysis of resected soft tissue sarcomas of pelvic retroperitoneal structures. J. Surg. Oncol. 2016, 113, 103–107. [Google Scholar] [CrossRef]
- Bremjit, P.J.; Jones, R.L.; Chai, X.; Kane, G.; Rodler, E.T.; Loggers, E.T.; Pollack, S.M.; Pillarisetty, V.G.; Mann, G.N. A contemporary large single-institution evaluation of resected retroperitoneal sarcoma. Ann. Surg. Oncol. 2014, 21, 2150–2158. [Google Scholar] [CrossRef]
- Komatsu, S.; Demizu, Y.; Sulaiman, N.S.; Terashima, K.; Suga, M.; Kido, M.; Toyama, H.; Tokumaru, S.; Okimoto, T.; Sasaki, R.; et al. Space-making particle therapy for sarcomas derived from the abdominopelvic region. Radiother. Oncol. 2020, 146, 194–199. [Google Scholar] [CrossRef]
- Tsugawa, D.; Komatsu, S.; Demizu, Y.; Sulaiman, N.S.; Suga, M.; Kido, M.; Toyama, H.; Okimoto, T.; Sasaki, R.; Fukumoto, T. Space-making particle therapy with surgical spacer placement in patients with sacral chordoma. J. Am. Coll. Surg. 2020, 230, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Wang, T.; Terashima, K.; Demizu, Y.; Anzai, M.; Suga, M.; Yamashita, T.; Suzuki, O.; Okimoto, T.; Sasaki, R.; et al. Innovative combination treatment to expand the indications of particle therapy: Spacer placement surgery using bio-absorbable polyglycolic acid spacer. J. Am. Coll. Surg. 2024, 238, 119–128. [Google Scholar] [CrossRef]
- Shiba, S.; Okamoto, M.; Tashiro, M.; Ogawa, H.; Osone, K.; Yanagawa, T.; Kohama, I.; Okazaki, S.; Miyasaka, Y.; Osu, N.; et al. Rectal dose-sparing effect with bioabsorbable spacer placement in carbon ion radiotherapy for sacral chordoma: Dosimetric comparison of a simulation study. J. Radiat. Res. 2021, 62, 549–555. [Google Scholar] [CrossRef]
- Matsumoto, S.; Lee, S.H.; Imai, R.; Inaniwa, T.; Matsufuji, N.; Fukahori, M.; Kohno, R.; Yonai, S.; Okonogi, N.; Yamada, S.; et al. Unresectable chondrosarcomas treated with carbon ion radiotherapy: Relationship between dose-averaged linear energy transfer and local recurrence. Anticancer Res. 2020, 40, 6429–6435. [Google Scholar] [CrossRef]
- Sakamoto, S.; Oike, T.; Kobayashi, D.; Miyasaka, Y.; Sakai, M.; Okamoto, M.; Shiba, S.; Tomizawa, K.; Darwis, N.D.M.; Yoshida, H.; et al. Low dose-averaged LET contributes to local recurrence of pancreatic cancer treated with carbon ion radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2025, 123, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, N.; Matsumoto, S.; Fukahori, M.; Furuichi, W.; Inaniwa, T.; Matsufuji, N.; Imai, R.; Yamada, S.; Kanematsu, N.; Tsuji, H. Dose-averaged linear energy transfer per se does not correlate with late rectal complications in carbon-ion radiotherapy. Radiother. Oncol. 2020, 153, 272–278. [Google Scholar] [CrossRef] [PubMed]
| n (%) | |
|---|---|
| Total number of patients | 76 (100) |
| Median age (years) | 61 |
| Sex | |
| Male | 44 (58) |
| Female | 32 (42) |
| Tumor status | |
| Naïve | 47 (62) |
| Post-operative recurrence | 20 (26) |
| Residual tumor after resection | 2 (2) |
| Solitary metastasis | 7 (9) |
| Histology | |
| Liposarcoma | 22 (29) |
| Well-differentiated | 1 |
| Myxoid | 3 |
| Pleomorphic | 3 |
| Dedifferentiated | 15 |
| MFH/UPS | 14 (18) |
| Leiomyosarcoma | 8 (11) |
| MPNST | 6 (8) |
| Others | 26 (34) |
| Location | |
| Upper abdomen | 45 (59) |
| Pelvis | 31 (41) |
| Total irradiation dose | |
| 73.6 | 1 (1) |
| 70.4 | 74 (98) |
| 64.0 | 1 (1) |
| Median maximum diameter (cm) | 10 (2.5–21) |
| Median PTV (cm3) | 508.6 |
| n (%) | |
|---|---|
| Total number of patients | 47 (100) |
| Median age (years) | 61 (14–83) |
| Sex | |
| Male | 25 (53) |
| Female | 22 (47) |
| Histology | |
| Liposarcoma | 7 (15) |
| Pleomorphic | 1 |
| Dedifferentiated | 6 |
| MFH/UPS | 10 (21) |
| Leiomyosarcoma | 5 (11) |
| MPNST | 6 (12) |
| Others | 19 (41) |
| Location | |
| Upper abdomen | 32 (70) |
| Pelvis | 15 (30) |
| Reasons for unresectability * | |
| Bone invasion | 16 |
| Arterial invasion | 21 |
| IVC invasion | 4 |
| Aging | 7 |
| Others | 7 |
| Median maximum diameter (cm) | 11 (4–21) |
| Median PTV (cm3) | 606 (67–2872) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, R.; Yonemoto, T.; Araki, N.; Takiyama, H.; Ikawa, H.; Yamada, S.; Ishikawa, H. Carbon Ion Radiotherapy for Retroperitoneal Sarcoma: A Single-Institution Study. Cancers 2025, 17, 3395. https://doi.org/10.3390/cancers17203395
Imai R, Yonemoto T, Araki N, Takiyama H, Ikawa H, Yamada S, Ishikawa H. Carbon Ion Radiotherapy for Retroperitoneal Sarcoma: A Single-Institution Study. Cancers. 2025; 17(20):3395. https://doi.org/10.3390/cancers17203395
Chicago/Turabian StyleImai, Reiko, Tsukasa Yonemoto, Nobuhito Araki, Hirotoshi Takiyama, Hiroaki Ikawa, Shigeru Yamada, and Hitoshi Ishikawa. 2025. "Carbon Ion Radiotherapy for Retroperitoneal Sarcoma: A Single-Institution Study" Cancers 17, no. 20: 3395. https://doi.org/10.3390/cancers17203395
APA StyleImai, R., Yonemoto, T., Araki, N., Takiyama, H., Ikawa, H., Yamada, S., & Ishikawa, H. (2025). Carbon Ion Radiotherapy for Retroperitoneal Sarcoma: A Single-Institution Study. Cancers, 17(20), 3395. https://doi.org/10.3390/cancers17203395







