The Interplay Between lncRNAs–microRNAs Network Dysregulation and Cellular Hallmarks of Thyroid Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. LncRNAs and the Hallmarks of Cancer in Thyroid Cancer
3.1.1. Sustaining Proliferative Signaling
3.1.2. Evading Growth Suppressors
3.1.3. Resisting Cell Death
3.1.4. Enabling Replicative Immortality
3.1.5. Inducing Angiogenesis
3.1.6. Activating Invasion and Metastasis
3.1.7. Deregulating Cellular Energetics
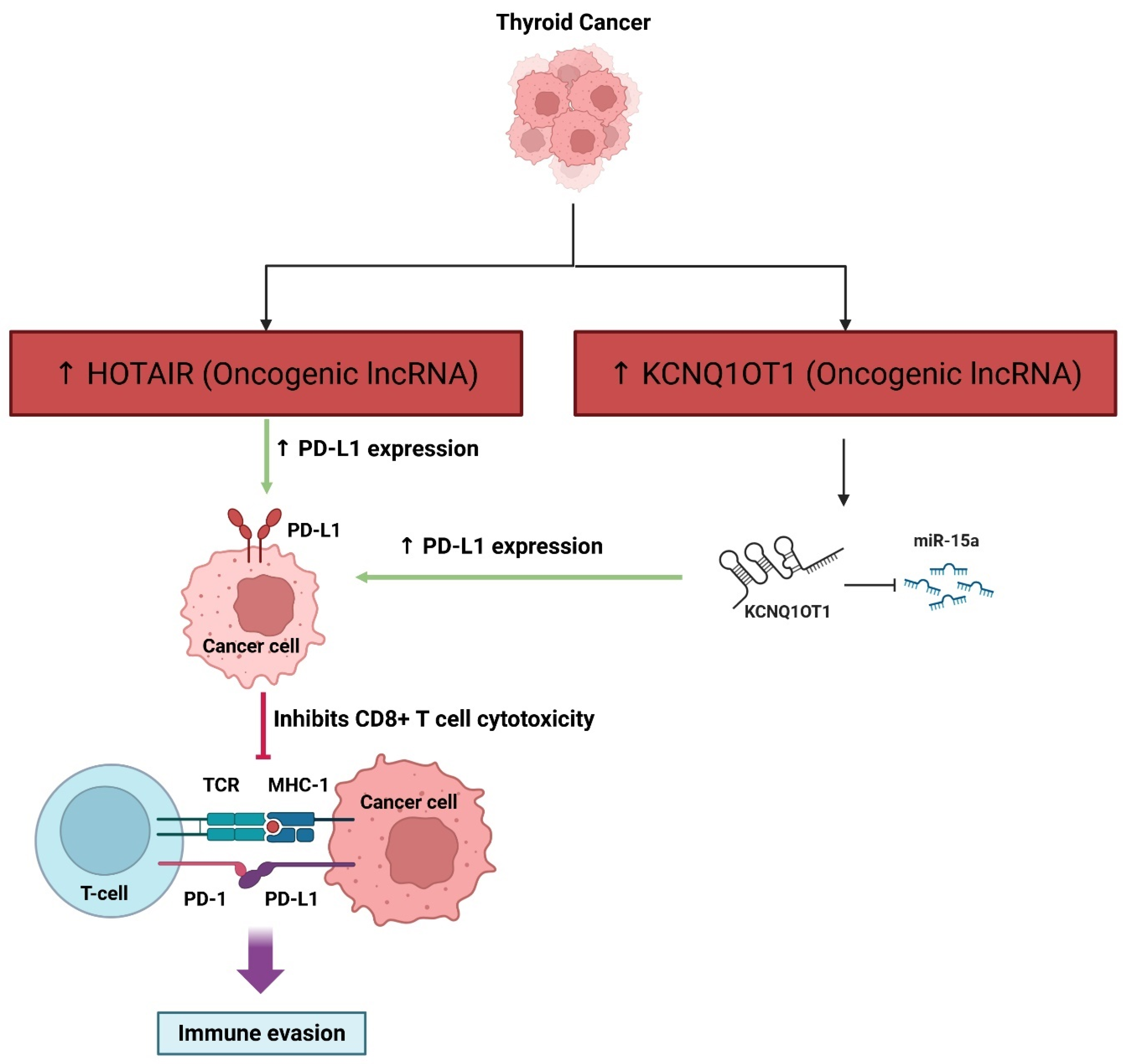
3.1.8. Avoiding Immune Destruction
3.1.9. Genome Instability and Mutation
3.1.10. Tumor-Promoting Inflammation
3.2. Clinical Implications of lncRNA Dysregulation in Thyroid Cancer
3.3. lncRNA–miRNA Crosstalk in Cancer Immunity
4. Discussion
4.1. Future Perspectives
4.2. Clinical Evidence and Translational Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATC | Anaplastic Thyroid Carcinoma |
| ASO | Antisense Oligonucleotide |
| ceRNA | Competing Endogenous RNA |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CTLA-4 | Cytotoxic T-Lymphocyte–Associated Protein 4 |
| EMT | Epithelial–Mesenchymal Transition |
| ERK | Extracellular Signal-Regulated Kinase |
| FTC | Follicular Thyroid Carcinoma |
| HIF | Hypoxia-Inducible Factor |
| IL-6 | Interleukin-6 |
| lncRNA | Long Non-Coding RNA |
| MAPK | Mitogen-Activated Protein Kinase |
| miRNA | microRNA |
| MTC | Medullary Thyroid Carcinoma |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3K | Phosphoinositide 3-Kinase |
| PTC | Papillary Thyroid Carcinoma |
| scRNA-seq | Single-Cell RNA Sequencing |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAM | Tumor-Associated Macrophage |
| TC | Thyroid Cancer |
| TERT | Telomerase Reverse Transcriptase |
| TME | Tumor Microenvironment |
| VEGF | Vascular Endothelial Growth Factor |
| Wnt | Wingless/Integrated |
References
- Lyu, Z.; Zhang, Y.; Sheng, C.; Huang, Y.; Zhang, Q.; Chen, K. Global burden of thyroid cancer in 2022: Incidence and mortality estimates from GLOBOCAN. Chin. Med. J. 2024, 137, 2567–2576. [Google Scholar] [CrossRef]
- Forma, A.; Kłodnicka, K.; Pająk, W.; Flieger, J.; Teresińska, B.; Januszewski, J.; Baj, J. Thyroid Cancer: Epidemiology, Classification, Risk Factors, Diagnostic and Prognostic Markers, and Current Treatment Strategies. Int. J. Mol. Sci. 2025, 26, 5173. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Mete, O.; Baloch, Z.W. The 2022 WHO classification of thyroid tumors: Novel concepts in nomenclature and grading. Endocr. Relat. Cancer 2023, 30, e220293. [Google Scholar] [CrossRef] [PubMed]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Yu, W.; Zhang, Y.; Ao, X.; Wang, J. Long non-coding RNAs: Biogenesis, functions, and clinical significance in gastric cancer. Mol. Ther. Oncolytics 2021, 23, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Houfani, A.A.; Foster, L.J. Pivotal functions and impact of long con-coding RNAs on cellular processes and genome integrity. J. Biomed. Sci. 2024, 31, 52. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell. Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Gonzalez, I.; Munita, R.; Agirre, E.; Dittmer, T.A.; Gysling, K.; Misteli, T.; Luco, R.F. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat. Struct. Mol. Biol. 2015, 22, 370–376. [Google Scholar] [CrossRef]
- Somasundaram, K.; Gupta, B.; Jain, N.; Jana, S. LncRNAs divide and rule: The master regulators of phase separation. Front. Genet. 2022, 13, 2022. [Google Scholar] [CrossRef]
- Naseer, Q.A.; Malik, A.; Zhang, F.; Chen, S. Exploring the enigma: History, present, and future of long non-coding RNAs in cancer. Discov. Oncol. 2024, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wu, H.; Yin, D.; Fang, W.; Zhou, L. Integrative analysis of ceRNA networks and immune cell infiltration in thyroid cancer for enhanced diagnostic and prognostic insights. Sci. Rep. 2025, 15, 12190. [Google Scholar] [CrossRef]
- Zhang, D.; Pei, S.; Feng, Z.; Xia, G. Functions and mechanisms of lncRNAs in immune escape and their application in immunotherapy for colorectal cancer. J. Transl. Med. 2025, 23, 689. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Ma, X.; Liu, L.; Liu, J.; Yin, Y.; Li, H.; Chen, Y.; Zhang, X.; Zhang, L.; et al. LncRNA TINCR impairs the efficacy of immunotherapy against breast cancer by recruiting DNMT1 and downregulating MiR-199a-5p via the STAT1–TINCR-USP20-PD-L1 axis. Cell Death Dis. 2023, 14, 76. [Google Scholar] [CrossRef]
- Mu, D.; Han, B.; Huang, H.; Zheng, Y.; Zhang, J.; Shi, Y. Unraveling the advances of non-coding RNAs on the tumor microenvironment: Innovative strategies for cancer therapies. J. Transl. Med. 2025, 23, 614. [Google Scholar] [CrossRef]
- Tabatabaeian, H.; Peiling Yang, S.; Tay, Y. Non-Coding RNAs: Uncharted Mediators of Thyroid Cancer Pathogenesis. Cancers 2020, 12, 3264. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Wang, D.; Cao, X.; Wang, S.; Liu, Y.; Wang, J.; Li, P.; He, Q. The Emerging Landscapes of Long Noncoding RNA in Thyroid Carcinoma: Biological Functions and Clinical Significance. Front. Oncol. 2021, 11, 2021. [Google Scholar] [CrossRef]
- Dong, X.; Jin, C.; Chen, D.; Chen, Y.; Ye, Z.-Q.; Zhang, X.; Huang, X.; Zhang, W.; Gu, D.-N. Genomic Instability-Related LncRNA Signature Predicts the Prognosis and Highlights LINC01614 Is a Tumor Microenvironment-Related Oncogenic lncRNA of Papillary Thyroid Carcinoma. Front. Oncol. 2021, 11, 2021. [Google Scholar] [CrossRef]
- Guo, K.; Qian, K.; Shi, Y.; Sun, T.; Wang, Z. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis. 2021, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Abedimanesh, S.; Safaralizadeh, R.; Jahanafrooz, Z.; Najafi, S.; Amini, M.; Nazarloo, S.S.; Mahdavi, S.Z.B.; Baradaran, B.; Jebelli, A.; Mokhtarzadeh, A.A. Interaction of noncoding RNAs with hippo signaling pathway in cancer cells and cancer stem cells. Noncoding RNA Res. 2024, 9, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Wang, G.; Wei, L.; Yang, H. p53-associated miRNAs repress lncRNA ZFAS1 to retard the proliferation of papillary thyroid carcinoma. Endokrynol. Pol. 2024, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, Y.; Yu, Y.; Zhong, C.; Lang, Q.; Liang, Z.; Lv, C.; Xu, F.; Tian, Y. Long Noncoding RNA H19: A Novel Therapeutic Target Emerging in Oncology via Regulating Oncogenic Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 796740. [Google Scholar] [CrossRef]
- Yuan, X.; Yuan, H.; Zhang, N.; Liu, T.; Xu, D. Thyroid carcinoma-featured telomerase activation and telomere maintenance: Biology and translational/clinical significance. Clin. Transl. Med. 2022, 12, e1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, Y.; Chen, W.; Dong, H.; Cui, B.; Liu, C.; Liu, Z.; Wang, F.; Du, J. Regulation and Therapeutic Application of Long Non-Coding RNA in Tumor Angiogenesis. Technol. Cancer. Res. Treat. 2024, 23, 15330338241273239. [Google Scholar] [CrossRef]
- Su, Y.; Mei, L.; Jiang, T.; Wang, Z.; Ji, Y. Novel role of lncRNAs regulatory network in papillary thyroid cancer. Biochem. Biophys. Rep. 2024, 38, 101674. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Wang, Y.; Hua, K.; Liu, S. The role of long noncoding RNA H19 in gynecological pathologies: Insights into gene regulation and immune modulation (Review). Int. J. Mol. Med. 2023, 52, 73. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Tian, L.; Zhang, F.; Lin, Z.; Gong, B.; Liu, T.; Teng, W. A novel signature to predict thyroid cancer prognosis and immune landscape using immune-related LncRNA pairs. BMC Med. Genom. 2022, 15, 183. [Google Scholar] [CrossRef]
- Yuan, J.; Song, Y.; Pan, W.; Li, Y.; Xu, Y.; Xie, M.; Shen, Y.; Zhang, N.; Liu, J.; Hua, H. LncRNA SLC26A4-AS1 suppresses the MRN complex-mediated DNA repair signaling and thyroid cancer metastasis by destabilizing DDX5. Oncogene 2020, 39, 6664–6676. [Google Scholar]
- Baba, S.K.; Baba, S.K.; Mir, R.; Elfaki, I.; Algehainy, N.; Ullah, M.F.; Barnawi, J.; Altemani, F.H.; Alanazi, M.; Mustafa, S.K.; et al. Long non-coding RNAs modulate tumor microenvironment to promote metastasis: Novel avenue for therapeutic intervention. Front. Cell Dev. Biol. 2023, 11, 2023. [Google Scholar] [CrossRef]
- Dadafarin, S.; Rodríguez, T.C.; Carnazza, M.A.; Tiwari, R.K.; Moscatello, A.; Geliebter, J. MEG3 expression indicates lymph node metastasis and presence of cancer-associated fibroblasts in papillary thyroid cancer. Cells 2022, 11, 3181. [Google Scholar] [CrossRef]
- Wang, C.; Yan, G.; Zhang, Y.; Jia, X.; Bu, P. Long non-coding RNA MEG3 suppresses migration and invasion of thyroid carcinoma by targeting of Rac1. Neoplasma 2015, 62, 541–549. [Google Scholar] [CrossRef]
- Fagin, J.A.; Krishnamoorthy, G.P.; Landa, I. Pathogenesis of cancers derived from thyroid follicular cells. Nat. Rev. Cancer 2023, 23, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Cabanillas, M.E. Genomic alterations in thyroid cancer: Biological and clinical insights. Nat. Rev. Endocrinol. 2024, 20, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Zaballos, M.A.; Santisteban, P. Key signaling pathways in thyroid cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Borkhuu, O.; Bao, W.; Yang, Y.-T. Signaling pathways in thyroid cancer and their therapeutic implications. J. Clin. Med. Res. 2016, 8, 284. [Google Scholar] [CrossRef]
- Miro, C.; Nappi, A.; Cicatiello, A.G.; Di Cicco, E.; Sagliocchi, S.; Murolo, M.; Belli, V.; Troiani, T.; Albanese, S.; Amiranda, S. Thyroid hormone enhances angiogenesis and the Warburg effect in squamous cell carcinomas. Cancers 2021, 13, 2743. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.S.; Lai, H.Y.; Whang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid hormone, cancer, and apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [CrossRef]
- Hejazi, M.; Bahojb Mahdavi, S.Z.; Abedimanesh, S.; Heshmat, R.; Larijani, B.; Mokhtarzadeh, A.A.; Shafiee, G.; Tavangar, S.M. LINC00162 silencing enhances sorafenib sensitivity and inhibits thyroid cancer cells progression through modulation of MAPK signaling and apoptosis. Sci. Rep. 2025, 15, 29726. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Jin, X.; Guo, H.; Li, Y. Long non-coding RNA H19 knockdown inhibits the cell viability and promotes apoptosis of thyroid cancer cells through regulating the PI3K/AKT pathway. Exp. Ther. Med. 2019, 18, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Yao, Y.; Liu, A.; Shi, L.; Chen, D.; Tang, L.; Yang, G.; Liang, X.; Peng, J.; Shao, C. lncRNA H19 binds VGF and promotes pNEN progression via PI3K/AKT/CREB signaling. Endocr. Relat. Cancer 2019, 26, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Yang, G.; Wan, L.; Yin, F.; Li, H.; Yin, D. LncRNA SOCS2-AS1 promotes the progression of papillary thyroid cancer by destabilizing p53 protein. Biochem Biophys Res Commun 2023, 669, 95–102. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Wei, D.; Zhai, L.; Liu, L.; Pan, C.; Han, Z.; Liu, H.; Zhong, W.; Jiang, X. LncRNA ZFAS1 promotes invasion of medullary thyroid carcinoma by enhancing EPAS1 expression via miR-214-3p/UCHL1 axis. J. Cell Commun. Signal. 2024, 18, e12021. [Google Scholar] [CrossRef]
- Pang, R.; Yang, S. lncRNA DUXAP8 inhibits papillary thyroid carcinoma cell apoptosis via sponging the miR-20b-5p/SOS1 axis. Oncol. Rep. 2021, 45, 64. [Google Scholar] [CrossRef]
- Yuan, Q.; Fan, Y.; Liu, Z.; Wang, X.; Jia, M.; Geng, Z.; Zheng, J.; Lu, X. miR-744–5p mediates lncRNA HOTTIP to regulate the proliferation and apoptosis of papillary thyroid carcinoma cells. Exp. Cell Res. 2020, 392, 112024. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, J.; Li, X. lncRNA RUNDC3A-AS1 Regulates Proliferation and Apoptosis of Thyroid Cancer Cells via the miR-151b/SNRPB Axis. Int. J. Endocrinol. 2022, 2022, 9433434. [Google Scholar] [CrossRef]
- Ji, L.; Fan, X.; Zhou, F.; Gu, J.; Deng, X. lncRNA RPL34-AS1 inhibits cell proliferation and invasion while promoting apoptosis by competitively binding miR-3663-3p/RGS4 in papillary thyroid cancer. J. Cell. Physiol. 2020, 235, 3669–3678. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Y.; Yang, X.; Wu, Z.; Xu, X.; Kang, F.; Liu, Y. LncRNA ATP1A1-AS1 inhibits cell proliferation and promotes cell apoptosis in thyroid carcinoma by regulating the miR-620/IRF2BP2 axis. Am. J. Med. Sci. 2023, 365, 73–83. [Google Scholar] [CrossRef]
- Li, L.; Lin, X.; Xu, P.; Jiao, Y.; Fu, P. LncRNA GAS5 sponges miR-362-5p to promote sensitivity of thyroid cancer cells to 131I by upregulating SMG1. IUBMB Life 2020, 72, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-X.; Yang, S.-L.; Jia, Y.; Wu, J.-C.; Xu, Z.; Zhang, H. Epigenetic regulation of papillary thyroid carcinoma by long non-coding RNAs. Semin. Cancer Biol. 2022, 83, 253–260. [Google Scholar] [CrossRef]
- Chan, I.L. Regulation of Telomerase in Normal and Cancer Stem Cells. In Proceedings of the 11th International Conference on Bioinformatics Research and Applications, Milan, Italy, 13–15 September 2024; pp. 78–84. [Google Scholar]
- McKelvey, B.A.; Umbricht, C.B.; Zeiger, M.A. Telomerase reverse transcriptase (TERT) regulation in thyroid cancer: A review. Front. Endocrinol. 2020, 11, 485. [Google Scholar] [CrossRef]
- Samimi, H.; Sajjadi-Jazi, S.M.; Seifirad, S.; Atlasi, R.; Mahmoodzadeh, H.; Faghihi, M.A.; Haghpanah, V. Molecular mechanisms of long non-coding RNAs in anaplastic thyroid cancer: A systematic review. Cancer Cell Int. 2020, 20, 352. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Liu, H.; Chen, S.-R. Mechanisms of long non-coding RNAs in cancers and their dynamic regulations. Cancers 2020, 12, 1245. [Google Scholar] [CrossRef]
- Dumbović, G.; Braunschweig, U.; Langner, H.K.; Smallegan, M.; Biayna, J.; Hass, E.P.; Jastrzebska, K.; Blencowe, B.; Cech, T.R.; Caruthers, M.H. Nuclear compartmentalization of TERT mRNA and TUG1 lncRNA is driven by intron retention. Nat. Commun. 2021, 12, 3308. [Google Scholar] [CrossRef]
- Ye, M.; Dong, S.; Hou, H.; Zhang, T.; Shen, M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol. Ther. Nucleic Acids 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Lei, C.; Xiao, H.; Yang, J.; Luo, Q.; Hu, L.; Chen, F.; Long, M.; Zhang, H. lncRNA ACVR2B-AS1 modulates thyroid cancer progression by regulating miR-195-5p. Discov. Oncol. 2025, 16, 1–11. [Google Scholar]
- Matsuse, M.; Mitsutake, N. TERT promoter mutations in thyroid cancer. Endocr. J. 2023, 70, 1035–1049. [Google Scholar] [CrossRef]
- Seo, D.H.; Lee, S.G.; Choi, S.M.; Kim, H.Y.; Park, S.; Jung, S.G.; Jo, Y.S.; Lee, J. Promoter mutation-independent TERT expression is related to immune-enriched milieu in papillary thyroid cancer. Endocr. Relat. Cancer 2024, 31, e240068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; De Souza, C.; Kumar, V.E.; Nambiar, R.; Hao, D.; Zhu, X.; Luo, Y.; Liu, S.; Zhang, L.; Zhu, J. Long non-coding RNA signatures as predictors of prognosis in thyroid cancer: A narrative review. Ann. Transl. Med. 2021, 9, 359. [Google Scholar] [CrossRef]
- Lim, Y.W.S.; Xiang, X.; Garg, M.; Le, M.T.; Wong, A.L.-A.; Wang, L.; Goh, B.-C. The double-edged sword of H19 lncRNA: Insights into cancer therapy. Cancer Lett. 2021, 500, 253–262. [Google Scholar]
- Ghasemian, M.; Poodineh, J. A review on the biological roles of LncRNA PTCSC3 in cancerous and non-cancerous disorders. Cancer Cell Int. 2023, 23, 184. [Google Scholar]
- Si, L.; Yang, Z.; Ding, L.; Zhang, D. Regulatory effects of lncRNAs and miRNAs on the crosstalk between autophagy and EMT in cancer: A new era for cancer treatment. J. Cancer Res. Clin. Oncol. 2022, 148, 547–564. [Google Scholar]
- Zhao, J.; Du, P.; Cui, P.; Qin, Y.; Hu, C.E.; Wu, J.; Zhou, Z.; Zhang, W.; Qin, L.; Huang, G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 2018, 37, 4094–4109. [Google Scholar]
- Zhao, J.H.; Wang, B.; Wang, X.H.; Wang, J.R.; Xu, C.W. Influence of lncRNA ANRIL on neuronal apoptosis in rats with cerebral infarction by regulating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci 2019, 23, 10092–10100. [Google Scholar] [CrossRef]
- Xia, M.; Duan, L.-J.; Lu, B.-N.; Pang, Y.-Z.; Pang, Z.-R. LncRNA AFAP1-AS1/miR-27b-3p/VEGF-C axis modulates stemness characteristics in cervical cancer cells. Chin. Med. J. 2021, 134, 2091–2101. [Google Scholar]
- Liang, S.; Ren, K.; Li, B.; Li, F.; Liang, Z.; Hu, J.; Xu, B.; Zhang, A. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1α/VEGF signal pathway. Mol. Cell. Biochem. 2020, 465, 1–11. [Google Scholar]
- Yang, B.; Tian, H.; Xiao, C. lncRNA NONHSAT021963, which upregulates VEGF in A549 cells, mediates PM2. 5 exposure-induced angiogenesis in Shenyang, China. Mol. Cell. Toxicol. 2020, 16, 1–10. [Google Scholar]
- Hussen, B.M.; Azimi, T.; Abak, A.; Hidayat, H.J.; Taheri, M.; Ghafouri-Fard, S. Role of lncRNA BANCR in human cancers: An updated review. Front. Cell. Dev. Biol. 2021, 9, 689992. [Google Scholar]
- Liu, Y.; Khan, S.; Li, L.; Ten Hagen, T.L.; Falahati, M. Molecular mechanisms of thyroid cancer: A competing endogenous RNA (ceRNA) point of view. Biomed. Pharmacother. 2022, 146, 112251. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, Y.; Luo, Y. The role of long non-coding RNAs in the tumor immune microenvironment. Front. Immunol. 2022, 13, 851004. [Google Scholar] [CrossRef]
- Hejazi, M.; Jafari, T.; Yari, A.; Heshmat, R.; Larijani, B.; Ahvaz, S.; Pourbagherian, O.; Tavangar, S.M.; Shafiee, G.; Mokhtarzadeh, A.A. A novel long non-coding RNA, PICSAR, promotes thyroid cancer progression through the hsa-miR-320A/hsa-miR-485/RAPGEFL1 axis. Med. Oncol. 2025, 42, 448. [Google Scholar] [CrossRef]
- Dai, W.; Jin, X.; Han, L.; Huang, H.; Ji, Z.; Xu, X.; Tang, M.; Jiang, B.; Chen, W. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/β-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020, 11, 743. [Google Scholar]
- Chen, Y.; Gao, H.; Li, Y. Inhibition of LncRNA FOXD3-AS1 suppresses the aggressive biological behaviors of thyroid cancer via elevating miR-296-5p and inactivating TGF-β1/Smads signaling pathway. Mol. Cell. Endocrinol. 2020, 500, 110634. [Google Scholar] [CrossRef]
- Qi, F.; Tang, J.; Cai, Z.; Wang, G.; Wang, Z. Long non-coding RNA CATIP antisense RNA 1 (lncRNA CATIP-AS1) downregulation contributes to the progression and metastasis of thyroid cancer via epithelial–mesenchymal transition (EMT) pathway. Bioengineered 2022, 13, 7592–7606. [Google Scholar] [CrossRef]
- Bracken, C.; Goodall, G.; Gregory, P. RNA regulatory mechanisms controlling TGF-β signaling and EMT in cancer. Semin. Cancer Biol. 2024, 102–103, 4–16. [Google Scholar] [CrossRef]
- Rodrigues-Junior, D.; Moustakas, A. Unboxing the network among long non-coding RNAs and TGF-β signaling in cancer. Upsala J. Med. Sci. 2024, 129, e10614. [Google Scholar] [CrossRef]
- Kang, Y.E.; Shong, M.; Kim, J.M.; Koo, B.S. Prognostic Significance of Sirtuins Expression in Papillary Thyroid Carcinoma. Int. J. Thyroidol. 2018, 11, 143–151. [Google Scholar] [CrossRef]
- Senousy, M.A.; Shaker, O.G.; Ayeldeen, G.; Radwan, A.F. Association of lncRNA MEG3 rs941576 polymorphism, expression profile, and its related targets with the risk of obesity-related colorectal cancer: Potential clinical insights. Sci. Rep. 2024, 14, 10271. [Google Scholar] [CrossRef] [PubMed]
- Gugnoni, M.; Kashyap, M.K.; Wary, K.K.; Ciarrocchi, A. lncRNAs: The unexpected link between protein synthesis and cancer adaptation. Mol. Cancer 2025, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Golla, U.; Sesham, K.; Dallavalasa, S.; Manda, N.K.; Unnam, S.; Sanapala, A.K.; Nalla, S.; Kondam, S.; Kumar, R. ABHD11-AS1: An emerging long non-coding RNA (lncRNA) with clinical significance in human malignancies. Noncoding RNA 2022, 8, 21. [Google Scholar]
- Alamoudi, A.A. The Role of Non-Coding RNAs in MYC-Mediated Metabolic Regulation: Feedback Loops and Interactions. Noncoding RNA 2025, 11, 27. [Google Scholar] [PubMed]
- Huo, N.; Cong, R.; Sun, Z.-J.; Li, W.-C.; Zhu, X.; Xue, C.-Y.; Chen, Z.; Ma, L.-Y.; Chu, Z.; Han, Y.-C. STAT3/LINC00671 axis regulates papillary thyroid tumor growth and metastasis via LDHA-mediated glycolysis. Cell Death Dis. 2021, 12, 799. [Google Scholar] [CrossRef]
- Shi, L.; Duan, R.; Sun, Z.; Jia, Q.; Wu, W.; Wang, F.; Liu, J.; Zhang, H.; Xue, X. LncRNA GLTC targets LDHA for succinylation and enzymatic activity to promote progression and radioiodine resistance in papillary thyroid cancer. Cell Death Differ. 2023, 30, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, H.; Wang, Q.; Song, Z.; Wang, J. Chemotherapy-mediated lncRNA-induced immune cell plasticity in cancer immunopathogenesis. Int. Immunopharmacol. 2024, 141, 112967. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, T.; Li, Y.; Huang, X. LncRNAs act as modulators of macrophages within the tumor microenvironment. Carcinogenesis 2024, 45, 363–377. [Google Scholar] [CrossRef]
- Uddin, M.N.; Wang, X. The landscape of long non-coding RNAs in tumor stroma. Life Sci. 2021, 264, 118725. [Google Scholar] [CrossRef]
- Febrero, B.; Ruiz-Manzanera, J.J.; Ros-Madrid, I.; Hernández, A.M.; Orenes-Piñero, E.; Rodríguez, J.M. Tumor microenvironment in thyroid cancer: Immune cells, patterns, and novel treatments. Head Neck 2024, 46, 1486–1499. [Google Scholar] [CrossRef]
- Arshi, A.; Mahmoudi, E.; Raeisi, F.; Dehghan Tezerjani, M.; Bahramian, E.; Ahmed, Y.; Peng, C. Exploring potential roles of long non-coding RNAs in cancer immunotherapy: A comprehensive review. Front. Immunol. 2024, 15, 1446937. [Google Scholar] [CrossRef]
- Huang, D.; Chen, J.; Yang, L.; Ouyang, Q.; Li, J.; Lao, L.; Zhao, J.; Liu, J.; Lu, Y.; Xing, Y. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 2018, 19, 1112–1125. [Google Scholar]
- Tuersong, T.; Shataer, M.; Chen, Y.; Chen, G.; Li, X.; Lei, L.; Younusi, A.; Ma, L. Extracellular vesicle-LncRNA HOTAIR modulates esophageal cancer chemoresistance and immune microenvironment via miR-375/CDH2 pathway. J. Cell Commun. Signal. 2025, 19, e70014. [Google Scholar]
- Chen, Q.-H.; Li, B.; Liu, D.-G.; Zhang, B.; Yang, X.; Tu, Y.-L. LncRNA KCNQ1OT1 sponges miR-15a to promote immune evasion and malignant progression of prostate cancer via up-regulating PD-L1. Cancer Cell Int. 2020, 20, 394. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Chen, Y.; Ou, Q.; Wang, X.; Tang, N. LncRNA MIAT correlates with immune infiltrates and drug reactions in hepatocellular carcinoma. Int. Immunopharmacol. 2020, 89, 107071. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.; Esposito, F.; Capone, M.; Pallante, P.; Fusco, A. Noncoding RNAs in thyroid-follicular-cell-derived carcinomas. Cancers 2022, 14, 3079. [Google Scholar]
- Taniue, K.; Akimitsu, N. The functions and unique features of LncRNAs in cancer development and tumorigenesis. Int. J. Mol. Sci. 2021, 22, 632. [Google Scholar] [CrossRef]
- Lu, W.; Xu, Y.; Xu, J.; Wang, Z.; Ye, G. Identification of differential expressed lnc RNA s in human thyroid cancer by a genome-wide analyses. Cancer Med. 2018, 7, 3935–3944. [Google Scholar]
- Liyanarachchi, S.; Li, W.; Yan, P.; Bundschuh, R.; Brock, P.; Senter, L.; Ringel, M.D.; De La Chapelle, A.; He, H. Genome-wide expression screening discloses long noncoding RNAs involved in thyroid carcinogenesis. J. Clin. Endocrinol. Metab. 2016, 101, 4005–4013. [Google Scholar] [CrossRef]
- Demin, D.; Murashko, M.; Uvarova, A.; Stasevich, E.; Shyrokova, E.; Gorlachev, G.E.; Korneev, K.; Ustiugova, A.; Tkachenko, E.A.; Kostenko, V.V.; et al. Adversary of DNA integrity: A long non-coding RNA stimulates driver oncogenic chromosomal rearrangement in human thyroid cells. bioRxiv 2023, 152, 1452–1462. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Rong, H.; Zhang, P.; Huo, Z.; Li, Y.; Xiao, H.; Guan, H.; Li, H. Long noncoding RNA FAM111A-DT promotes aggressiveness of papillary thyroid cancer via activating NF-κB signaling. J. Endocrinol. Investig. 2025, 48, 1121–1136. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Y.; Peng, J.; Thakur, A.; Bai, N.; Yang, K.; Xu, Z. Decoding roles of exosomal lncRNAs in tumor-immune regulation and therapeutic potential. Cancers 2022, 15, 286. [Google Scholar] [PubMed]
- Egranov, S.D.; Hu, Q.; Lin, C.; Yang, L. LncRNAs as tumor cell intrinsic factors that affect cancer immunotherapy. RNA Biol. 2020, 17, 1625–1627. [Google Scholar] [CrossRef]
- Sang, L.-J.; Ju, H.-Q.; Liu, G.-P.; Tian, T.; Ma, G.-L.; Lu, Y.-X.; Liu, Z.-X.; Pan, R.-L.; Li, R.-H.; Piao, H.-L. LncRNA CamK-A regulates Ca2+-signaling-mediated tumor microenvironment remodeling. Mol. Cell 2018, 72, 71–83.e77. [Google Scholar] [CrossRef] [PubMed]
- Cormier, F.; Housni, S.; Dumont, F.; Villard, M.; Cochand-Priollet, B.; Mercier-Nomé, F.; Perlemoine, K.; Bertherat, J.; Groussin, L. NF-κB signaling activation and roles in thyroid cancers: Implication of MAP3K14/NIK. Oncogenesis 2023, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-L.; Zheng, W.-J.; Xu, L.; Zhou, Q.-Y.; Chen, J. Identification of potential LncRNAs as papillary thyroid carcinoma biomarkers based on integrated bioinformatics analysis using TCGA and RNA sequencing data. Sci. Rep. 2023, 13, 4350. [Google Scholar] [CrossRef]
- Içduygu, F.; Akgun, E.; Sengul, D.; Ozgoz, A.; Alp, E. Expression of SOX2OT, DANCR and TINCR long non-coding RNAs in papillary thyroid cancer and its effects on clinicopathological features. Mol. Med. Rep. 2022, 25, 1–10. [Google Scholar] [CrossRef]
- Guo, C.; Li, H.; Pan, N.; Xu, S.; Zeng, Q.; Zhou, B.; Wang, J. Identification of prognostic signature with seven LncRNAs for papillary thyroid carcinoma. Adv. Med. Sci. 2022, 67, 103–113. [Google Scholar] [CrossRef]
- Kim, D.; Yu, J.; Kim, J.; Hwang, Y.-A.; Kim, J.K.; Ku, C.; Yoon, J.; Kwak, J.; Nam, K.; Lee, E.J. Use of long non-coding RNAs for the molecular diagnosis of papillary thyroid cancer. Front. Oncol. 2022, 12, 924409. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Mohammadzadeh, R.; Amini, M.; Najafi, S.; Baradaran, B.; Bahojb Mahdavi, S.Z.; Yari, A.; Mokhtarzadeh, A.A. Improvement of carboplatin chemosensitivity in lung cancer cells by siRNA-mediated downregulation of DLGAP1-AS2 expression. Sci. Rep. 2025, 15, 7971. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-M.; Zhao, J.-Y.; Lv, F.-F.; Yang, X.-B.; Wang, H.-J. A potential nine-lncRNAs signature identification and nomogram diagnostic model establishment for papillary thyroid cancer. Pathol. Oncol. Res. 2022, 28, 1610012. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Liu, B.; Lv, Y.; Man, J.; Liang, M.; Qiao, H. Analysis of lncRNA-miRNA-mRNA interactions identified a novel biomarker LINC00657 to improve prognosis prediction of papillary thyroid carcinoma. Int. Immunopharmacol. 2024, 137, 112432. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Barwal, T.S.; Sharma, U.; Rana, M.K.; Bazala, S.; Singh, I.; Murmu, M.; Kapoor, H.S.; Thakur, S.; Jain, M.; Jain, A. A diagnostic and prognostic value of blood-based circulating long non-coding RNAs in thyroid, pancreatic and ovarian cancer. Crit. Rev. Oncol. Hematol. 2022, 171, 103598. [Google Scholar]
- Rao, Y.; Liu, H.; Yan, X.; Wang, J. In silico analysis identifies differently expressed lncRNAs as novel biomarkers for the prognosis of thyroid cancer. Comput. Math. Methods Med. 2020, 2020, 3651051. [Google Scholar] [CrossRef]
- Tous, C.; Muñoz-Redondo, C.; Gavilán, A.; Bravo-Gil, N.; Baco-Antón, F.; Navarro-González, E.; Antiñolo, G.; Borrego, S. Delving into the role of lncRNAs in papillary thyroid cancer: Upregulation of LINC00887 promotes cell proliferation, growth and invasion. Int. J. Mol. Sci. 2024, 25, 1587. [Google Scholar] [CrossRef]
- Yi, D.; Zhang, D.; He, J. Long non-coding RNA LIFR-AS1 suppressed the proliferation, angiogenesis, migration and invasion of papillary thyroid cancer cells via the miR-31-5p/SIDT2 axis. Cell Cycle 2021, 20, 2619–2637. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, R.; Huang, N.; Luo, C. Long non-coding RNA ASMTL-AS1 inhibits tumor growth and glycolysis by regulating the miR-93-3p/miR-660/FOXO1 axis in papillary thyroid carcinoma. Life Sci. 2020, 244, 117298. [Google Scholar] [CrossRef]
- Guo, K.; Chen, L.; Wang, Y.; Qian, K.; Zheng, X.; Sun, W.; Sun, T.; Wu, Y.; Wang, Z. Long noncoding RNA RP11-547D24. 1 regulates proliferation and migration in papillary thyroid carcinoma: Identification and validation of a novel long noncoding RNA through integrated analysis of TCGA database. Cancer Med. 2019, 8, 3105–3119. [Google Scholar] [CrossRef]
- Huang, C.; Su, X.; Zhou, D.-L.; Xu, B.-H.; Liu, Q.; Zhang, X.; Tang, T.; Yang, X.-H.; Ye, Z.-L.; He, C.-Y. A diagnostic and predictive lncRNA lnc-MPEG1-1 promotes the proliferation and metastasis of papillary thyroid cancer cells by occupying miR-766-5p. Mol. Ther. Nucleic Acids 2022, 28, 408–422. [Google Scholar]
- Liu, Y.; Shi, P.; Fang, H.; Zhao, Z.; Yang, F.; Zhang, J.; Jing, S.; Geng, C. Long non-coding RNA GHET1 promotes thyroid cancer cell proliferation and invasion. Transl. Cancer Res. 2021, 10, 4148. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cui, C.; Chen, J.; Hu, Z.; Wang, Y.; Hu, D. Long non-coding RNA UCA1 promotes papillary thyroid cancer cell proliferation via miR-204-mediated BRD4 activation. Mol. Med. Rep. 2018, 18, 3059–3067. [Google Scholar] [PubMed]
- Liu, Y.; Zhang, H.; Wang, H.; Du, J.; Dong, P.; Liu, M.; Lin, Y. Long non-coding RNA DUXAP8 promotes the cell proliferation, migration, and invasion of papillary thyroid carcinoma via miR-223-3p mediated regulation of CXCR4. Bioengineered 2021, 12, 496–506. [Google Scholar] [CrossRef]
- Xiao, J.; Bing, Z.; Xiao, G.; Guan, Y.; Luan, J. Long non-coding (lnc) RNA PAPAS overexpression inhibits tumor growth in papillary thyroid carcinoma by downregulating lncRNA HOTTIP. Oncol. Lett. 2020, 19, 2281–2285. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Huang, Y.; Gang, Q.; Liu, M.; Zhang, H.; Shen, S.; Qi, Y.; Zhang, J. The prognostic value and potential immune mechanisms of lncRNAs related to immunogenic cell death in papillary thyroid carcinoma. J. Inflamm. Res. 2024, ume 17, 1995–2008. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Z.; Xu, L.; Sun, L.; Song, H.; Yin, H.; He, F. lncRNA SNHG3 acts as a novel tumor suppressor and regulates tumor proliferation and metastasis via AKT/mTOR/ERK pathway in papillary thyroid carcinoma. J. Cancer 2020, 11, 3492. [Google Scholar] [CrossRef]
- Yang, M.; Huang, S.; Zhao, Y.; Xie, B.; Hu, X.; Cai, Y. Novel LncRNA AK023507 inhibits cell metastasis and proliferation in papillary thyroid cancer through β-catenin/Wnt signaling pathway. Biochem. Biophys. Res. Commun. 2023, 655, 104–109. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J. Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis. Open Life Sci. 2020, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, D.; Chen, L.; Gao, S.; Huang, X. Long non-coding RNA BRM promotes proliferation and invasion of papillary thyroid carcinoma by regulating the microRNA-331-3p/SLC25A1 axis. Oncol. Lett. 2020, 19, 3071–3078. [Google Scholar] [CrossRef]
- Shi, P.; Liu, Y.; Zhang, M.; Yang, J.; Jing, S.; Yang, D.; Liu, F.; Wu, Y.; Shi, H.; Geng, C. Cervical carcinoma high-expressed long non-coding RNA 1 promotes papillary thyroid carcinoma cell proliferation and invasion. Transl. Cancer Res. 2021, 10, 4158. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moya, J.; Wert-Lamas, L.; Acuña-Ruíz, A.; Fletcher, A.; Wert-Carvajal, C.; McCabe, C.J.; Santisteban, P.; Riesco-Eizaguirre, G. Identification of an interactome network between lncRNAs and miRNAs in thyroid cancer reveals SPTY2D1-AS1 as a new tumor suppressor. Sci. Rep. 2022, 12, 7706. [Google Scholar] [CrossRef]
- Huang, C.; Duan, Z.; Chen, B.; Xia, H.; Wang, G. LncRNA LINC00969 modified by METTL3 attenuates papillary thyroid cancer progression in an m6A-dependent manner. Adv. Clin. Exp. Med. 2025, 34, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, J.; Yin, S.; Ma, S.; Wang, J.; Yi, H. LncRNA FAM230B promotes the metastasis of papillary thyroid cancer by sponging the miR-378a-3p/WNT5A axis. Biochem. Biophys. Res. Commun. 2021, 546, 83–89. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, W.; Wang, Z.; Dong, W.; He, L.; Zhang, T.; Shao, L.; Zhang, H. ATF2-induced lncRNA GAS8-AS1 promotes autophagy of thyroid cancer cells by targeting the miR-187-3p/ATG5 and miR-1343-3p/ATG7 axes. Mol. Ther. Nucleic Acids 2020, 22, 584–600. [Google Scholar] [CrossRef]
- Hazafa, A.; Mumtaz, M.; Farooq, M.F.; Bilal, S.; Chaudhry, S.N.; Firdous, M.; Naeem, H.; Ullah, M.O.; Yameen, M.; Mukhtiar, M.S. CRISPR/Cas9: A powerful genome editing technique for the treatment of cancer cells with present challenges and future directions. Life Sci. 2020, 263, 118525. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Alibakhshi, A.; Hashemi, M.; Hejazi, M.; Hosseini, V.; de la Guardia, M.; Ramezani, M. Biodegradable nano-polymers as delivery vehicles for therapeutic small non-coding ribonucleic acids. J. Control. Release 2017, 245, 116–126. [Google Scholar] [CrossRef]
- Grillone, K.; Caridà, G.; Luciano, F.; Cordua, A.; Di Martino, M.T.; Tagliaferri, P.; Tassone, P. A systematic review of non-coding RNA therapeutics in early clinical trials: A new perspective against cancer. J. Transl. Med. 2024, 22, 731. [Google Scholar] [CrossRef]
- Luzón-Toro, B.; Fernández, R.M.; Martos-Martínez, J.; Rubio-Manzanares-Dorado, M.; Antiñolo, G.; Borrego, S. LncRNA LUCAT1 as a novel prognostic biomarker for patients with papillary thyroid cancer. Sci. Rep. 2019, 9, 14374. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Wu, C.; Yan, M.; Wu, H.; Wang, J.; Yang, X.; Shao, Q. Construction and investigation of lncRNA-associated ceRNA regulatory network in papillary thyroid cancer. Oncol. Rep. 2018, 39, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Y. LncRNA H19 is a potential biomarker and correlated with immune infiltration in thyroid carcinoma. Clin. Exp. Med. 2023, 23, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, H.; Zhao, Y.; Li, C.; Liang, Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 279. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, H.; Guo, Z.; Dong, Z.; Yu, Y.; Zheng, J.; Ma, Y.; Sun, H.; Zhang, Q.; Zhang, J. Identification of m6A-related lncRNAs LINC02471 and DOCK9-DT as potential biomarkers for thyroid cancer. Int. Immunopharmacol. 2024, 133, 112050. [Google Scholar] [CrossRef]
- Gong, Y.; Gong, D.; Liu, S.; Gong, X.; Xiong, J.; Zhang, J.; Jiang, L.; Liu, J.; Zhu, L.; Luo, H.; et al. Deciphering the role of NcRNAs in Pancreatic Cancer immune evasion and drug resistance: A new perspective for targeted therapy. Front. Immunol. 2024, 15, 2024. [Google Scholar] [CrossRef]
- Zheng, J.; Dou, R.; Zhang, X.; Zhong, B.; Fang, C.; Xu, Q.; Di, Z.; Huang, S.; Lin, Z.; Song, J. LINC00543 promotes colorectal cancer metastasis by driving EMT and inducing the M2 polarization of tumor associated macrophages. J. Transl. Med. 2023, 21, 153. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Liao, M.; Zhang, Q.; Yang, M. LncRNA MIR155HG induces M2 macrophage polarization and drug resistance of colorectal cancer cells by regulating ANXA2. Cancer Immunol. Immunother. 2022, 71, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Zhang, X.; Li, C.; Zhang, J.; Zhou, W. LncRNA HLA-F-AS1 promotes colorectal cancer metastasis by inducing PFN1 in colorectal cancer-derived extracellular vesicles and mediating macrophage polarization. Cancer Gene Ther. 2021, 28, 1269–1284. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.; Li, M.; Jin, Z.; Chen, G.; Gan, C. Long non-coding RNA NBR2 suppresses the progression of colorectal cancer by downregulating miR-19a to regulate M2 macrophage polarization. J. Physiol. Investig. 2023, 66, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.; Prakash, M.D.; Cox, M.; Wilson, K.; Boer, J.C.; Cauchi, J.A.; Plebanski, M. Therapeutic cancer vaccines—T cell responses and epigenetic modulation. Front. Immunol. 2019, 9, 3109. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; He, J.; Chen, Q.; Yuan, M.; Zeng, X.; Li, Y.; Zeng, Y.; He, M.; Zhou, Q.; Feng, D. ELFN1-AS1 promotes GDF15-mediated immune escape of colorectal cancer from NK cells by facilitating GCN5 and SND1 association. Discov. Oncol. 2023, 14, 56. [Google Scholar]
- Withers, D.R.; Hepworth, M.R.; Wang, X.; Mackley, E.C.; Halford, E.E.; Dutton, E.E.; Marriott, C.L.; Brucklacher-Waldert, V.; Veldhoen, M.; Kelsen, J. Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat. Med. 2016, 22, 319–323. [Google Scholar] [CrossRef]
- Li, L.; Xiong, F.; Wang, Y.; Zhang, S.; Gong, Z.; Li, X.; He, Y.; Shi, L.; Wang, F.; Liao, Q. What are the applications of single-cell RNA sequencing in cancer research: A systematic review. J. Exp. Clin. Cancer Res. 2021, 40, 163. [Google Scholar] [CrossRef]
- Wang, Q.; Zhi, Y.; Zi, M.; Mo, Y.; Wang, Y.; Liao, Q.; Zhang, S.; Gong, Z.; Wang, F.; Zeng, Z. Spatially resolved transcriptomics technology facilitates cancer research. Adv. Sci. 2023, 10, 2302558. [Google Scholar] [CrossRef]
- Lyu, P.; Gu, X.; Wang, F.; Sun, H.; Zhou, Q.; Yang, S.; Yuan, W. Advances in targeting cancer-associated fibroblasts through single-cell spatial transcriptomic sequencing. Biomark. Res. 2024, 12, 73. [Google Scholar]
- Orrapin, S.; Thongkumkoon, P.; Udomruk, S.; Moonmuang, S.; Sutthitthasakul, S.; Yongpitakwattana, P.; Pruksakorn, D.; Chaiyawat, P. Deciphering the biology of circulating tumor cells through single-cell RNA sequencing: Implications for precision medicine in cancer. Int. J. Mol. Sci. 2023, 24, 12337. [Google Scholar] [CrossRef] [PubMed]
- Aprile, M.; Costa, V.; Cimmino, A.; Calin, G.A. Emerging role of oncogenic long noncoding RNA as cancer biomarkers. Int. J. Cancer 2023, 152, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, Y.; Liu, B.; Zhao, M. Expression of lncRNA-HOTAIR in the serum of patients with lymph node metastasis of papillary thyroid carcinoma and its impact. Oncol. Lett. 2020, 20, 907–913. [Google Scholar] [PubMed]
- Winkle, M.; El-Daly, S.; Fabbri, M.; Calin, G. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Huang, L. The tumor therapeutic potential of long non-coding RNA delivery and targeting. Acta Pharm. Sin. B 2022, 13, 1371–1382. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sin. B 2020, 11, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.; Valeri, N.; Hahne, J. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Coan, M.; Haefliger, S.; Ounzain, S.; Johnson, R. Targeting and engineering long non-coding RNAs for cancer therapy. Nat. Rev. Genet. 2024, 25, 578–595. [Google Scholar] [CrossRef]
- Amodio, N.; Raimondi, L.; Juli, G.; Stamato, M.A.; Caracciolo, D.; Tagliaferri, P.; Tassone, P. MALAT1: A druggable long non-coding RNA for targeted anti-cancer approaches. J. Hematol. Oncol. 2018, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]





| LncRNA | Hallmark Affected | Mechanism | Experimental Model | Main Findings | Ref. |
|---|---|---|---|---|---|
| MEG3 | Invasion and Metastasis; TME/CAF remodeling | Tumor-suppressive; stromal-enriched MEG3 in CAFs associated with LNM; post-transcriptional repression of Rac1 (3′UTR) | Bulk and single-cell RNA-seq; in vitro | MEG3 signal predicts lymph node metastasis and CAF infiltration; overexpression suppresses migration/invasion via Rac1 downregulation | [31,32] |
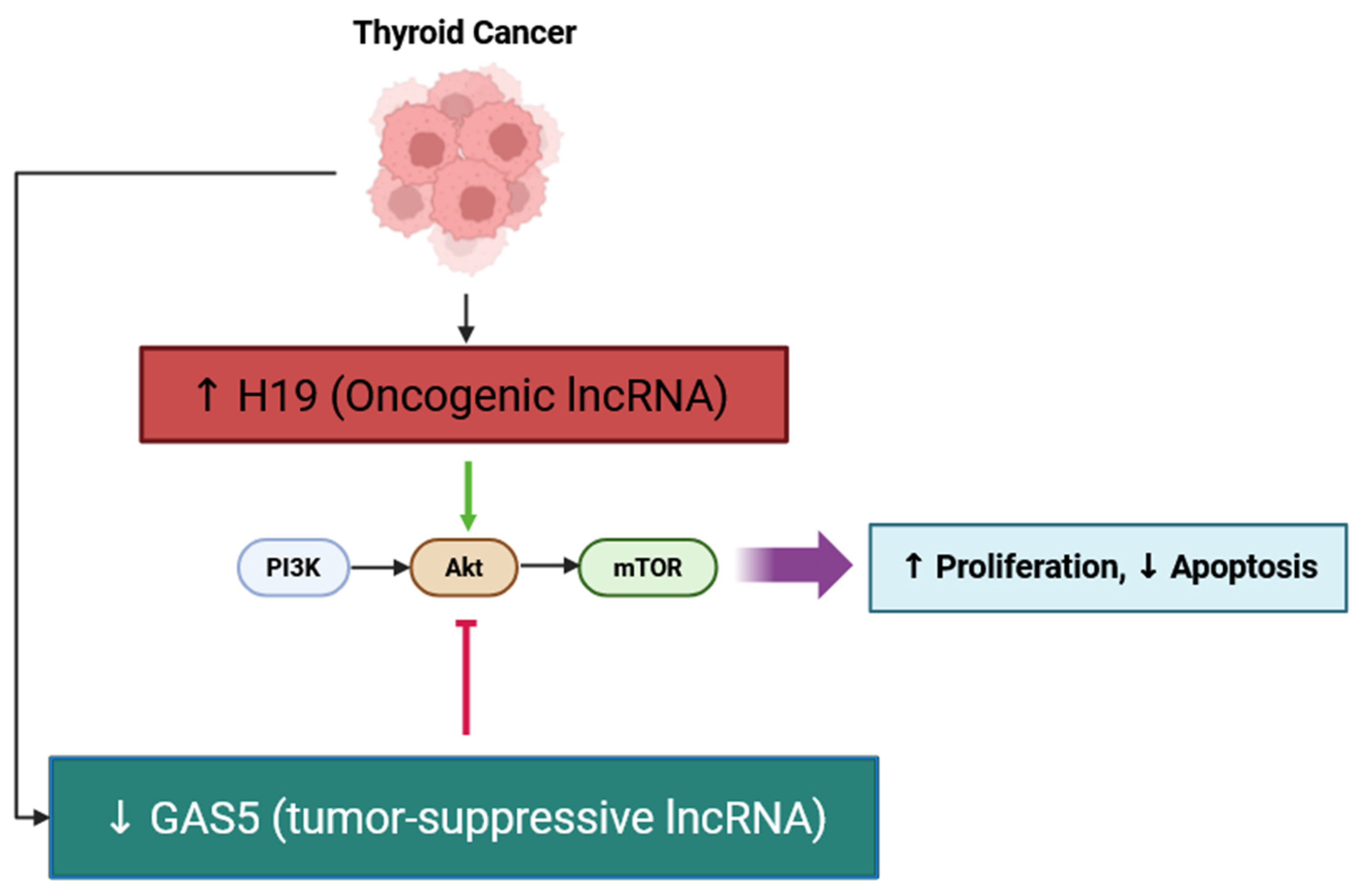
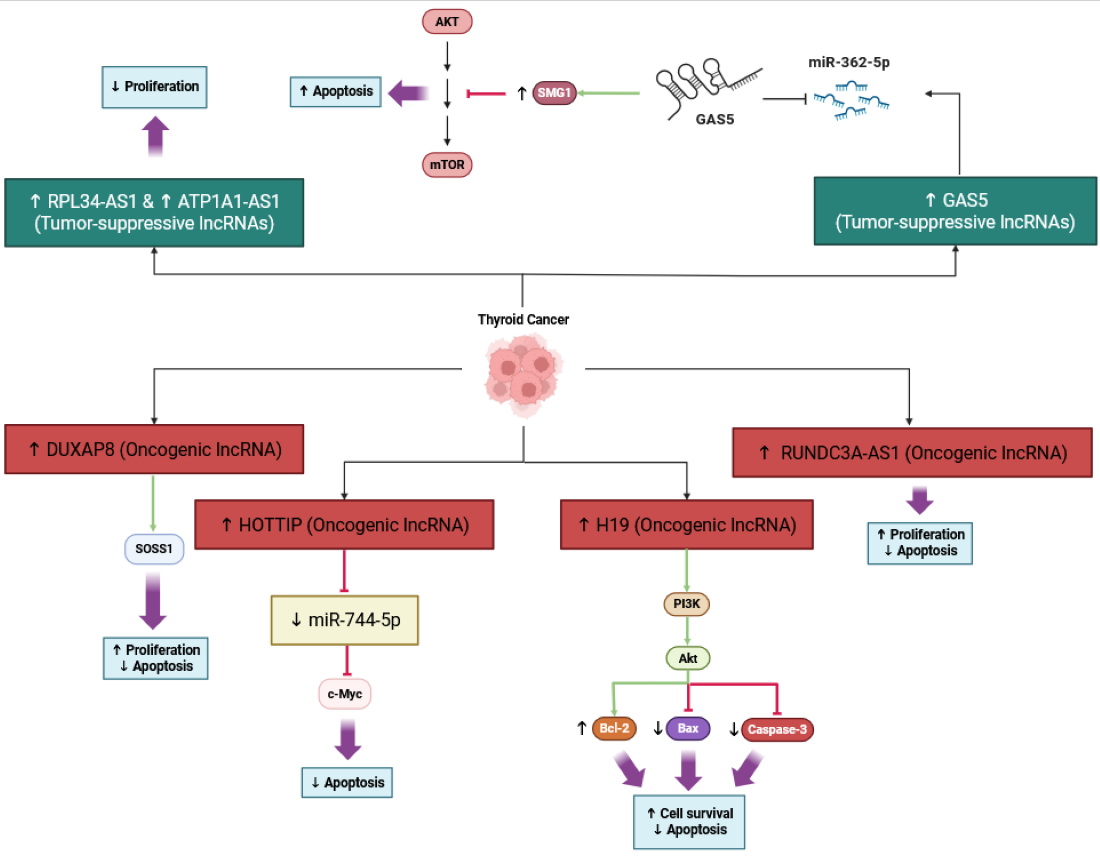
| H19 | Proliferation, Apoptosis, Angiogenesis, Immune Infiltration | Activates PI3K/AKT; ↑ VEGF; ↑ immune infiltration | In vitro; tissues | Overexpression linked to increased proliferation/apoptosis resistance and immune cell infiltration signatures in thyroid carcinoma | [41,61] |
| KCNQ1OT1 | Immune Evasion | Sponges miR-15a → ↑ PD-L1 | In vitro | Oncogenic; promotes growth and motility via miRNA sponging to de-repress target oncogenes | [93] |
| GAS5 | Apoptosis, Therapy Sensitivity | Sponges miR-362-5p → ↑ SMG1; Akt/mTOR inhibition | In vitro | Tumor-suppressive; enhances apoptosis and radioiodine sensitivity | [50] |
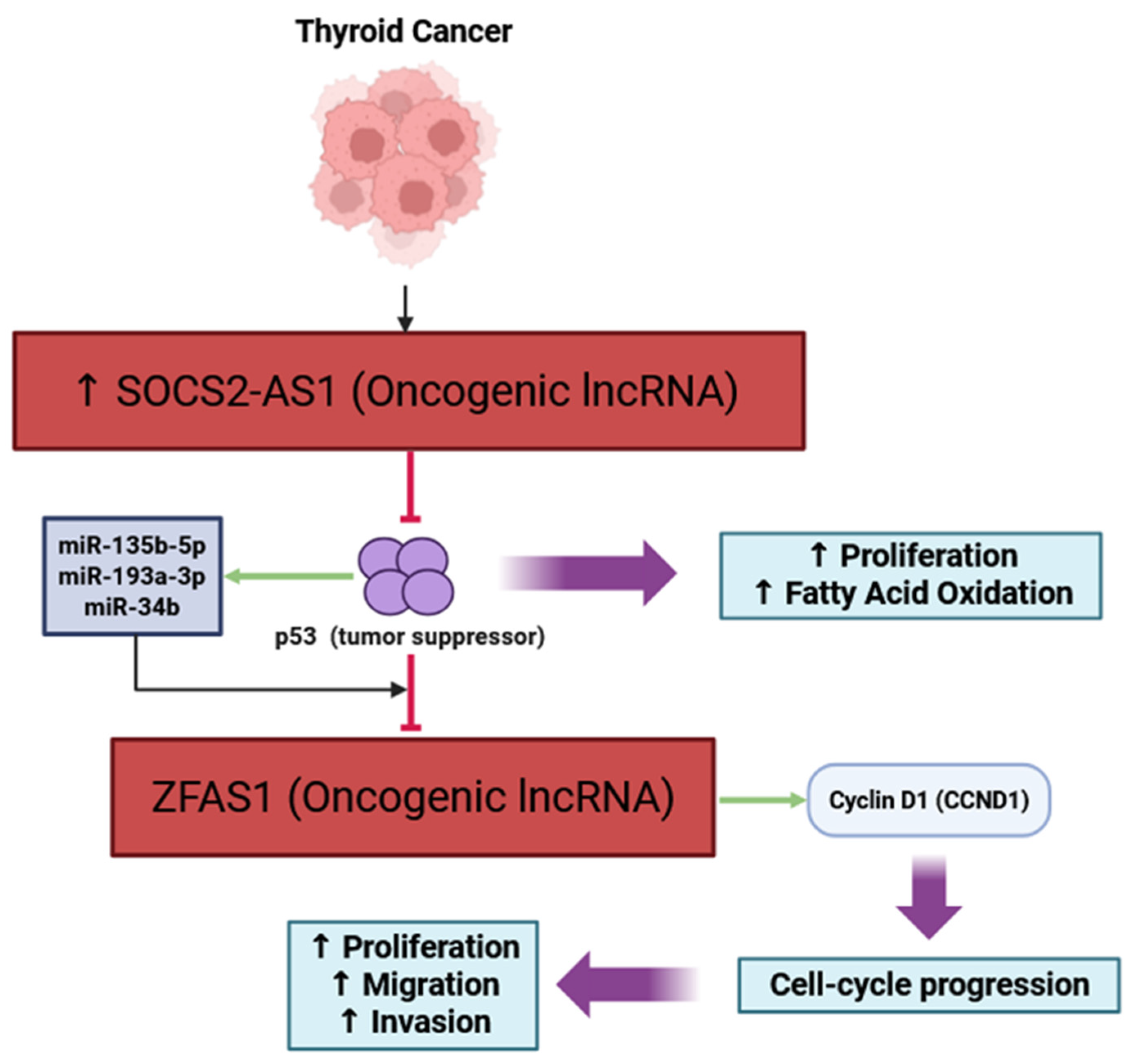
| SOCS2-AS1 | Proliferation, FAO | Degrades p53; ↑ fatty acid oxidation | In vitro | Oncogenic; promotes proliferation and metabolism | [43] |
| ZFAS1 | Proliferation, EMT | Repressed by p53; regulates miRNAs | In vitro | Oncogenic; promotes proliferation/invasion | [22] |
| HOTTIP | Apoptosis Resistance | Sponges miR-744-5p → ↑ c-Myc | In vitro | Oncogenic; inhibits apoptosis | [46] |
| RUNDC3A-AS1 | Proliferation, Apoptosis | miR-151b/SNRPB axis | In vitro | Oncogenic; promotes growth, inhibits apoptosis | [47] |
| RPL34-AS1 | Apoptosis | Modulates miRNAs | In vitro | Tumor-suppressive; induces apoptosis | [48] |
| ATP1A1-AS1 | Apoptosis | Regulates downstream effectors | In vitro | Tumor-suppressive; apoptosis induction | [49] |
| TUG1 | Replicative Immortality | ↑ TERT | In vitro | Oncogenic; promotes telomerase activity | [56] |
| FOXD2-AS1 | Replicative Immortality | Sponges miR-7-5p → ↑ TERT | In vitro | Oncogenic; enhances immortality | [56] |
| ACVR2B-AS1 | Replicative Immortality | Sponges miR-195-5p → ↑ FGF2 | In vitro | Oncogenic; proliferation, poor prognosis | [58] |
| ABHD11-AS1 | Proliferation, Angiogenesis | Modulates PI3K/Akt, EMT | In vitro; tissues | Oncogenic; angiogenesis and proliferation | [61] |
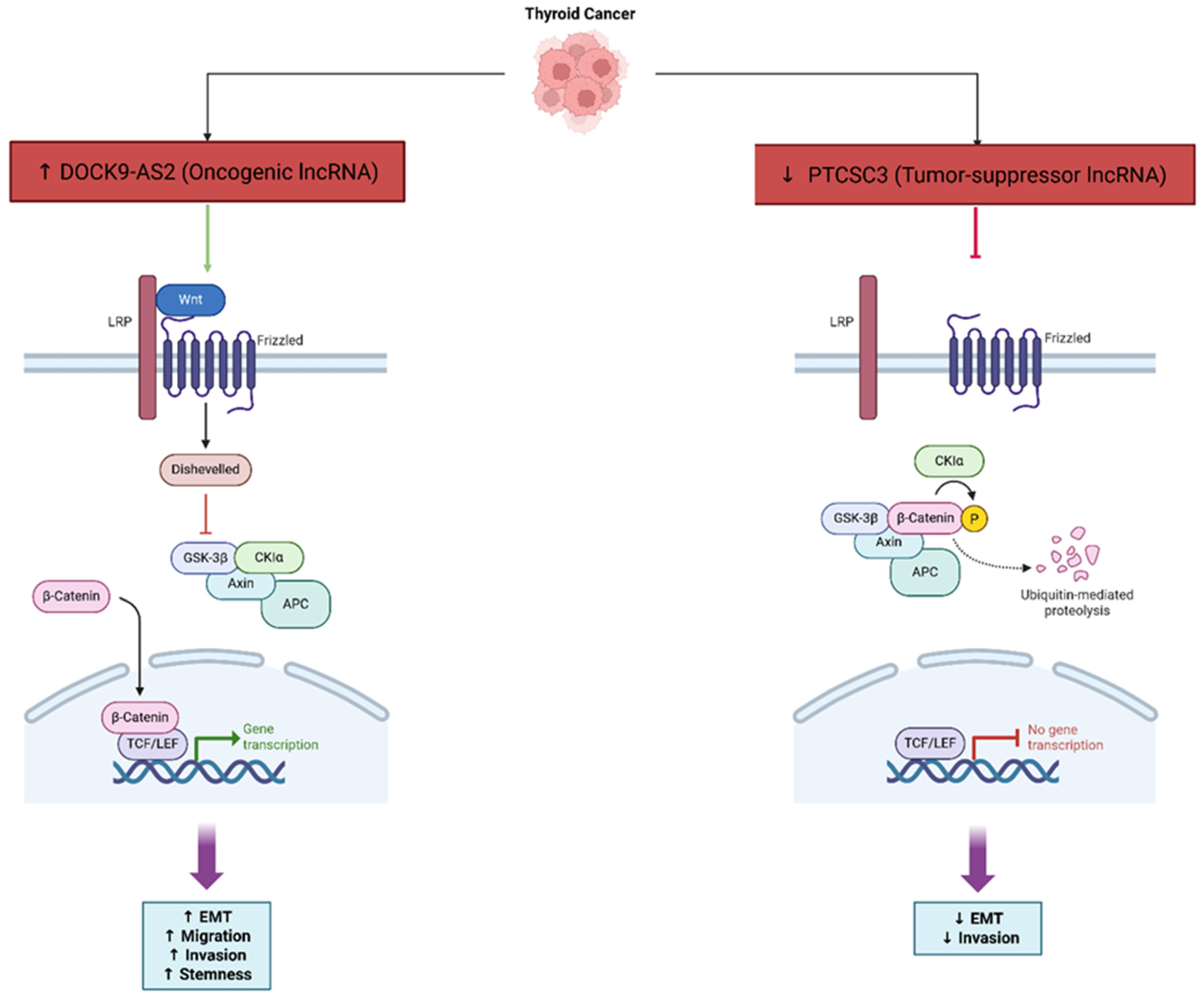
| PTCSC3 | Invasion, Angiogenesis | Suppresses Wnt/β-catenin | In vitro | Tumor-suppressive; inhibits EMT and angiogenesis | [63] |
| ANRIL | Angiogenesis | Activates NF-κB → ↑ VEGF | Rat model; cross-cancer | Oncogenic; angiogenesis | [66] |
| AFAP1-AS1 | Angiogenesis, Stemness | Sponges miR-27b-3p → ↑ VEGF-C | In vitro | Oncogenic; CSC properties, angiogenesis | [67] |
| SNHG1 | Angiogenesis | HIF-1α/VEGF axis | In vitro (hypoxia) | Oncogenic; enhances angiogenesis | [68] |
| BANCR | EMT, Invasion | MAPK regulation | In vitro | Oncogenic; promotes invasion | [70] |
| DOCK9-AS2 | EMT, Stemness | SP1 binding; sponges miR-1972 → ↑ CTNNB1 | In vitro; exosomes | Oncogenic; EMT, stemness, metastasis | [74] |
| PVT1 | Metabolism, Angiogenesis | Stabilizes STAT3; glycolysis regulation | In vitro | Oncogenic; glycolysis and VEGFA activation | [27,65] |
| LINC00671 | Metabolic Reprogramming | Downregulates LDHA | In vitro | Tumor-suppressive; suppresses glycolysis | [84] |
| GLTC | Metabolic Reprogramming | LDH1 succinylation → ↑ lactate | In vitro | Oncogenic; drives glycolysis, radioiodine resistance | [85] |
| HOTAIR | Immune Evasion | ↑ PD-L1; exosomal | In vitro | Oncogenic; T cell suppression | [92] |
| NKILA | Immune Evasion | Induces CTL/T helper AICD | In vitro | Oncogenic; immune escape | [91] |
| MIAT | Immune Evasion, Prognosis | ↑ PD-1/PD-L1/CTLA4; EZH2 axis | In vitro; tissues | Oncogenic; poor survival and immune suppression | [19,94] |
| SLC26A4-AS1 | Genome Instability | Alters MRN complex → instability | In vitro | Tumor-suppressive; loss → metastasis | [29] |
| CamK-A | Inflammation, Angiogenesis | Activates PNCK → NF-κB | In vitro | Oncogenic; promotes inflammatory TME | [103,104] |
| LINC00887 | Proliferation, Invasion; Immune Evasion | Activates Wnt/β-catenin and Hippo; upregulates PD-L1 | In vitro (CRISPR knockdown) | Oncogenic; promotes PTC growth, invasion, and immune evasion via Wnt/Hippo and PD-L1 upregulation. | [115] |
| LIFR-AS1 | Proliferation, Angiogenesis, Invasion | Sponges miR-31-5p to upregulate SIDT2 | In vitro; in vivo (xenograft) | Tumor-suppressive; inhibits proliferation and angiogenesis by sponging miR-31-5p and upregulating SIDT2. | [116] |
| ASMTL-AS1 | Proliferation; Metabolic Reprogramming | Sponges miR-93-3p and miR-660 to upregulate FOXO1 | In vitro | Tumor-suppressive; inhibits growth and glycolysis via miR-93-3p/miR-660/FOXO1 axis. | [117] |
| RP11-547D24.1 | Proliferation, Apoptosis, Invasion | Unclear; tumor suppressor via TCGA analysis | In vitro (loss-of-function) | Tumor-suppressive; loss enhances proliferation and invasion while reducing apoptosis. | [118] |
| lnc-MPEG1-1 | Proliferation, Metastasis | Sponges miR-766-5p | In vitro; clinical samples | Oncogenic; promotes proliferation and metastasis by sponging miR-766-5p. | [119] |
| GHET1 | Proliferation, Invasion | Modulates cell-cycle/apoptosis; exact target unclear | In vitro; clinical samples | Oncogenic; enhances invasion and proliferation, linked to aggressive features. | [120] |
| UCA1 | Proliferation | Sponges miR-204 to upregulate BRD4 | In vitro; in vivo (mice) | Oncogenic; promotes cell proliferation via miR-204/BRD4 axis. | [121] |
| DUXAP8 | Proliferation, Invasion | Sponges miR-223-3p to upregulate CXCR4 | In vitro | Oncogenic; sponges miR-223-3p to increase CXCR4, promoting invasion and proliferation. | [122] |
| PAPAS | Proliferation | Downregulates oncogenic HOTTIP | In vitro; patient tissues | Tumor-suppressive; represses HOTTIP to inhibit proliferation. | [123] |
| LINC00924 | Proliferation, Invasion; Apoptosis | ICD-related; may impact immune microenvironment | In vitro; bioinformatic analysis | Tumor-suppressive; reduces proliferation, invasion, and increases apoptosis; may affect immune microenvironment. | [124] |
| SNHG3 | Proliferation, Metastasis | Inhibits AKT/mTOR/ERK pathway | In vitro; in vivo | Tumor-suppressive; inhibits growth and metastasis by regulating AKT/mTOR/ERK. | [125] |
| AK023507 | Proliferation, Metastasis | Inhibits Wnt/β-catenin signaling | In vitro | Tumor-suppressive; downregulates Wnt/β-catenin to inhibit proliferation and invasion. | [126] |
| LINC01614 | Proliferation, Invasion | Genomic instability-related; modulates TME | In vitro; patient data | Oncogenic; promotes PTC progression and alters tumor microenvironment. | [18] |
| LINC00704 | Proliferation, Invasion | Sponges miR-204-5p to upregulate HMGB1 | In vitro | Oncogenic; drives growth and motility via miR-204-5p/HMGB1 axis. | [127] |
| lncRNA BRM | Proliferation, Invasion | Sponges miR-331-3p to upregulate SLC25A1 | In vitro | Oncogenic; promotes PTC progression via miR-331-3p/SLC25A1 axis. | [128] |
| CCHE1 | Proliferation, Invasion | Activates ERK/MAPK pathway | In vitro; patient tissues | Oncogenic; activates ERK/MAPK pathway, promoting aggressive features. | [129] |
| LncRNA | Clinical Application | Expression Pattern | Patient Correlation | Diagnostic/Prognostic Value | Therapeutic Targeting | Ref. |
|---|---|---|---|---|---|---|
| ACVR2B-AS1 | Prognostic biomarker | Upregulated | High expression correlates with metastasis and poor survival | Elevated levels predict poor prognosis | siRNA knockdown affects miR-195-5p and FGF2 pathway | [58] |
| LUCAT1 | Prognostic biomarker and therapeutic target | Upregulated | Associated with advanced tumor stage and poor survival | Independent predictor of poor outcome | siRNA knockdown inhibits JAK-STAT pathway and tumor proliferation | [137] |
| SLC26A4-AS1 | Prognostic marker for metastasis | Downregulated | Low expression linked to lymph node metastasis and poor survival | Low levels predict higher metastatic risk and poor prognosis | Restoration may suppress EMT via DDX5 degradation | [29] |
| LINC00284 | Tumor suppressor; prognostic marker in panel | Downregulated | High expression associated with improved survival | Included in 3-lncRNA prognostic signature | Potential modulation via miR-205/E2F1 axis | [138] |
| LINC00704 | Prognostic biomarker and therapy target | Upregulated | High expression associated with aggressiveness and shorter survival | Independent predictor of poor outcome | siRNA-mediated silencing reduces cell growth and induces apoptosis | [97] |
| H19 | Diagnostic biomarker and immune infiltration marker | Downregulated | Loss of expression linked to poor OS; high expression linked to increased immune infiltration | Diagnostic and prognostic implications based on expression and immune infiltration | Suggested restoration or demethylation may be beneficial | [139] |
| lnc-MPEG1-1 | Diagnostic and predictive lncRNA | Upregulated | High expression correlates with lymph node metastasis | Part of a predictive nomogram for lymph node metastasis | Knockdown inhibits EMT via miR-766-5p axis | [119] |
| XIST | Therapeutic and diagnostic target | Upregulated | High expression positively correlates with MET and tumor progression | High XIST expression marks aggressive tumors | Silencing restores miR-34a and suppresses MET/PI3K/AKT pathway | [140] |
| LINC02471 and DOCK9-DT | Prognostic and immune-related markers | Upregulated | High expression associated with immune cell infiltration and risk scoring | Included in a prognostic model (m6A-related lncRNAs) | No direct targeting; potential through m6A modulation | [141] |
| MIAT | Prognostic biomarker and therapeutic target | Upregulated | High expression correlates with EZH2 and poorer survival | Predicts recurrence and overall survival | Knockdown affects miR-150-5p/EZH2 axis | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejazi, M.; Heshmat, R.; Shafiee, G.; Larijani, B.; Mokhtarzadeh, A.A.; Ebrahimi, V.; Tavangar, S.M. The Interplay Between lncRNAs–microRNAs Network Dysregulation and Cellular Hallmarks of Thyroid Cancer. Cancers 2025, 17, 3373. https://doi.org/10.3390/cancers17203373
Hejazi M, Heshmat R, Shafiee G, Larijani B, Mokhtarzadeh AA, Ebrahimi V, Tavangar SM. The Interplay Between lncRNAs–microRNAs Network Dysregulation and Cellular Hallmarks of Thyroid Cancer. Cancers. 2025; 17(20):3373. https://doi.org/10.3390/cancers17203373
Chicago/Turabian StyleHejazi, Maryam, Ramin Heshmat, Gita Shafiee, Bagher Larijani, Amir Ali Mokhtarzadeh, Vida Ebrahimi, and Seyed Mohammad Tavangar. 2025. "The Interplay Between lncRNAs–microRNAs Network Dysregulation and Cellular Hallmarks of Thyroid Cancer" Cancers 17, no. 20: 3373. https://doi.org/10.3390/cancers17203373
APA StyleHejazi, M., Heshmat, R., Shafiee, G., Larijani, B., Mokhtarzadeh, A. A., Ebrahimi, V., & Tavangar, S. M. (2025). The Interplay Between lncRNAs–microRNAs Network Dysregulation and Cellular Hallmarks of Thyroid Cancer. Cancers, 17(20), 3373. https://doi.org/10.3390/cancers17203373






