Ovarian Cancer in the Era of Precision Surgery and Targeted Therapies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. DNA Extraction, Library Preparation and Sequencing
2.3. Data Analyses
2.4. Statistical Analyses
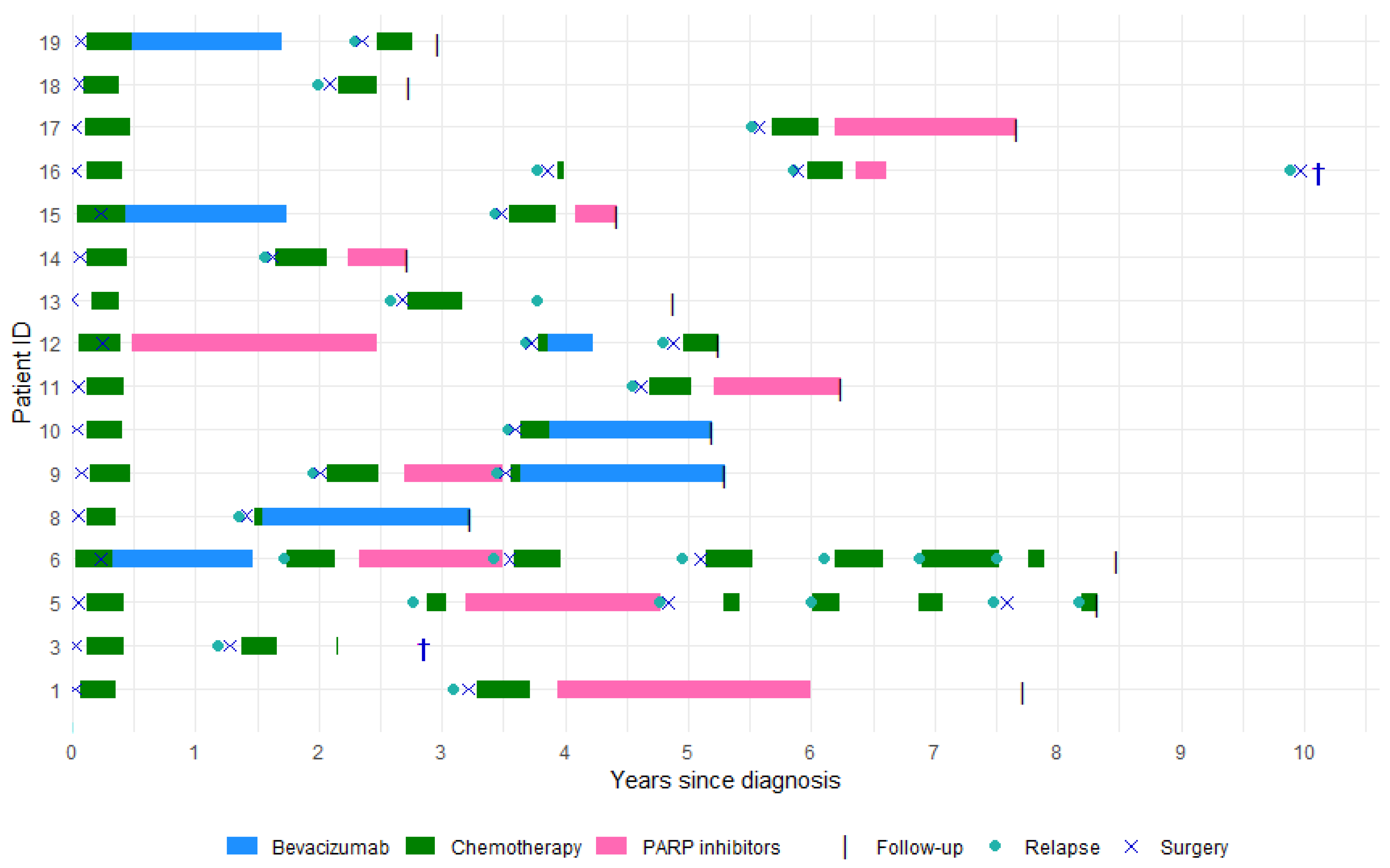
3. Results
3.1. Patients
3.2. Treatments
3.3. Pathogenic and Likely Pathogenic Mutations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMG | American College of Medical Genetics |
| AGO | Arbeitsgemeinschaft Gynäkologische Onkologie |
| AI | Artificial Intelligence |
| BARD1 | BRCA1 Associated RING Domain 1 |
| BRCA1/2 | Breast Cancer Gene 1/2 |
| CA125 | Cancer Antigen 125 |
| CI | Confidence Interval |
| CNV | Copy Number Variations |
| CT | Computed Tomography |
| DNA | Deoxyribonucleic Acid |
| ECOG | Eastern Cooperative Oncology Group |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| FIGO | International Federation of Gynecology and Obstetrics |
| GOG | Gynecologic Oncology Group |
| GOVEC | Gynecologic Oncology and Pelvis Mass Study |
| HGSC | High-grade Serous Carcinoma |
| HR | Hazard Ratio |
| HRD | Homologous Recombination Deficiency |
| Indel | Insertion-Deletion |
| MA | Massachusetts |
| MNV | Multi Nucleotide Variation |
| NF1 | Neurofibromin 1 |
| NOTCH2 | Neurogenic Locus Notch Homolog Protein 2 |
| OCA-Plus | Oncomine Comprehensive Assay Plus |
| OS | Overall Survival |
| PARP | Poly (ADP-ribose) Polymerase |
| PFS | Progression Free Survival |
| PDX | Patient-Derived Xenograft |
| PET-CT | Positron Emission Tomography-Computed Tomography |
| RAD51D | RAD51 Paralog D |
| RSC | Research Simplified Chemistry |
| SNV | Single Nucleotide Variation |
| SNP | Single Nucleotide Polymorphism |
| UK | United Kingdom |
References
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.; Pothuri, B.; Ray-Coquard, I.; Tan, D.; Bellet, E.; Oaknin, A.; et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Ovarian Cancer Continue Including Fallopian Tube Cancer and Primary Peritoneal Cancer; National Comprehensive Cancer Network: Philadelphia, PA, USA, 2024. [Google Scholar]
- Dansk Gynækologisk Cancer Gruppe (DGCG). Ovariecancer-Kirurgi Ved Recidiv; Dansk Gynækologisk Cancer Gruppe: Copenhagen, Denmark, 2022. [Google Scholar]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Dansk Gynækologisk Cancer Gruppe (DGCG). Ovariecancer-Edicinsk Recidivbehandling; Dansk Gynækologisk Cancer Gruppe: Copenhagen, Denmark, 2023. [Google Scholar]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Swisher, E.M.; Kwan, T.T.; Oza, A.M.; Tinker, A.V.; Ray-Coquard, I.; Oaknin, A.; Coleman, R.L.; Aghajanian, C.; Konecny, G.E.; O’mAlley, D.M.; et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Kondrashova, O.; Topp, M.; Nesic, K.; Lieschke, E.; Ho, G.Y.; Harrell, M.I.; Zapparoli, G.V.; Hadley, A.; Holian, R.; Boehm, E.; et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Smith, P.; Bradley, T.; Gavarró, L.M.; Goranova, T.; Ennis, D.P.; Mirza, H.B.; De Silva, D.; Piskorz, A.M.; Sauer, C.M.; Al-Khalidi, S.; et al. The copy number and mutational landscape of recurrent ovarian high-grade serous carcinoma. Nat. Commun. 2023, 14, 1–15. [Google Scholar] [CrossRef]
- Vestergaard, L.K.; Oliveira, D.N.P.; Poulsen, T.S.; Høgdall, C.K.; Høgdall, E.V. Oncomine TM Comprehensive Assay v3 vs. Oncomine TM Comprehensive Assay Plus. Cancers 2021, 13, 5230. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, S.; Smeets, D.; Moisse, M.; Braicu, E.I.; Vanderstichele, A.; Zhao, H.; Van Nieuwenhuysen, E.; Berns, E.; Sehouli, J.; Zeillinger, R.; et al. Genetic heterogeneity after first-line chemotherapy in high-grade serous ovarian cancer. Eur. J. Cancer 2016, 53, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Yfat, K.; Mariam, K.; Mario, B.; Hal, H.; Dana, J.; Lina, S.; Ilan, B.; Gregory, P.; Limor, H. Germline BRCA mutation carriers are more likely to undergo cytoreductive surgery for relapsed, platinum sensitive, ovarian cancer. Gynecol. Oncol. 2022, 167, 256–260. [Google Scholar] [CrossRef]
- Harter, P.; Hahmann, M.; Lueck, H.J.; Poelcher, M.; Wimberger, P.; Ortmann, O.; Canzler, U.; Richter, B.; Wagner, U.; Hasenburg, A.; et al. Surgery for recurrent ovarian cancer: Role of peritoneal carcinomatosis: Exploratory analysis of the DESKTOP i trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 1324–1330. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Friedman, R.L.; Spirtos, N.M. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer 2000, 88, 144–153. [Google Scholar] [CrossRef]
- Chi, D.S.; McCaughty, K.; Diaz, J.P.; Huh, J.; Schwabenbauer, S.; Hummer, A.J.; Venkatraman, E.S.; Aghajanian, C.; Sonoda, Y.; Abu-Rustum, N.R.; et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006, 106, 1933–1939. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Eriksson, A.G.; Yagel, I.; Chang, S.J.; Lim, M.C. Surgery for Recurrent Epithelial Ovarian Cancer. Curr. Oncol. Rep. 2024, 26, 46–54. [Google Scholar] [CrossRef]
- Hall, M.; Savvatis, K.; Nixon, K.; Kyrgiou, M.; Hariharan, K.; Padwick, M.; Owens, O.; Cunnea, P.; Campbell, J.; Farthing, A.; et al. Maximal-Effort Cytoreductive Surgery for Ovarian Cancer Patients with a High Tumor Burden: Variations in Practice and Impact on Outcome. Ann. Surg. Oncol. 2019, 26, 2943–2951. [Google Scholar] [CrossRef]
- Nikolaidi, A.; Papadopoulou, E.; Haidopoulos, D.; Liontos, M.; Fountzilas, E.; Tsaousis, G.; Goula, K.; Tsolaki, E.; Christopoulou, A.; Binas, I.; et al. Molecular Alterations in Paired Epithelial Ovarian Tumors in Patients Treated with Neoadjuvant Chemotherapy. Cancers 2024, 16, 3580. [Google Scholar] [CrossRef]
- Zalaznick, H.; Clegg, B.; Cogan, E.; Perry, M.; Trost, J.; Mancini-DiNardo, D.; Gutin, A.; Lanchbury, J.; Timms, K. Rates of homologous recombination deficiency across different subtypes of ovarian cancer and in pre- and post-neoadjuvant chemotherapy tumor samples (139.5). Gynecol. Oncol. 2022, 166, S86–S87. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Phillips, M.R.; Kaiser, P.; Thabane, L.; Bhandari, M.; Chaudhary, V. Retina Evidence Trials International Alliance Study Group. Risk of bias: Why measure it, and how? Eye 2022, 36, 346–348. [Google Scholar] [CrossRef]
- Muzzey, D.; Evans, E.A.; Lieber, C. Understanding the Basics of NGS: From Mechanism to Variant Calling. Curr. Genet. Med. Rep. 2015, 3, 158–165. [Google Scholar] [CrossRef]
- Altmann, A.; Weber, P.; Bader, D.; Preuss, M.; Binder, E.B.; Müller-Myhsok, B. A beginners guide to SNP calling from high-Throughput DNA-sequencing data. Hum. Genet. 2012, 131, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Kordowitzki, P.; Lange, B.; Elias, K.M.; Haigis, M.C.; Mechsner, S.; Braicu, I.E.; Sehouli, J. Transforming treatment paradigms: Focus on personalized medicine for high-grade serous ovarian cancer. CA Cancer J. Clin. 2025, 75, 436–460. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | Secondary Cytoreductive Surgery | |
|---|---|---|
| Age in years median (range) | 64 (41–74) | 67 (46–77) |
| CA125 median (range) | 195 (14–8890) | 35 (6–490) |
| BMI median (range) | 24 (18–34) | 24 (18–35) |
| Performance score | ||
| 0 | 9 (56%) | 14 (87.5%) |
| 1 | 6 (38%) | 2 (12.5%) |
| 2 | 1 (6%) | - |
| FIGO stage | ||
| I-II | 4 (25%) | - |
| III-IV | 12 (75%) | - |
| Surgery | ||
| Primary debulking | 13 (81%) | - |
| Interval debulking | 3 (19%) | - |
| Residual tumor after surgery | ||
| 0 | 16 (100%) | 14 (87.5%) |
| Unresectable | 0 | 2 (12.5%) |
| Platinum response | ||
| >12 months (sensitive) | 14 (87.5%) | - |
| 6 – ≤ 12 months (partial sensitive) | 2 (12.5%) | - |
| Follow-up in months | ||
| Time to first relapse median (range) | - | 32 (14–66) |
| Total follow-up time median (range) | - | 63 (33–122) |
| Patient ID | Diagnosis | Relapse |
| 1 | BRCA2 (c.7876T>C p.Trp2626Arg exon 17) TP53 (c.422G>A p.Cys141Tyr exon 5) | BRCA2 TP53 |
| 3 | TP53 (c.810T>G p.Phe270Leu exon 8) | TP53 |
| 5 | BRCA2(c. 37G>T p.Glu13Ter exon 2) | BRCA2 |
| 6 | BRCA1 (c.115T>G p.Cys39Gly exon 3) TP53 (c.1028delA p.Glu343GlyfsTer2 exon 10) | BRCA1 TP53 |
| 8 | FANCD2 (c.935delT p.Leu312CysfsTer10 exon 12) | FANCD2 |
| 9 | ILR7 (c.636T>A p.Tyr212Ter exon 5) TP53 (c.659A>G p.Tyr220Cys exon 6) | ILR7 TP53 NOTCH2 (c.6575_6605del p.Pro2192LeufsTer20 exon 34) |
| 10 | TP53 (c.916C>T p.Arg306Ter exon 8) | TP53 |
| 11 | TP53 (c.839G>A p.Arg280Lys exon 8) | TP53 |
| 12 | BRCA2 (c.5903C>G p.Ser1968Ter exon 11) TP53 (c.150_151insT p.Glu51Ter exon 4) | BRCA2 TP53 |
| 13 | TP53 (c.536A>G p.His179Arg exon 5) | TP53 |
| 14 | TP53 (c.742C>T p.Arg248Trp exon7) | TP53 |
| 15 | TP53 (c.734G>A p.Gly245Asp exon 7) | TP53 |
| 16 | BRCA2 (c.5346G>A p.Trp1782Ter exon 21) RB1 (c.37_40delGCCG p.Ala13ProfsTer51 exon1) TP53 (c.339_341delCTT p.Phe113del exon 4) | BRCA2 RB1 TP53 |
| 17 | BRCA2 (c.2454delT p.Gln819LysfsTer6 exon 11) TP53 (c.731G>A p.Gly244Asp exon 7) | BRCA2 TP53 |
| 18 | TP53 (c.659A>C p.Tyr220Ser exon 6) | TP53 |
| 19 | TP53 (c.1109C>T p.Arg337Cys exon 10) | TP53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisman, Y.; Poulsen, T.S.; Schnack, T.H.; Høgdall, C.; Høgdall, E. Ovarian Cancer in the Era of Precision Surgery and Targeted Therapies. Cancers 2025, 17, 3371. https://doi.org/10.3390/cancers17203371
Sisman Y, Poulsen TS, Schnack TH, Høgdall C, Høgdall E. Ovarian Cancer in the Era of Precision Surgery and Targeted Therapies. Cancers. 2025; 17(20):3371. https://doi.org/10.3390/cancers17203371
Chicago/Turabian StyleSisman, Yagmur, Tim Svenstrup Poulsen, Tine Henrichsen Schnack, Claus Høgdall, and Estrid Høgdall. 2025. "Ovarian Cancer in the Era of Precision Surgery and Targeted Therapies" Cancers 17, no. 20: 3371. https://doi.org/10.3390/cancers17203371
APA StyleSisman, Y., Poulsen, T. S., Schnack, T. H., Høgdall, C., & Høgdall, E. (2025). Ovarian Cancer in the Era of Precision Surgery and Targeted Therapies. Cancers, 17(20), 3371. https://doi.org/10.3390/cancers17203371







