The Role of MUC1 in Gastric Cancer Development
Simple Summary
Abstract
1. Introduction
2. Gastric Cancer
3. Mucins
4. MUC1
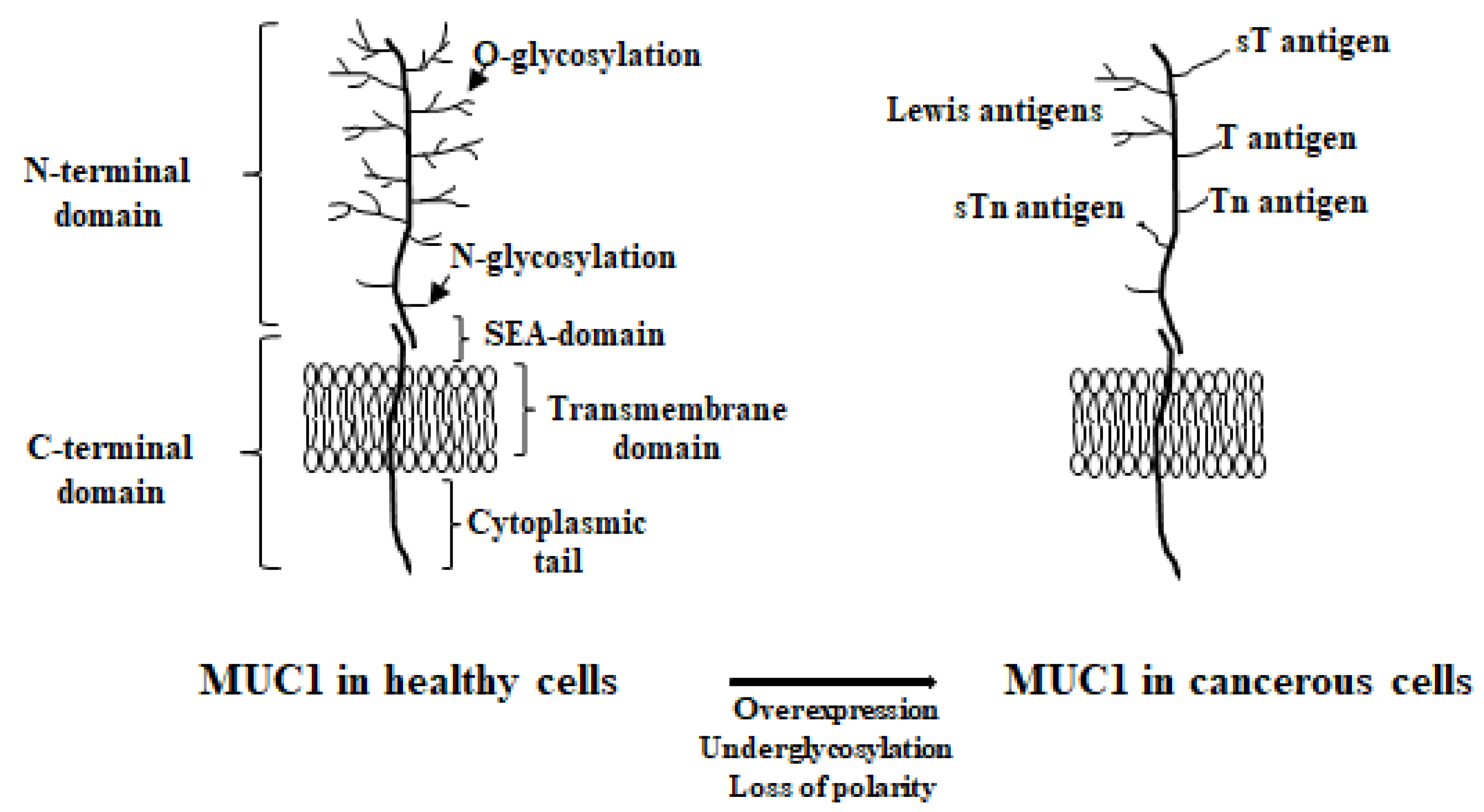
4.1. MUC1’s Structure
4.2. MUC1’s General Function
5. MUC1 in Gastric Cancer
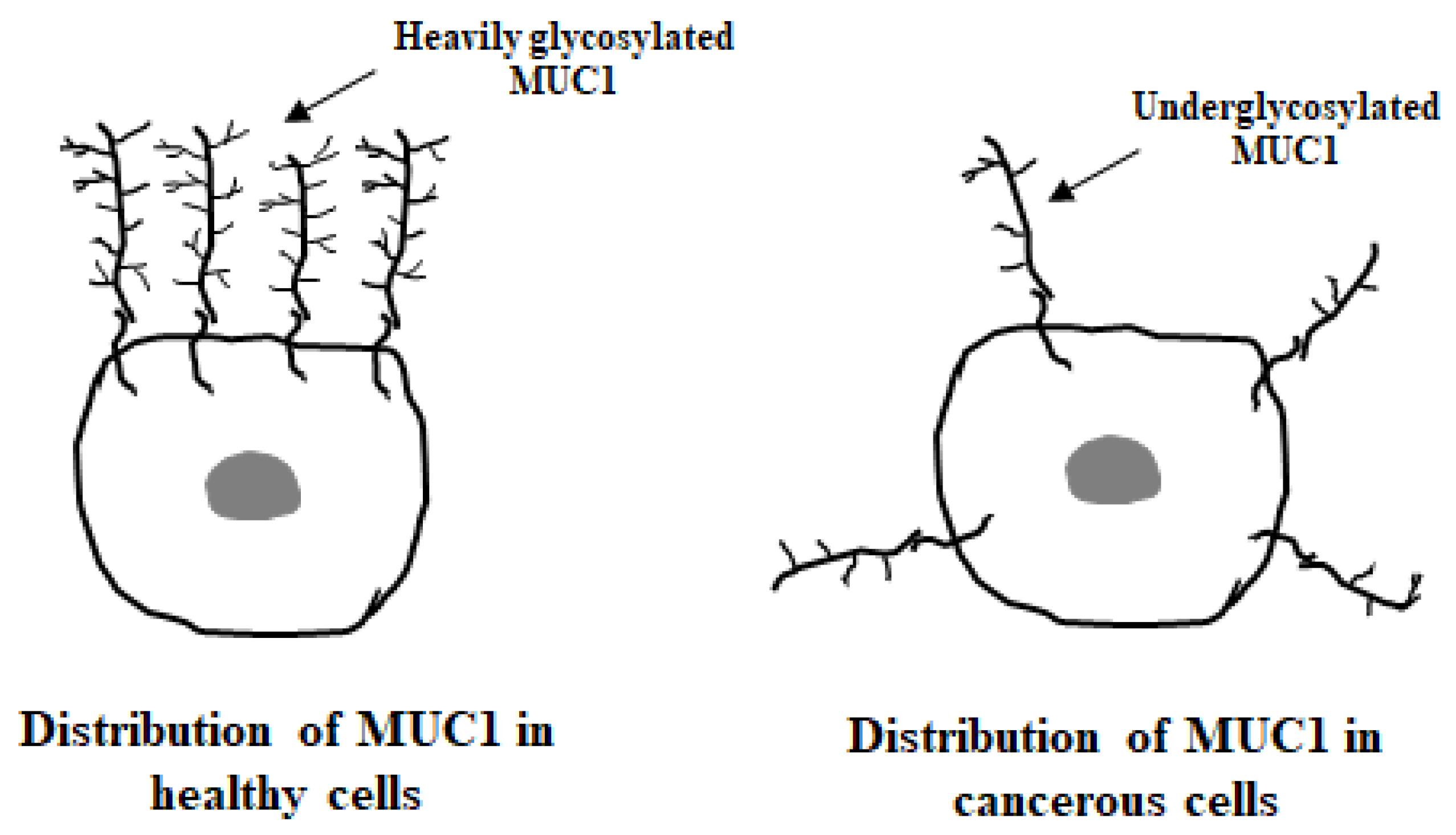
5.1. MUC1 Localization
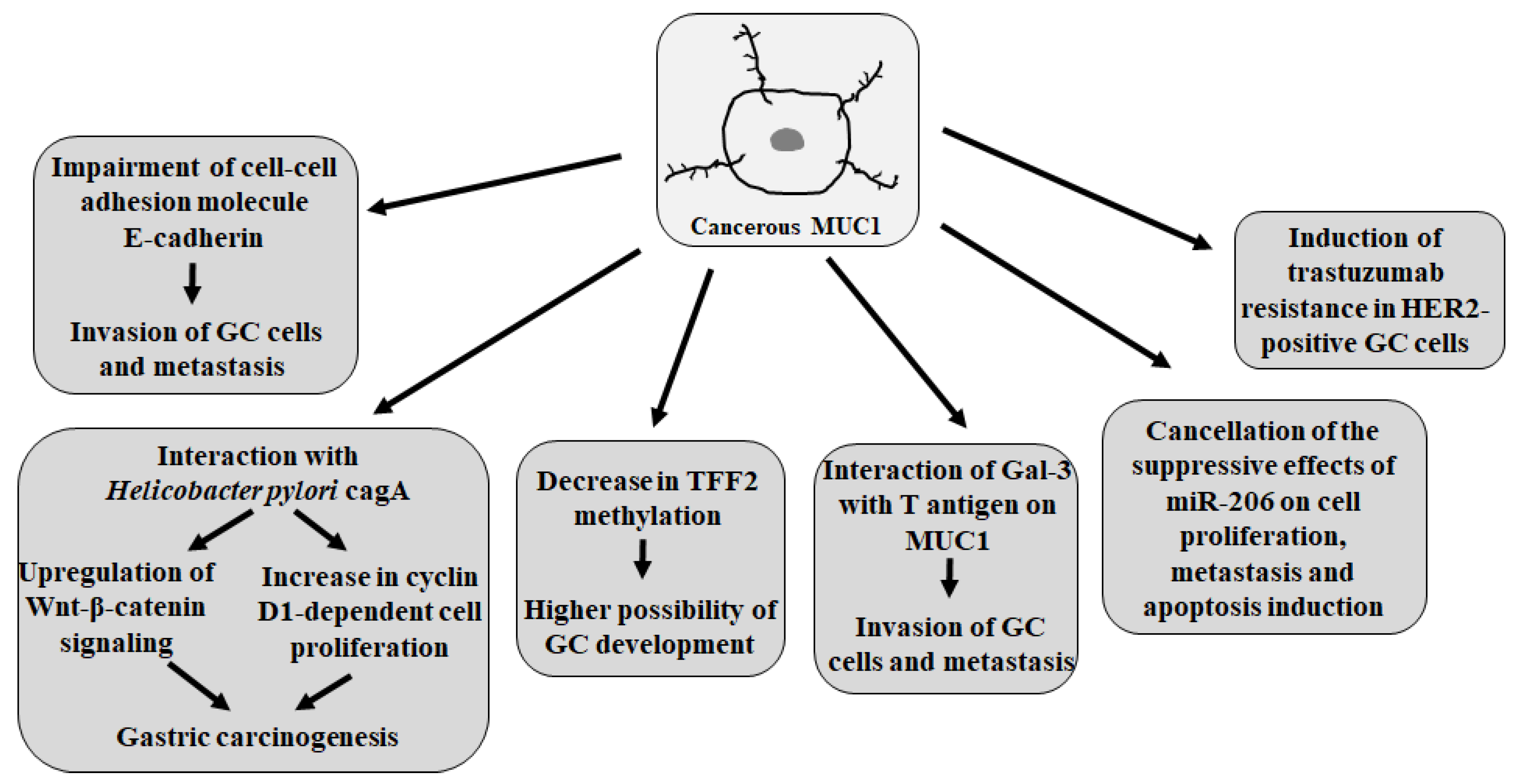
5.2. MUC1 Action in GC
5.3. VNTR Polymorphism of MUC1 Gene and the Risk of Stomach Cancer
5.4. MUC1 as Prognostic Factor
5.5. MUC1 as Therapeutic Target
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, S.; Boyle, T.; Ahmed, M.; Lee, S.H.; Benyamin, B.; Hypponen, E. Lifestyle, genetic risk and coincidence of cancer: A prospective cohort study of 13 cancer types. Int. J. Epidemiol. 2023, 52, 817–826. [Google Scholar] [CrossRef]
- Lopez-Plaza, B.; Loria-Kohen, V.; Gonzalez-Rodriguez, L.G.; Fernandez-Cruz, E. Diet and lifestyle in cancer prevention. Nutr. Hosp. 2022, 39, 74–77. [Google Scholar]
- Scott, J.; Basanta, D.; Marusyk, A. Darwinism, Not Mutationalism, for New Cancer Therapies. In Rethinking Cancer, A New Paradigm for the Postgenomics Era; Strauss, B., Bertolaso, M., Emberg, I., Bissell, M.J., Eds.; The MIT Press: Cambridge, MA, USA; London, UK, 2021; pp. 187–205. [Google Scholar]
- Strauss, B.; Bertolaso, M.; Emberg, I.; Bissell, M.J. Rethinking Cancer, A New Paradigm for the Postgenomics Era; The MIT Press: Cambridge, MA, USA; London, UK, 2021. [Google Scholar]
- Sackman, R. Rethinking Cancer: Non-Traditional Approaches to the Theories, Treatments, and Prevention of Cancer; Square One Publishers: Garden City Park, NY, USA, 2003; p. 111. [Google Scholar]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Balady, G.J. Survival of the fittest—More evidence. N. Engl. J. Med. 2002, 346, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Gendler, S.J. MUC1, the renaissance molecule. J. Mammary Gland. Biol. Neoplasia 2001, 6, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Kim, Y.I.; Pecha, R.L.; Keihanian, T.; Mercado, M.; Pena-Munoz, S.V.; Lang, K.; Van Buren, G.; Dhingra, S.; Othman, M.O. MUC1 expressions and its prognostic values in US gastric cancer patients. Cancers 2023, 15, 998. [Google Scholar] [CrossRef]
- Wang, X.T.; Kong, F.B.; Mai, W.; Li, L.; Pang, L.M. MUC1 immunohistochemical expression as a prognostic factor in gastric cancer: Meta-analysis. Dis. Markers 2016, 2016, 9421571. [Google Scholar] [CrossRef]
- Yang, W.I.; Zhap, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef]
- Petryszyn, P.; Chapelle, N.; Matysiak-Budnik, T. Gastric cancer: Where are we heading? Dig. Dis. 2020, 38, 280–285. [Google Scholar] [CrossRef]
- Bao, X.; Ye, H.; Chen, Z.; Chen, W.; Xiao, Y.; Wu, X.; Li, Z. C1GALT1-mediated O-glycan T antigen increase enhances the migration and invasion ability of gastric cancer cells. Biochem. Biophys. Res. Commun. 2024, 734, 150641. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Wei, Z.J.; Xu, A.M.; Zang, J.H. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int. J. Surg. 2018, 56, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Identification of novel biomarkers, MUC5AC, MUC1, KRT7, GAPDH, CD44 for gastric cancer. Med. Oncol. 2020, 37, 34. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Wang, Y.A. Gastric cancer immunosuppressive microenvironment heterogeneity: Implications for therapy development. Trends Cancer 2024, 10, 627–642. [Google Scholar] [CrossRef]
- Van Custem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Koemans, W.J.; Lurvink, R.J.; Grootscholten, C.; Verhoven, R.H.A.; Hingh, I.H.; Sandick, J.W. Synchronous peritoneal metastases of gastric cancer origin: Incidence, treatment and survival of a nationwide Dutch cohort. Gastric Cancer 2021, 24, 800–809. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Patel, T.H.; Cecchini, M. Targeted therapies in advanced gastric cancer. Curr. Treat. Options Oncol. 2020, 21, 70. [Google Scholar] [CrossRef]
- Gautam, S.K.; Khan, P.; Natarajan, G.; Atri, P.; Aithal, A.; Ganti, A.K.; Batra, S.K.; Nasser, M.W.; Jain, M. Mucins as potential biomarkers for early detection of cancer. Cancers 2023, 15, 1640. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Dang, N.D.T.; Vu, Q.V.; Dang, A.K.; Ta, T.V. A model for gastric cancer risk prediction based on MUC1 polymorphisms and health-risk behaviors in a Vietnamese population. In Vivo 2023, 37, 2347–2356. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Bian, S.; Wang, Y.; Zhou, Y.; Wang, W.; Guo, L.; Wen, l.; Fu, W.; Zhou, X.; Tang, F. Integrative single-cell multiomics analyses dissect molecular signatures of intratumoral heterogeneities and differentiation states of human gastric cancer. Natl. Sci. Rev. 2023, 10, nwad094. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hormazabal, P.; Retamales-Ortega, R.; Musleh, M.; Bustamante, M.; Stambuk, J.; Pisano, R.; Valladares, H.; Lanzarini, E.; Chlong, H.; Suazo, J.; et al. Polymorphisms PSCA rs2294008, IL-4 rs2243250 and MUC1 rs4072037 are associated with gastric cancer in a high risk population. Mol. Biol. Rep. 2020, 47, 9239–9243. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Persson, C.; Hou, L.; Zheng, Z.; Yeager, M.; Liossowska, J.; Chanock, S.J.; Chow, W.H.; Ye, W. A comprehensive analysis of common genetic variation in MUC1, MUC5AC, MUC6 genes and risk of stomach cancer. Cancer Causes Control 2010, 21, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, I.; Yook, J.H.; Song, K.; Kim, B.S. Association between the MUC1 rs4072037 polymorphism and risk of gastric cancer and clinical outcomes. J. Gastric Cancer 2020, 20, 127–138. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Ge, Y.; Ma, G.; Liu, H.; Lin, Y.; Zhang, G.; Du, M.; Wang, M.; Chu, H.; Zhang, H.; Zhang, Z. MUC1 is associated with TFF2 methylation in gastric cancer. Clin. Epigenetics 2020, 12, 37. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2013, 42, 211–217. [Google Scholar] [CrossRef]
- Lopez, M.J.; Carbajal, J.; Alfaro, A.L.; Saravia, L.G.; Zanabria, D.; Araujo, J.M.; Quispe, L.; Zevallos, A.; Buleje, J.L.; Cho, C.E.; et al. Characteristics of gastric cancer around the world. Crit. Rev. Oncol. Hematol. 2023, 181, 103841. [Google Scholar] [CrossRef]
- Tan, P.; Yeoh, K.G. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology 2015, 149, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu-Burdowski, J.; Park, J. Treatment of early gastric cancer. Surg. Clin. N. Am. 2025, 105, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Peleteiro, B.; Malvezzi, M.; Bosetti, C.; Bertuccio, P.; Levi, F.; Negri, E.; La Vecchia, C.; Lunet, N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur. J. Cancer 2014, 50, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef]
- Brockhausen, I.; Melamed, J. Mucins as anti-cancer targets: Perspectives of the glycobiologist. Glycoconj. J. 2021, 38, 459–474. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef]
- Singh, P.K.; Hollingsworth, M.A. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006, 16, 467–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Lei, C.; Li, W.; Han, J.; Zhang, J.; Zhang, Y. A sweet warning: Mucin-type O-glycans in cancer. Cells 2022, 11, 3666. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Magalhaes, A.; Gomes, J.; Peixoto, A.; Gaiteiro, C.; Fernandes, E.; Santos, L.L.; Reis, C.A. Protein glycosylation in gastric and colorectal cancers: Toward cancer detection and targeted therapeutics. Cancer Lett. 2017, 387, 32–45. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Hauselmann, I.; Borsig, L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014, 4, 28. [Google Scholar] [CrossRef]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polonia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Burchell, J.M.; Beatson, R.; Graham, R.; Taylor-Papadimitriou, J.; Tajadura-Ortega, V. O-linked mucin-type glycosylation in breast cancer. Biochem. Soc. Trans. 2018, 46, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2016, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, R.; Taravati, A.; Hashemi-Soeth, M.B. Association of MUC1 5640G>A and PSCA 5057C>T polymorphisms with the risk of gastric cancer in Northern Iran. MBC Med. Genet. 2020, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, D.M.; Cudic, M. Tumor-associated O-glycans of MUC1: Carriers of the glycol-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Qing, L.; Li, Q.; Dong, Z. MUC1: An emerging target in cancer treatment and diagnosis. Bull. Cancer 2022, 109, 1202–1216. [Google Scholar] [CrossRef]
- Cascio, S.; Finn, O.J. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules 2016, 6, 39. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Ju, T.; Heimburg-Molinaro, J.; Cummings, R.D. Simple sugars to complex disease-mucin-type O-glycans in cancer. Adv. Cancer Res. 2015, 126, 53–135. [Google Scholar]
- Parry, S.; Hanish, F.G.; Leir, S.H.; Sutton-Smith, M.; Morris, H.R.; Dell, A.; Harris, A. N-Glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology 2006, 16, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sandrine, I.K.; Yang, M.; Tu, J.; Yuan, X. MUC1 and MUC16: Critical for immune modulation in cancer therapeutics. Front. Immunol. 2024, 15, 1356913. [Google Scholar] [CrossRef] [PubMed]
- Piyush, T.; Rhodes, J.M.; Yu, L.G. MUC1 O-glycosylation contributes to anoikis resistance in epithelial cancer cells. Cell Death Discov. 2017, 3, 17044. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Mensdorff-Pouilly, S.; Goletz, S. What makes MUC1 a tumor antigen? Tumor Biol. 2005, 26, 217–220. [Google Scholar] [CrossRef]
- Pinto, R.; Carvalho, A.S.; Conze, T.; Magalhaes, A.; Picco, G.; Burchell, J.M.; Taylor-Papadimitriou, J.; Reis, C.A.; Almeida, R.; Mandel, U.; et al. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell. Mol. Med. 2012, 16, 1474–1484. [Google Scholar] [CrossRef]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-Tn in cancer: (How) did we miss the target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef]
- Cazet, D.; Julien, S.; Bobowski, M.; Burchell, J.; Delannoy, P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010, 12, 204. [Google Scholar] [CrossRef]
- Sindrewicz, P.; Lian, L.Y.; Yu, L.G. Interaction of the oncofetal Thomsen-Friedenreich antigen with galectins in cancer progression and metastasis. Front. Oncol. 2016, 6, 79. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Linden, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Giraldi, L.; Michelazzo, M.B.; Arzani, D.; Persiani, R.; Pastorina, R.; Boccia, S. MUC1, MUC5AC, and MUC6 polymorphisms, Helicobacter pylori infection, and gastric cancer: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2018, 27, 323–330. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Every, A.L.; Skene, C.D.; Linden, S.K.; Chionh, Y.T.; Swierczak, A.; McAuley, J.; Harbour, S.; Kaparakis, M.; Ferrero, R.; et al. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 2007, 133, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.S.; Yung, K.K.L. MUC1: Structure, function, and clinic application in epithelial cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, J.P.M.; Strijbis, K. Transmembrane Mucins: Signaling receptors at the intersection of inflammation and cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef]
- Marczynski, M.; Jiang, K.; Blakeley, M.; Srivastava, V.; Vilaplana, F.; Crouzier, T.; Lieleg, O. Structural alterations of mucins are associated with losses in functionality. Biomacromolecules 2021, 22, 1600–1613. [Google Scholar] [CrossRef]
- Li, W.; Zhang, N.; Jin, C.; Long, M.D.; Rajabi, H.; Yasumizu, Y.; Fushimi, A.; Yamashita, N.; Hagiwara, M.; Zheng, R.; et al. MUC1-C drives stemness in progression of colitis to colorectal cancer. JCI Insight 2020, 5, e137112. [Google Scholar] [CrossRef]
- Kufe, D.M. MUC1-C in chronic inflammation and carcinogenesis; emergence as a target for cancer treatment. Carcinogenesis 2020, 41, 1173–1183. [Google Scholar] [CrossRef]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.L.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef]
- Rajabi, H.; Tagde, A.; Alam, M.; Bouillez, A.; Pitroda, S.; Suzuki, Y.; Kufe, D. DNA methylation by DNMT1 and DNMT3b methyltransferases is driven by the MUC1-C oncoprotein in human carcinoma cells. Oncogene 2016, 35, 6439–6445. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Maeda, T.; Bouillez, A.; Alam, M.; Tagde, A.; Hinohara, K.; Suzuki, Y.; Markert, T.; Miyo, M.; Komura, K.; et al. MUC1-C activates BMI1 in human cancer cells. Oncogene 2017, 36, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Liao, X.; Lv, Y.; Pang, Z.; Wang, Y.; Li, Q.; Liao, Y.; Ye, Q.; Chen, G.; Zhao, K.; et al. MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR-dependent manner. Cell Death Dis. 2017, 8, e2980. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cang, W.; Li, Q.; Liao, X.; Zhan, M.; Deng, H.; Li, S.; Jin, W.; Pang, Z.; Qiu, X.; et al. Erlotinib overcomes paclitaxel-resistant cancer stem cells by blocking the EGFR-CREB/GRbeta-IL-6 axis in MUC1-positive cervical cancer. Oncogenesis 2019, 8, 70. [Google Scholar] [CrossRef]
- Hagiwara, M.; Yasumizu, Y.; Yamashita, N.; Rajabi, H.; Fushimi, A.; Long, M.D.; Li, W.; Bhattacharya, A.; Ahmad, R.; Oya, M.; et al. MUC1-C activates the BAF (mSWI/SNF) complex in prostate cancer stem cells. Cancer Res. 2021, 81, 1111–1122. [Google Scholar] [CrossRef]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef]
- Zhang, H.K.; Zhang, Q.M.; Zhao, T.H.; Li, Y.Y.; Yi, Y.F. Expression of mucins and E-cadherin in gastric carcinoma and their clinical significance. World J. Gastroenterol. 2004, 10, 3044–3047. [Google Scholar] [CrossRef]
- Saeki, N.; Sakamoto, H.; Yoshida, T. Mucin 1 gene (MUC1) and gastric-cancer susceptibility. Int. J. Mol. Sci. 2014, 15, 7958–7973. [Google Scholar] [CrossRef]
- Ohno, T.; Aihara, R.; Kamiyama, Y.; Mochiki, E.; Asao, T.; Kuwano, H. Prognostic significance of combined expression of MUC1 and adhesion molecules in advanced gastric cancer. Eur. J. Cancer 2006, 42, 256–263. [Google Scholar] [CrossRef]
- Chan, A.O.O. E-cadherin in gastric cancer. World J. Gastroenterol. 2006, 12, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kitajima, Y.; Sato, S.; Miyazaki, K. Combined evaluation of mucin antigen and E-cadherin expression may help select patients with gastric cancer suitable for minimally invasive therapy. Br. J. Surg. 2003, 90, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Ng, G.Z.; Sutton, P. How host regulation of Helicobacter pylori-induced gastritis protects against peptic ulcer disease and gastric cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G514–G520. [Google Scholar] [CrossRef] [PubMed]
- Vinall, L.E.; King, M.; Novelli, M.; Green, C.A.; Daniels, G.; Hilkens, J.; Sarner, M.; Swallow, D.M. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology 2002, 123, 41–49. [Google Scholar] [CrossRef]
- Chiba, T.; Marusawa, H.; Seno, H.; Watanabe, N. Mechanism for gastric cancer development by Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2008, 23, 1175–1181. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Guang, W.; Ding, H.; Czinn, S.J.; Blanchard, T.G. Helicobacter pylori and gastric inflammation: Role of MUC1 mucin. J. Pediatr. Biochem. 2012, 2, 125–132. [Google Scholar] [CrossRef]
- Boltin, D.; Niv, Y. Mucins in gastric cancer—An update. J. Gastrointest. Dig. Syst. 2013, 3, 15519. [Google Scholar] [CrossRef]
- Guang, W.; Czinn, S.J.; Blanchard, T.G.; Kim, K.C.; Lillehoj, E.P. Genetic regulation of MUC1 expression by Helicobacter pylori in gastric cancer cells. Biochem. Biophys. Res. Commun. 2014, 445, 145–150. [Google Scholar] [CrossRef]
- Linden, S.K.; Sheng, Y.H.; Every, A.L.; Miles, K.M.; Skoog, E.C.; Florin, T.H.J.; Sutton, P.; McGuckin, M.A. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009, 5, e1000617. [Google Scholar] [CrossRef]
- Ng, G.Z.; Menheniott, T.R.; Every, A.L.; Stent, A.; Judd, L.M.; Chionh, Y.T.; Dhar, P.; Komen, J.C.; Giraud, A.S.; Wang, T.C.; et al. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut 2016, 65, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Semper, R.P.; Mejias-Luque, R.; Gross, C.; Anderl, F.; Muller, A.; Vieth, M.; Busch, D.H.; Prazeres da Costa, C.; Ruland, J.; Gross, O.; et al. Helicobacter pylori-induced IL-1beta secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J. Immunol. 2014, 193, 3566–3576. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, F.; Fonseca, A.; Caffrey, T.; Carvalho, F.; Mesquita, P.; Reis, C.; David, L.; Hollingsworth, M.A. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology 2005, 15, 511–517. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, an T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Fujii, C.; Harumiya, S.; Sato, Y.; Kawakubo, M.; Matoba, H.; Nakayama, J. α1,4-Linked N-acetylglucosamine suppresses gastric cancer development by inhibiting Mucin-1-mediated signaling. Cancer Sci. 2022, 113, 3852–3863. [Google Scholar] [CrossRef]
- Hoffman, W. Trefoil factors TFF (trefoil factyopr family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 2005, 62, 2932–2938. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Yang, X.; Liu, Y.; Shi, Y.; Ren, J.; Guleng, B. miRNA423-5p regulates cell proliferation and invasion by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett. 2014, 347, 98–104. [Google Scholar] [CrossRef]
- Sasaki, M.; Ikeda, H.; Nakanuma, Y. Expression profiles of MUC mucins and trefoil factor family (TFF) peptides in the intrahepatic biliary system: Physiological distribution and pathological significance. Prog. Histochem. Cytochem. 2007, 42, 61–110. [Google Scholar] [CrossRef]
- Longman, R.J.; Poulsom, R.; Corfield, A.P.; Warren, B.F.; Wright, N.A.; Thomas, M.G. Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. J. Histochem. Cytochem. 2006, 54, 1335–1348. [Google Scholar] [CrossRef]
- Deng, M.; Qin, Y.; Chen, X.; Wang, Q.; Wang, J. MiR-206 inhibits proliferation, migration, and invasion of gastric cancer cells by targeting the MUC1 gene. OncoTargets Ther. 2019, 12, 849–859. [Google Scholar] [CrossRef]
- Deng, M.; Jing, D.D.; Meng, X.J. Effect of MUC1 siRNA on drug resistance of gastric cancer cells to trastuzumab. Asian Pac. J. Cancer Prev. 2013, 14, 127–131. [Google Scholar] [CrossRef]
- Silva, F.; Carvalho, F.; Peixoto, A.; Seixas, M.; Almeida, R.; Carneiro, F.; Mesquita, P.; Figueiredo, C.; Nogueira, C.; Swallow, D.M.; et al. MUC1 gene polymorphism in the gastric carcinogenesis pathway. Eur. J. Hum. Genet. 2001, 9, 548–552. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, X.; Chang, J.; Tang, W.; Gan, L.; Wu, Z.; Li, J. MUC1 gene polymorphism rs4072037 and susceptibility to gastric cancer: A meta-analysis. Springerplus 2014, 3, 599. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zeng, M. Role of MUC1 rs4072037 polymorphism in gastric cancer: A meta-analysis. Int. J. Clin. Exp. Pathol. 2020, 13, 465–472. [Google Scholar] [PubMed]
- Shekarriz, R.; Jabbari, H.; Alikhani, R.; Alizadeh-Navaei, R.; Hashemi-Soteh, M.B. Association between MUC1 rs4072037 polymorphism and Helicobacter pylori in patients with gastric cancer. Caspian J. Intern. Med. 2024, 15, 132–140. [Google Scholar] [PubMed]
- Hu, N.; Wang, Z.; Song, X.; Wei, L.; Kim, B.S.; Freedman, N.D.; Baek, J.; Burdette, L.; Chang, J.; Chung, C.; et al. Genome wide association study of gastric adenocarcinoma in Asia: A comparison of associations between cardia and non-cardia tumours. Gut 2016, 65, 1611–1618. [Google Scholar] [CrossRef]
- Mocellin, S.; Verdi, D.; Pooley, K.A.; Nitti, D. Genetic variation and gastric cancer risk: A field synopsis and meta-analysis. Gut 2015, 64, 1209–1219. [Google Scholar] [CrossRef]
- Radziejewska, I. Galectin-3 and epithelial MUC1 mucin—Interactions supporting cancer development. Cancers 2023, 15, 2680. [Google Scholar] [CrossRef]
- Duarte, H.O.; Freitas, D.; Gomes, C.; Gomes, J.; Magalhaes, A.; Reis, C.A. Mucin-type O-glycosylation in gastric carcinogenesis. Biomolecules 2016, 6, 33. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; McKenzie, I.F.C. Cellular Mucins: Targets for immunotherapy. Crit. Rev. Immunol. 2017, 37, 421–437. [Google Scholar] [CrossRef]
- Yu, H.; Ye, C.; Li, J.; Pan, c.; Lin, W.; Chen, H.; Zhou, Z.; Ye, Y. An altered HLA-A0201-restricted MUC1 epitope that could induce more efficient anti-tumor effects against gastric cancer. Exp. Cell Res. 2020, 390, 111953. [Google Scholar] [CrossRef] [PubMed]
- Alsadoun, L.; Hassan, H.U.; Kalansuriya, I.; Bai, R.; Raut, Y.; Jameel, H.; Rehman, A.; Kadri, F.; Anika, N.N.; Khattak, A.U.; et al. Genetic markers of susceptibility in gastric cancer: A comprehensive systematic review. Cureus 2024, 16, e68358. [Google Scholar] [CrossRef] [PubMed]
- Dantas, R.N.; Souza, A.M.; Herrero, S.S.; Kassab, P.; Malheiros, C.A.; Lima, E.M. Association between PSCA, TNF-α, PARP1, and TP53 gene polymorphisms and gastric cancer susceptibility in the Brazilian population. Asian Pac. J. Cancer Prev. 2020, 21, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Durães, C.; Almeida, G.M.; Seruca, R.; Oliveira, C.; Carneiro, F. Biomarkers for gastric cancer: Prognostic, predictive or targets of therapy? Virchows Arch. 2014, 464, 367–378. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, H.K.; Kim, H.S.; Yang, H.K.; Kim, Y.I.; Kim, W.H. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: Their roles as prognostic indicators. Cancer 2001, 92, 1427–1434. [Google Scholar] [CrossRef]
- Baldus, S.E.; Zirbes, T.K.; Engel, S.; Hanish, F.G.; Monig, S.P.; Lorenzen, J.; Glossmann, J.; Fromm, S.; Thiele, J.; Pichlamaier, H.; et al. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int. J. Cancer 1998, 79, 133–138. [Google Scholar] [CrossRef]
- Liu, X.; Yi, C.; Wen, Y.; Radhakrishnan, P.; Tremayne, J.R.; Dao, T.; Johnson, K.R.; Hollingshworth, M.A. Interactions between MUC1 and p120 catenin regulate dynamic features of cell adhesion, motility, and metastasis. Cancer Res. 2014, 74, 1609–1620. [Google Scholar] [CrossRef]
- Tamura, Y.; Higashi, M.; Kitamoto, S.; Yokoyama, S.; Osako, M.; Horinouchi, M.; Shimizu, T.; Tabata, M.; Batra, S.K.; Goto, M.; et al. MUC4 and MUC1 expression in adenocarcinoma of the stomach correlates with vessel invasion and lymph node metastasis: An immunohistochemical study of early gastric cancer. PLoS ONE 2012, 7, e49251. [Google Scholar] [CrossRef]
- Wang, R.Q.; Fang, D.C. Alterations of MUC1 and MUC3 expression in gastric carcinoma: Relevance to patient clinicopathological features. J. Clin. Pathol. 2003, 56, 378–384. [Google Scholar] [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, D.; Spassova, M.K.; Querfelli, O.; Ragupathi, G.; Damani, P.; Livingston, P.O.; Danishefsky, S.J. From synthesis to biologics: Preclinical data on chemistry derived anticancer vaccine. J. Am. Chem. Soc. 2009, 131, 9298–9303. [Google Scholar] [CrossRef]
- Holmberg, L.A.; Sandmaier, B.M. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev. Vaccines 2004, 3, 655–663. [Google Scholar] [CrossRef]
- Gornowicz, A.; Szymanowski, W.; Bielawski, K.; Kałuża, Z.; Michalak, O.; Bielawska, A. Mucin 1 as a molecular target of a novel diisoquinoline derivative combined with anti-MUC1 antibody in AGS gastric cancer cells. Molecules 2021, 26, 6504. [Google Scholar] [CrossRef]
- Supruniuk, K.; Czarnomysy, R.; Muszyńska, A.; Radziejewska, I. Combined action of anti-MUC1 monoclonal antibody and pyrazole-Platinum(II) complexes reveals higher effectiveness towards apoptotic response in comparison with monotherapy in AGS gastric cancer cells. Pharmaceutics 2021, 13, 968. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziejewska, I. The Role of MUC1 in Gastric Cancer Development. Cancers 2025, 17, 3331. https://doi.org/10.3390/cancers17203331
Radziejewska I. The Role of MUC1 in Gastric Cancer Development. Cancers. 2025; 17(20):3331. https://doi.org/10.3390/cancers17203331
Chicago/Turabian StyleRadziejewska, Iwona. 2025. "The Role of MUC1 in Gastric Cancer Development" Cancers 17, no. 20: 3331. https://doi.org/10.3390/cancers17203331
APA StyleRadziejewska, I. (2025). The Role of MUC1 in Gastric Cancer Development. Cancers, 17(20), 3331. https://doi.org/10.3390/cancers17203331





