Multidisciplinary Prehabilitation Reduces Hospitalization Time and Suggests Improved Survival in Patients with Radiologically Diagnosed Lung Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Analysis Plan
3. Results
3.1. Demographics
3.2. Prehabilitation
3.3. Hospital Admissions and Length of Stay
3.3.1. Impact of Length of Stay by Treatment
3.3.2. Time to Inpatient Admission from Diagnosis
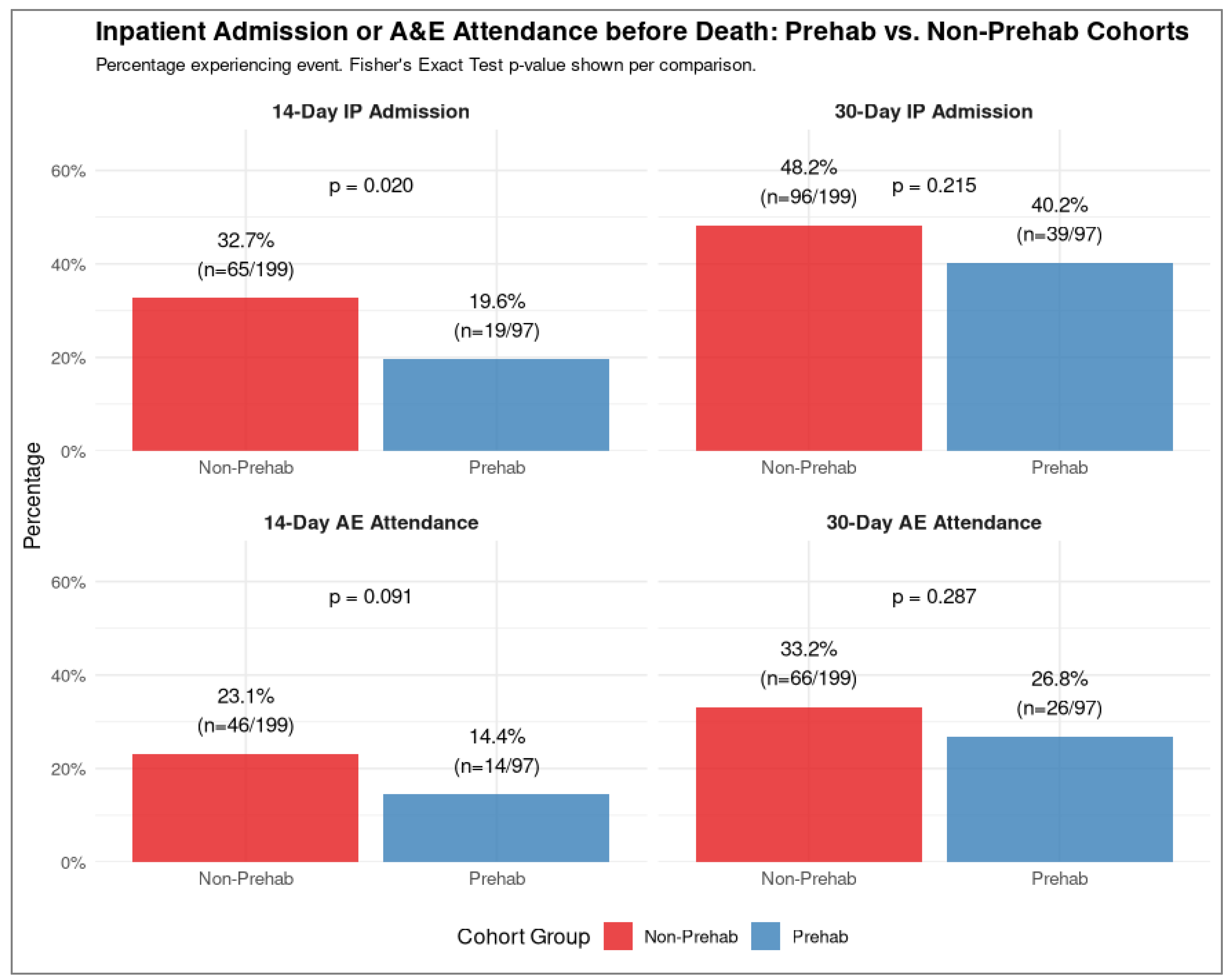
3.3.3. Admissions Within 14 and 30 Days of Death
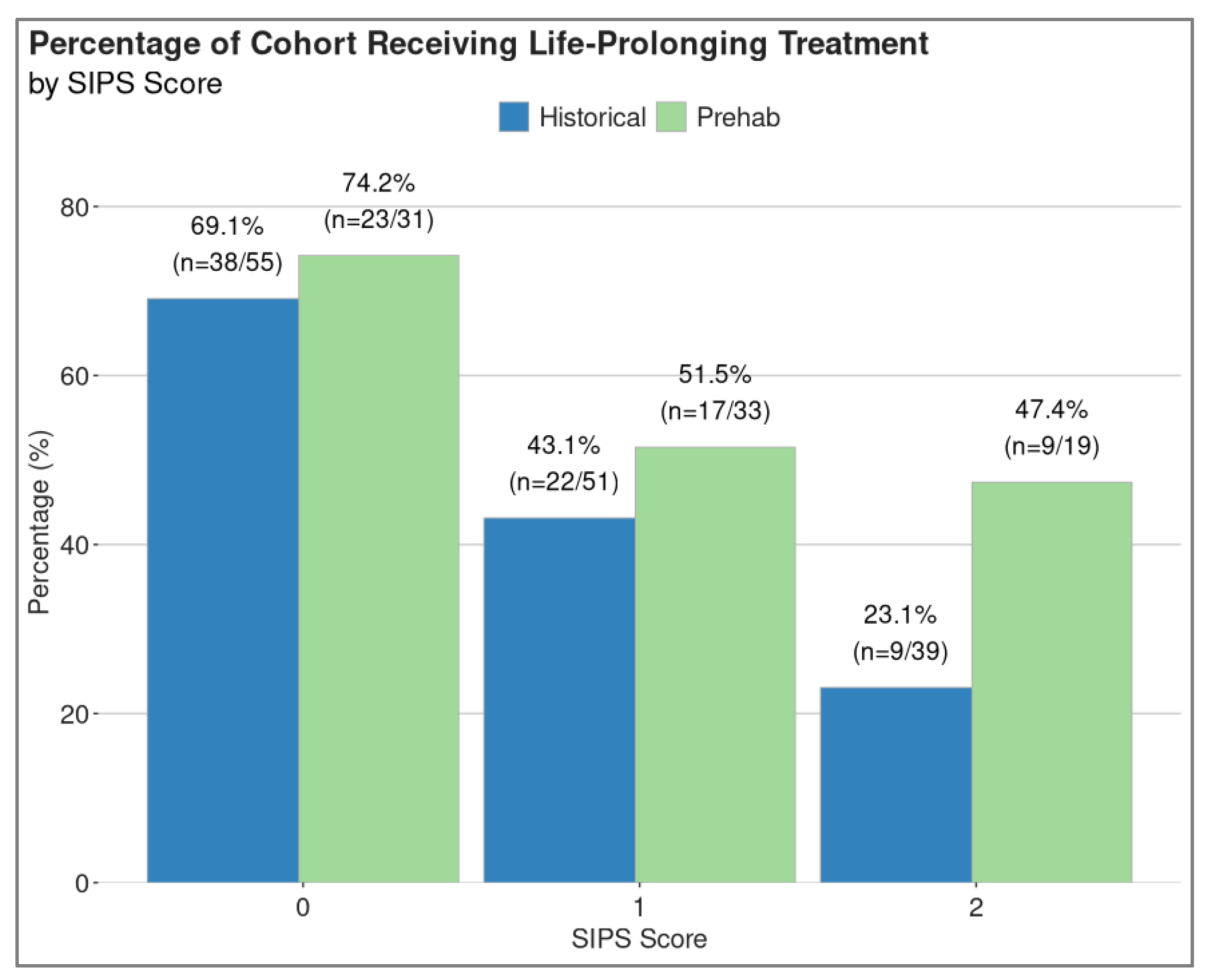
3.4. Treatment Rates
3.5. Inflammatory Status
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borg, M.; Løkke, A.; Ibsen, R.; Hilberg, O. Four decades of lung cancer: Trends in comorbidities and causes of death in a nationwide Danish cohort. Eur. J. Cancer 2025, 218, 115303. [Google Scholar] [CrossRef]
- Li, R.; Wu, J.; Ma, M.; Pei, J.; Song, Y.; Zhang, X.; Han, B. Comparison of PG-SGA, SGA and body-composition measurement in detecting malnutrition among newly diagnosed lung cancer patients in stage IIIB/IV and benign conditions. Med. Oncol. 2011, 28, 689–696. [Google Scholar] [CrossRef]
- Cancer Research UK. Lung Cancer Survival Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/survival#heading-Zero (accessed on 1 May 2025).
- Gould, M.K.; Munoz-Plaza, C.E.; Hahn, E.E.; Lee, J.S.; Parry, C.; Shen, E. Comorbidity Profiles and Their Effect on Treatment Selection and Survival among Patients with Lung Cancer. Ann. Am. Thorac. Soc. 2017, 14, 1571–1580. [Google Scholar] [CrossRef]
- Kiss, N.; Curtis, A. Current Insights in Nutrition Assessment and Intervention for Malnutrition or Muscle Loss in People with Lung Cancer: A Narrative Review. Adv. Nutr. 2022, 13, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.C.; et al. Overall Survival with Osimertinib in Resected. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Solomon, B.J.; Liu, G.; Felip, E.; Mok, T.S.K.; Soo, R.A.; Mazieres, J.; Shaw, A.T.; de Marinis, F.; Goto, Y.; Wu, Y.L.; et al. Lorlatinib Versus Crizotinib in Patients with Advanced. J. Clin. Oncol. 2024, 42, 3400–3409. [Google Scholar] [CrossRef]
- Wu, Y.L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in Resected. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Navani, N.; Conibear, J.; West, D. State of the Nation Report 2024, an Audit of Care Received by Patients Diagnosed with Lung Cancer in England and Wales During 2022. 2024. Available online: https://www.lungcanceraudit.org.uk/wp-content/uploads/2024/04/NLCA-State-of-the-Nation-Report-2024.pdf (accessed on 1 May 2025).
- Macmillan. Prehabilitation for People with Cancer. 2019. Available online: https://www.macmillan.org.uk/healthcare-professionals/cancer-pathways/prehabilitation (accessed on 1 May 2025).
- Scottish Government. Key Principles for Implementing Cancer Prehabilitation Across Scotland. Available online: https://www.prehab.nhs.scot/for-professionals/key-principles/ (accessed on 5 October 2022).
- Scottish Government. Cancer Strategy for Scotland 2023–2033; Scottish Government: Edinburgh, UK, 2023.
- Scottish Government. Cancer Action Plan for Scotland 2023–2026; Scottish Government: Edinburgh, UK, 2023.
- Rogers, L.J.; Bleetman, D.; Messenger, D.E.; Joshi, N.A.; Wood, L.; Rasburn, N.J.; Batchelor, T.J.P. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 155, 1843–1852. [Google Scholar] [CrossRef]
- Ljungqvist, O. ERAS—enhanced recovery after surgery: Moving evidence-based perioperative care to practice. J. Parenter. Enter. Nutr. 2014, 38, 559–566. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Kidd, G.; Gillis, C.; Branje, K.; Al-Bayati, M.; Baxi, A.; Grudzinski, A.L.; Boland, L.; Veroniki, A.A.; Wolfe, D.; et al. Relative efficacy of prehabilitation interventions and their components: Systematic review with network and component network meta-analyses of randomised controlled trials. BMJ 2025, 388, e081164. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Deans, M.; Walton, A.; Vallet, M.; Mencnarowksi, J.; McMillan, D.; Peacock, C.; Hall, P.; O’Brien, F.; Stares, M.; et al. Early prehabilitation reduces admissions and time in hospital in patients with newly diagnosed lung cancer. BMJ Support. Palliat. Care 2024, 15, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Stares, M.; Ding, T.E.; Stratton, C.; Thomson, F.; Baxter, M.; Cagney, H.; Cumming, K.; Swan, A.; Ross, F.; Barrie, C.; et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 2022, 7, 100445. [Google Scholar] [CrossRef]
- Gomez-Randulfe Rodriguez, M.; Phillips, I.; Gomes, F.; Mackean, M.; Stares, M. Validation of the Scottish Immunotherapy Prognostic Score (SIPS) in NSCLC patients treated with first-line pembrolizumab. ESMO Open 2024, 16, 3833. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, W.; Du, J.; Li, Y. Early Palliative Care in Advanced Non-Small Cell Lung Cancer Patients: A Meta-Analysis. Am. J. Hosp. Palliat. Care 2024, 42, 10499091241300530. [Google Scholar] [CrossRef]
- Berman, R.; Davies, A.; Cooksley, T.; Gralla, R.; Carter, L.; Darlington, E.; Scotté, F.; Higham, C. Supportive Care: An Indispensable Component of Modern Oncology. Clin. Oncol. 2020, 32, 781–788. [Google Scholar] [CrossRef]
- Monnery, D.; Benson, S.; Griffiths, A.; Cadwallader, C.; Hampton-Matthews, J.; Coackley, A.; Cooper, M.; Watson, A. Multi-professional-delivered enhanced supportive care improves quality of life for patients with incurable cancer. Int. J. Palliat. Nurs. 2018, 24, 510–514. [Google Scholar] [CrossRef]
- Taylor, S.; Vercell, A.; Sawyer, C.; Khatoon, B.; Coomber-Moore, J.; Yorke, J.; Mula, C.; Berman, R. Enhanced supportive care: Prospective cohort study of oncology patients and caregivers. BMJ Support. Palliat. Care 2024, 14, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.; Laird, B.J.A.; Minton, O.; Monnery, D.; Ahamed, A.; Boland, E.; Droney, J.; Vidrine, J.; Leach, C.; Scotté, F.; et al. The Rise of Supportive Oncology: A Revolution in Cancer Care. Clin. Oncol. 2023, 35, 213–215. [Google Scholar] [CrossRef]
- Kircher, C.E.; Hanna, T.P.; Tranmer, J.; Goldie, C.E.; Ross-White, A.; Moulton, E.; Flegal, J.; Goldie, C.L. Defining “early palliative care” for adults diagnosed with a life-limiting illness: A scoping review. BMC Palliat. Care 2025, 24, 93. [Google Scholar] [CrossRef] [PubMed]
- Tiberini, R.; Richardson, H. Rehabilitative Palliative Care: Enabling People to Live Fully Until They Die; Hospice UK: London, UK, 2015. [Google Scholar]
- Gourlay, E.; Banfill, K.; Merchant, Z.; Goodley, P.; Brown, L.; Murphy, J.; Moore, J.; Evison, M. Feasibility, acceptability and clinical outcomes of a real-world, regional lung cancer prehabilitation programme for patients undergoing curative intent radiotherapy. Lung Cancer 2025, 204, 108572. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Spiro, A.; McGough, C.; Norman, A.R.; Gillbanks, A.; Thomas, K.; Cunningham, D.; O’Brien, M.; Andreyev, H.J. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: A randomised controlled trial. J. Hum. Nutr. Diet. 2011, 24, 431–440. [Google Scholar] [CrossRef]
- Adizie, J.B.; Khakwani, A.; Beckett, P.; Navani, N.; West, D.; Woolhouse, I.; Harden, S.V. Stage III Non-small Cell Lung Cancer Management in England. Clin. Oncol. 2019, 31, 688–696. [Google Scholar] [CrossRef]
- Stares, M.; Brown, L.; Abhi, D.; Phillips, I. Prognostic Biomarkers of Systemic Inflammation in Non-Small Cell Lung Cancer: A Narrative review of challenges and opportunities. Cancers 2024, 16, 1508. [Google Scholar] [CrossRef]
- Manoukian, S.; Stewart, S.; Graves, N.; Mason, H.; Robertson, C.; Kennedy, S.; Pan, J.; Kavanagh, K.; Haahr, L.; Adil, M.; et al. Bed-days and costs associated with the inpatient burden of healthcare-associated infection in the UK. J. Hosp. Infect. 2021, 114, 43–50. [Google Scholar] [CrossRef]
- Burnett, C.; Bestall, J.C.; Burke, S.; Morgan, E.; Murray, R.L.; Greenwood-Wilson, S.; Williams, G.F.; Franks, K.N. Prehabilitation and Rehabilitation for Patients with Lung Cancer: A Review of Where we are Today. Clin. Oncol. 2022, 34, 724–732. [Google Scholar] [CrossRef]




| Category | Sub-Category | Historical Controls Number (%) | Prehabilitation Number (%) |
|---|---|---|---|
| Number of pts | 199 | 97 | |
| Age (median, range) | 70 (34–91) | 68 (38–89) | |
| Gender | Male | 101 (50.8%) | 42 (43.3%) |
| Female | 98 (49.2%) | 55 (56.7%) | |
| Diagnosis | Radiological (no biopsy) | 25 (12.6%) | 7 (7.2%) |
| Small cell lung cancer | 48 (24.1%) | 17 (17.5%) | |
| NSCLC | 126 (63.3%) | 72 (74.2%) | |
| Mesothelioma | 0 (0%) | 1 (1%) | |
| No. of patients with stage 3 disease | 72 (36%) | 30 (31%) | |
| No. of patients with stage 4 disease | 127 (64%) | 67 (69%) | |
| Inflammatory markers, SIPS | % Bloods available for SIPS | 145 (73%) | 83 (86%) |
| % SIPS 0 | 55 (37.9%) | 31 (37.3%) | |
| % SIPS 1 | 51 (35.2%) | 33 (39.8%) | |
| % SIPS 2 | 39 (26.9%) | 19 (22.9%) |
| Historical Controls (199 Patients) | Prehabilitation Cohort (97 Patients) | ||
|---|---|---|---|
| Treatment | % stage 3 receiving SACT | 31 (15.6%) | 16 (16.5%) |
| % stage 4 receiving SACT | 51 (25.6%) | 41 (42.3%) | |
| % stage 3 receiving radical RT (55–60 Gy in 20–30 doses) | 15 (7.5%) | 1 (1%) | |
| % stage 3 and 4 receiving high-dose palliative radiotherapy (36–39 Gy in 12–13 doses) | 7 (3.5%) | 2 (2.1%) | |
| % stage 3 and 4 receiving palliative radiotherapy (8–20 Gy in 1–5 doses) | 2 (1.0%) | 9 (9.3%) | |
| % stage 3 and 4 BSC | 90 (45.2%) | 27 (27.8%) | |
| % stage 3 patients receiving life-prolonging treatment | 52 (26.1%) | 18 (18.6%) | |
| % stage 4 patients receiving life-prolonging treatment | 55 (27.6%) | 43 (44.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, I.; McDougall, C.; Walton, A.; Stares, M.; Hall, P.; Grecian, R.; O’Brien, F.; Mencnarowski, J.; MacCormick, F.; McLean, G.; et al. Multidisciplinary Prehabilitation Reduces Hospitalization Time and Suggests Improved Survival in Patients with Radiologically Diagnosed Lung Cancer. Cancers 2025, 17, 3329. https://doi.org/10.3390/cancers17203329
Phillips I, McDougall C, Walton A, Stares M, Hall P, Grecian R, O’Brien F, Mencnarowski J, MacCormick F, McLean G, et al. Multidisciplinary Prehabilitation Reduces Hospitalization Time and Suggests Improved Survival in Patients with Radiologically Diagnosed Lung Cancer. Cancers. 2025; 17(20):3329. https://doi.org/10.3390/cancers17203329
Chicago/Turabian StylePhillips, Iain, Caleb McDougall, Abi Walton, Mark Stares, Peter Hall, Robert Grecian, Fiona O’Brien, Julie Mencnarowski, Fiona MacCormick, Gavin McLean, and et al. 2025. "Multidisciplinary Prehabilitation Reduces Hospitalization Time and Suggests Improved Survival in Patients with Radiologically Diagnosed Lung Cancer" Cancers 17, no. 20: 3329. https://doi.org/10.3390/cancers17203329
APA StylePhillips, I., McDougall, C., Walton, A., Stares, M., Hall, P., Grecian, R., O’Brien, F., Mencnarowski, J., MacCormick, F., McLean, G., Higgins, S., McMillan, D., Reid, C., Allan, L., Lim, B. J. L., & Barrie, C. (2025). Multidisciplinary Prehabilitation Reduces Hospitalization Time and Suggests Improved Survival in Patients with Radiologically Diagnosed Lung Cancer. Cancers, 17(20), 3329. https://doi.org/10.3390/cancers17203329






