P53 Mutation Induces Epithelial-to-Mesenchymal Transition (EMT) Associated with Stem Cell Properties and Tumorigenesis in Fallopian Tube Cells
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Experimental Animal
2.2. Cell Lines and Cell Culture
2.3. Adenovirus Infection to Inactivate Tumor Suppressor Genes
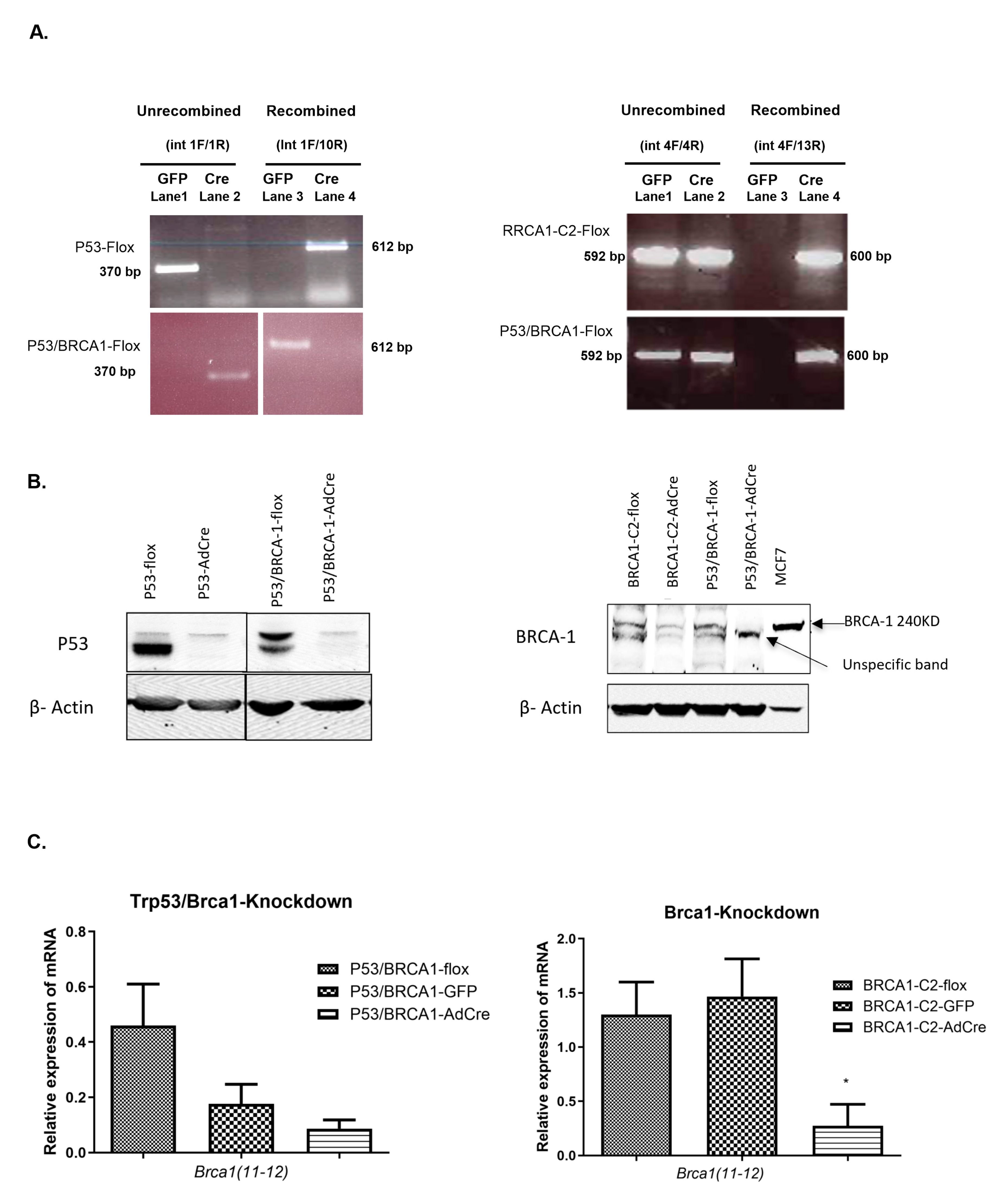
2.4. Detection of Recombination Using PCR
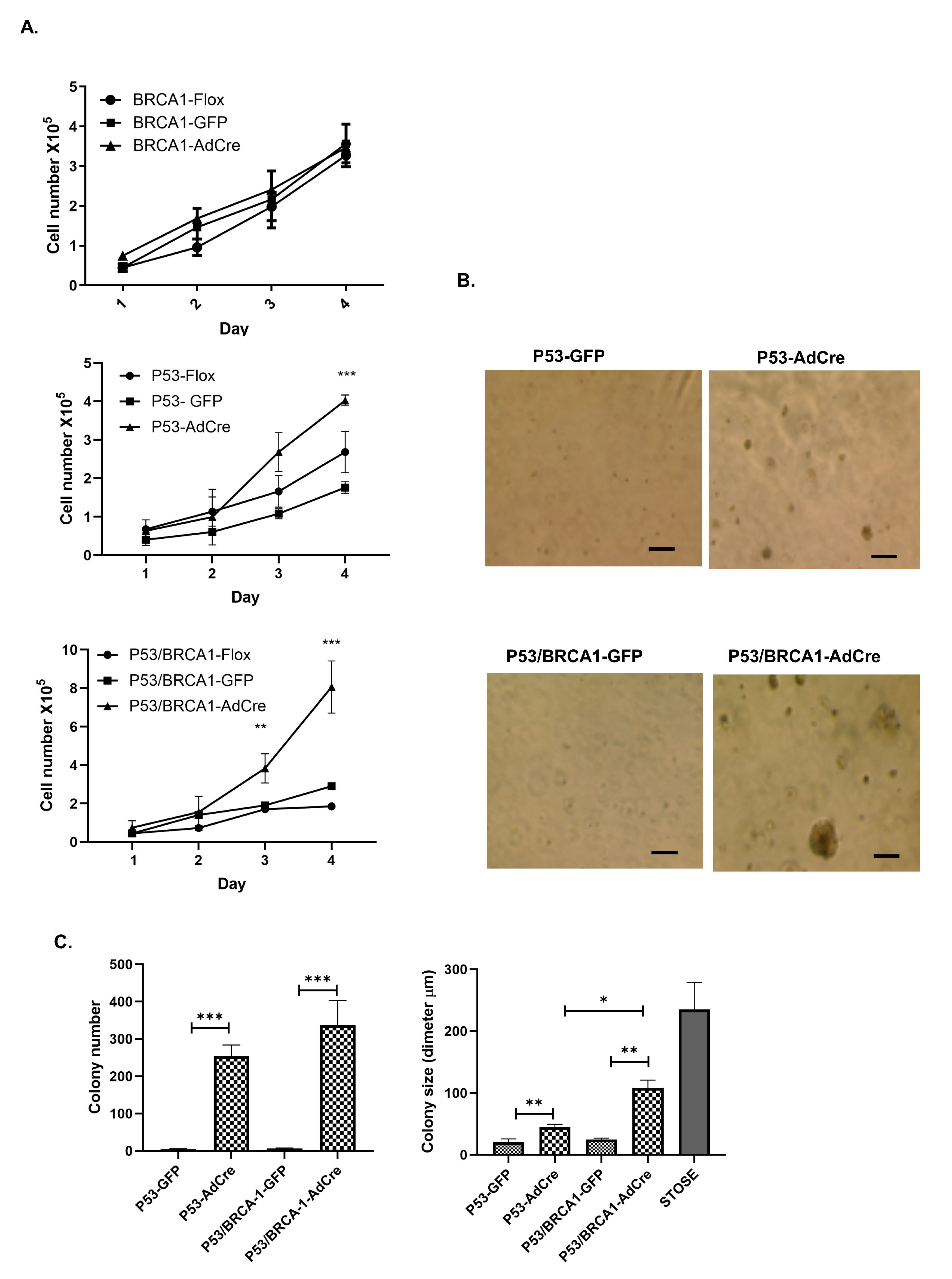
2.5. Proliferation Assay
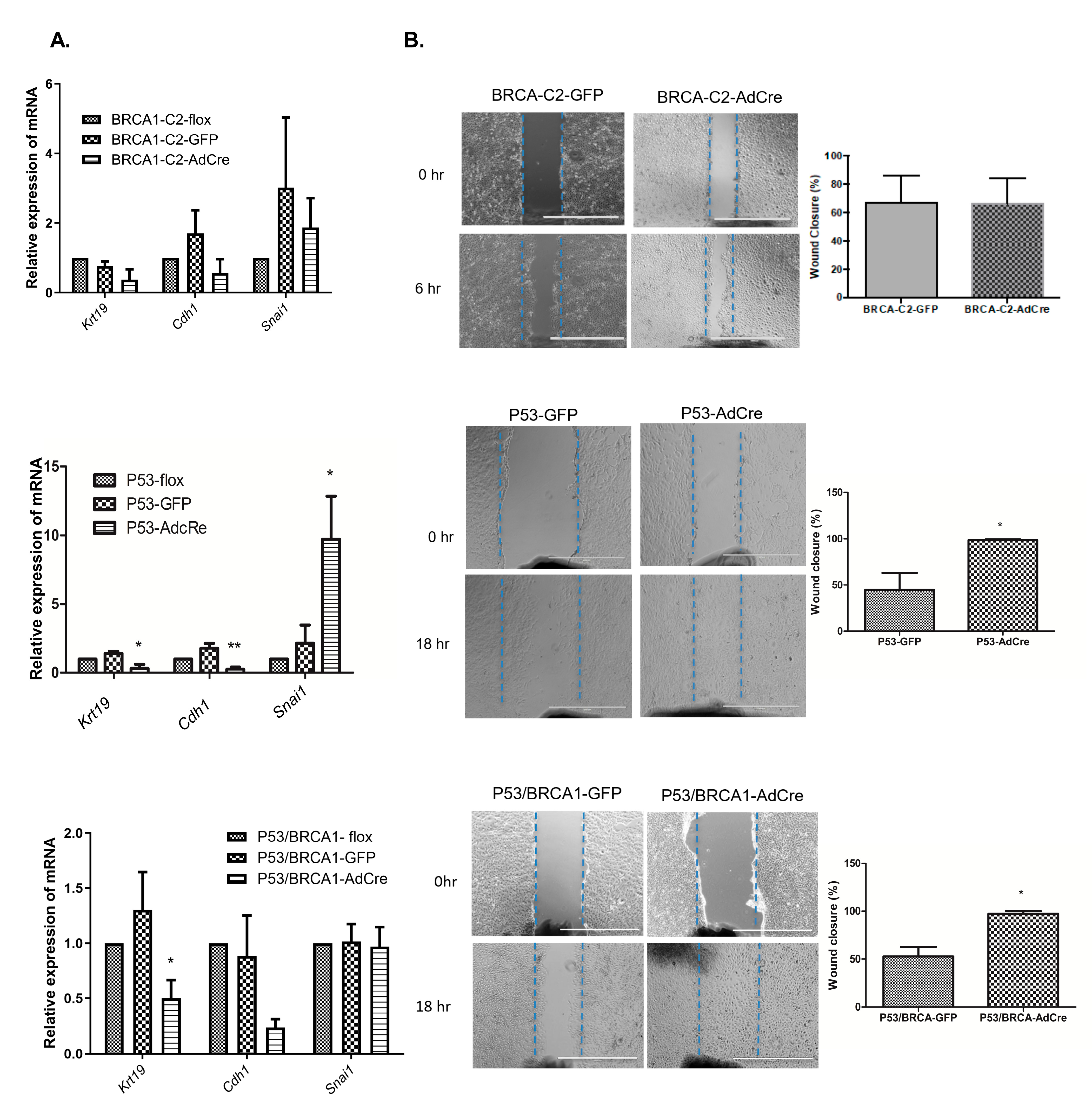
2.6. Migration Assay
2.7. Sphere Formation Assay
2.8. Colony Formation Assay in Soft Agar
2.9. Gene Expression Analysis
2.10. Western Blot Analysis
2.11. Flow Cytometry for SCA-1 Expression
2.12. Immunohistochemistry
2.13. Statistical Analyses
3. Results
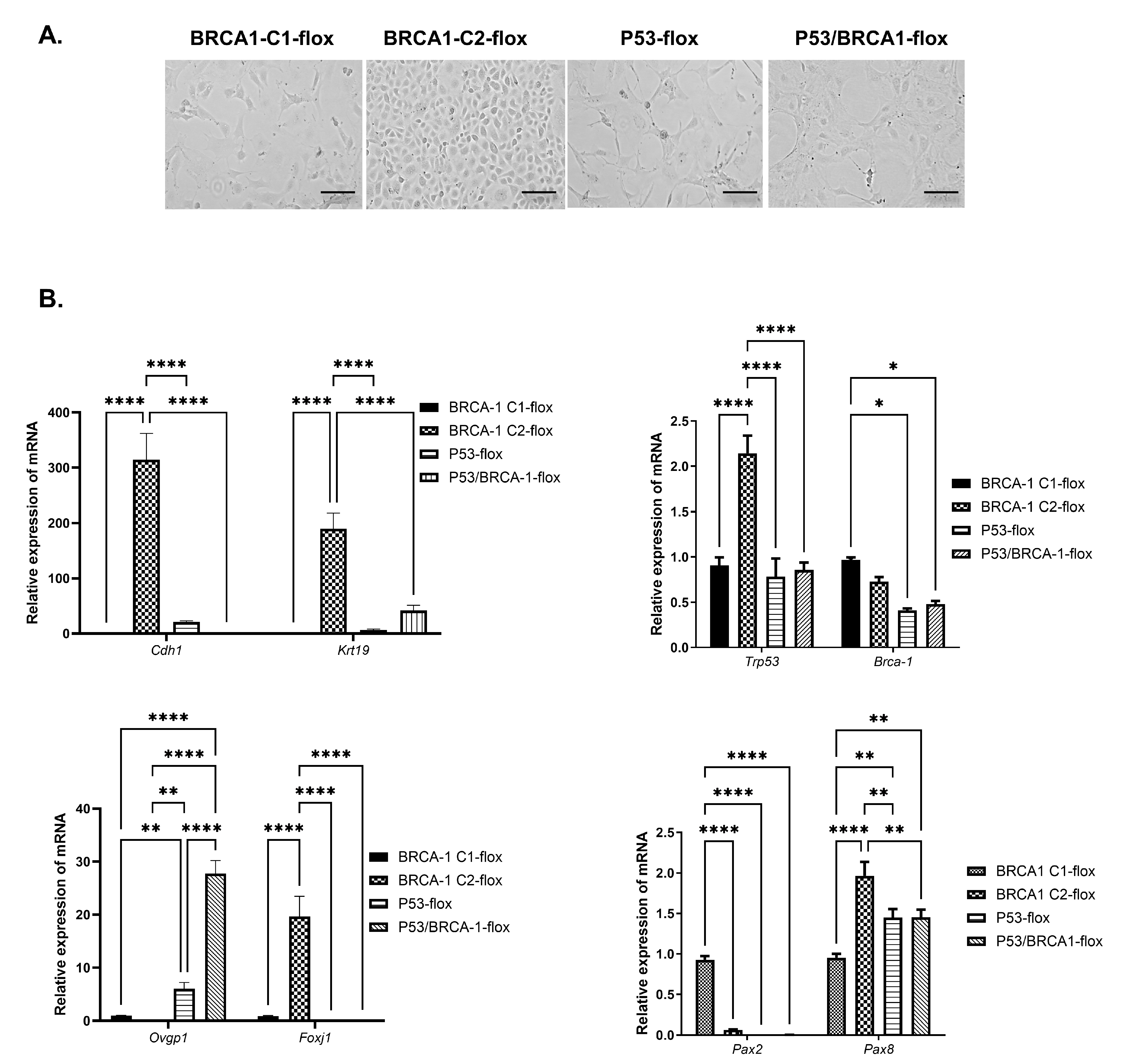
3.1. Isolation and Characterization of OVE Cells from Mice with Conditional Expression of Trp53 and/or Brca1
3.2. Loss of Trp53 Alone or Combined with Brca1 Induced OVE Cells to Undergo an Epithelial–Mesenchymal Transition
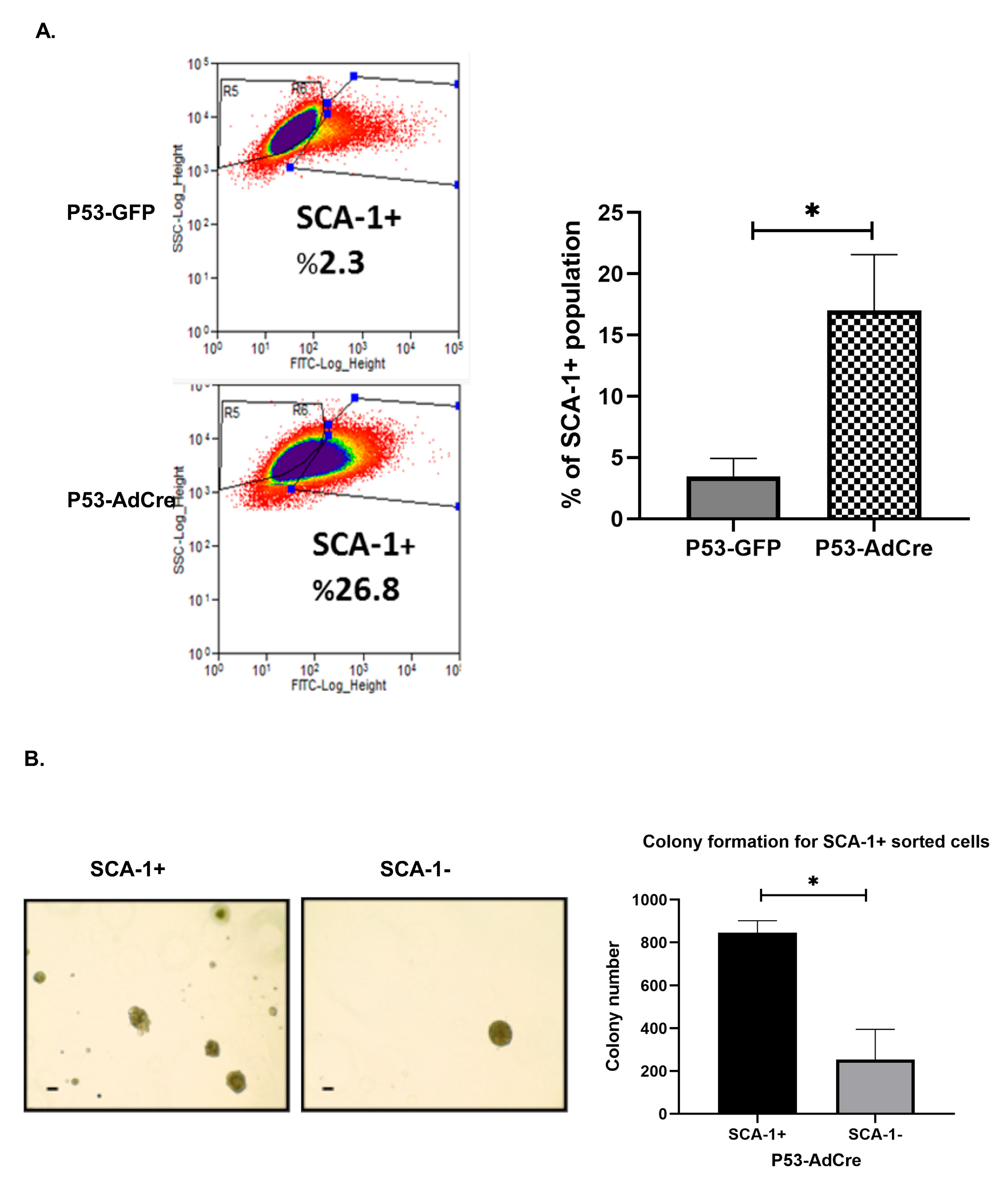
3.3. Loss of Trp53 Enhances Sphere-Forming Capacity and Expression of the Stem Cell Markers CD44 and SCA-1
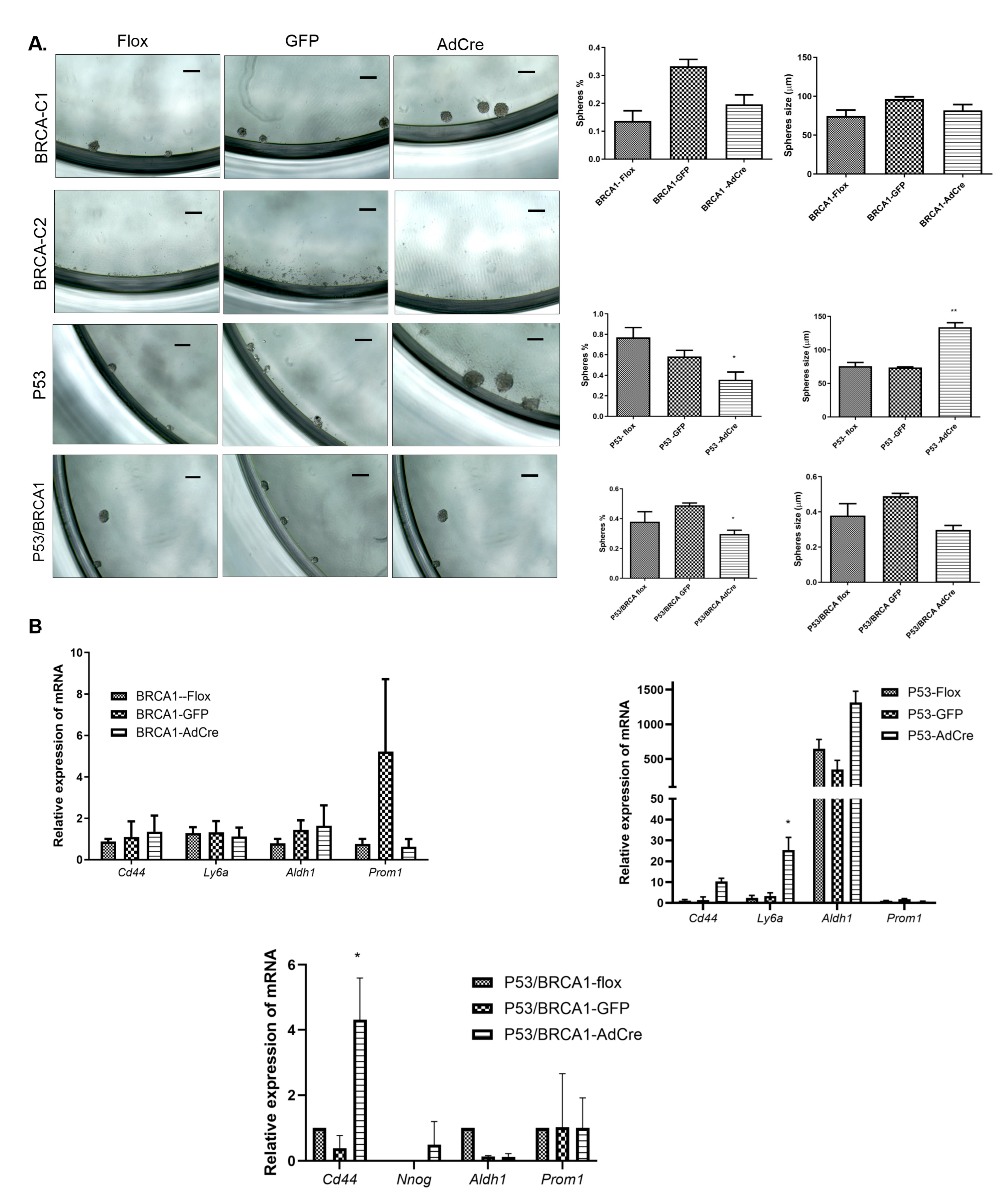
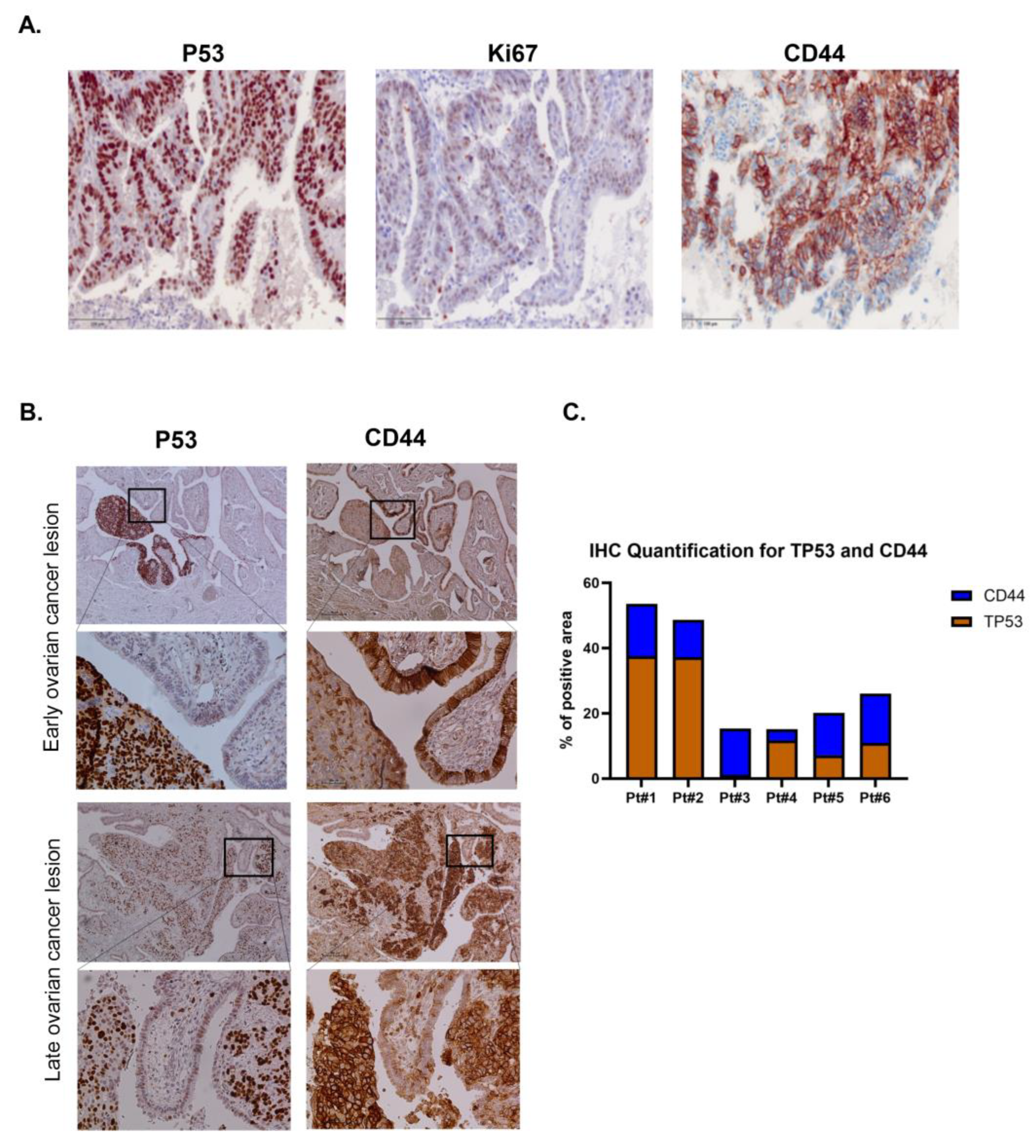
3.4. Trp53 Mutation Enhances the Stem Cell-Positive Population Associated with Tumorigenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, S.Y.; Lengyel, E.; Naora, H. The Mullerian HOXA10 Gene Promotes Growth of Ovarian Surface Epithelial Cells by Stimulating Epithelial–Stromal Interactions. Mol. Cell. Endocrinol. 2010, 317, 112–119. [Google Scholar] [CrossRef][Green Version]
- Idaikkadar, P.; Morgan, R.; Michael, A. HOX Genes in High Grade Ovarian Cancer. Cancers 2019, 11, 1107. [Google Scholar] [CrossRef]
- Chen, E.Y.; Mehra, K.; Mehrad, M.; Ning, G.; Miron, A.; Mutter, G.L.; Monte, N.; Quade, B.J.; McKeon, F.D.; Yassin, Y.; et al. Secretory Cell Outgrowth, PAX2 and Serous Carcinogenesis in the Fallopian Tube. J. Pathol. 2010, 222, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Fotopoulou, C.; Meyer, T. The Molecular Fingerprint of High Grade Serous Ovarian Cancer Reflects Its Fallopian Tube Origin. Int. J. Mol. Sci. 2013, 14, 6571–6596. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Tian, H.; Huang, H.S.; Hsu, C.F.; Liou, Y.; Wu, N.; Zhang, W.; Chu, T. Cellular Models of Development of Ovarian High-Grade Serous Carcinoma: A Review of Cell of Origin and Mechanisms of Carcinogenesis. Cell Prolif. 2021, 54, e13058. [Google Scholar] [CrossRef]
- Xiang, L.; Rong, G.; Zhao, J.; Wang, Z.; Shi, F. Identification of Candidate Genes Associated with Tubal Origin of High-Grade Serous Ovarian Cancer. Oncol. Lett. 2018, 15, 7769–7775. [Google Scholar] [CrossRef]
- Tong, G.X.; Chiriboga, L.; Hamele-Bena, D.; Borczuk, A.C. Expression of PAX2 in Papillary Serous Carcinoma of the Ovary: Immunohistochemical Evidence of Fallopian Tube or Secondary Mullerian System Origin? Mod. Pathol. 2007, 20, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Auersperg, N. The Origin of Ovarian Carcinomas: A Unifying Hypothesis. Int. J. Gynecol. Pathol. 2011, 30, 12–21. [Google Scholar] [CrossRef]
- Li, J.; Fadare, O.; Xiang, L.; Kong, B.; Zheng, W. Ovarian Serous Carcinoma: Recent Concepts on Its Origin and Carcinogenesis. J. Hematol. Oncol. 2012, 5, 8. [Google Scholar] [CrossRef]
- Callahan, M.J.; Crum, C.P.; Medeiros, F.; Kindelberger, D.W.; Elvin, J.A.; Garber, J.E.; Feltmate, C.M.; Berkowitz, R.S.; Muto, M.G. Primary Fallopian Tube Malignancies in BRCA-Positive Women Undergoing Surgery for Ovarian Cancer Risk Reduction. J. Clin. Oncol. 2007, 25, 3985–3990. [Google Scholar] [CrossRef]
- Lee, Y.; Miron, A.; Drapkin, R.; Nucci, M.R.; Medeiros, F.; Saleemuddin, A.; Garber, J.; Birch, C.; Mou, H.; Gordon, R.W.; et al. A Candidate Precursor to Serous Carcinoma That Originates in the Distal Fallopian Tube. J. Pathol. 2007, 211, 26–35. [Google Scholar] [CrossRef]
- Cass, I.; Walts, A.E.; Barbuto, D.; Lester, J.; Karlan, B. A Cautious View of Putative Precursors of Serous Carcinomas in the Fallopian Tubes of BRCA Mutation Carriers. Gynecol. Oncol. 2014, 134, 492–497. [Google Scholar] [CrossRef]
- Mehra, K.; Mehrad, M.; Ning, G.; Drapkin, R.; McKeon, F.D.; Xian, W. STICS, SCOUTs and p53 Signatures; A New Language for Pelvic Serous Carcinogenesis. Front. Biosci. 2011, 3, 625–634. [Google Scholar] [CrossRef][Green Version]
- Eisen, A.; Rebbeck, T.R.; Wood, W.C.; Weber, B.L. Prophylactic Surgery in Women with a Hereditary Predisposition to Breast and Ovarian Cancer. J. Clin. Oncol. 2000, 18, 1980–1995. [Google Scholar] [CrossRef]
- Kauff, N.D.; Satagopan, J.M.; Robson, M.E.; Scheuer, L.; Hensley, M.; Hudis, C.A.; Ellis, N.A.; Boyd, J.; Borgen, P.I.; Barakat, R.R.; et al. Risk-Reducing Salpingo-Oophorectomy in Women with a BRCA1 or BRCA2 Mutation. N. Engl. J. Med. 2002, 346, 1609–1615. [Google Scholar] [CrossRef]
- Choi, Y.H.; Terry, M.B.; Daly, M.B.; MacInnis, R.J.; Hopper, J.L.; Colonna, S.; Buys, S.S.; Andrulis, I.L.; John, E.M.; Kurian, A.W.; et al. Association of Risk-Reducing Salpingo-Oophorectomy with Breast Cancer Risk in Women with BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2021, 7, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Kim, J.; Coffey, D.M.; Creighton, C.J.; Yu, Z.; Hawkins, S.M.; Matzuk, M.M. High-Grade Serous Ovarian Cancer Arises from Fallopian Tube in a Mouse Model. Proc. Natl. Acad. Sci. USA 2012, 109, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Coffey, D.M.; Ma, L.; Matzuk, M.M. The Ovary Is an Alternative Site of Origin for High-Grade Serous Ovarian Cancer in Mice. Endocrinology 2015, 156, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wu, R.; Kuick, R.; Sessine, M.S.; Schulman, S.; Green, M.; Fearon, E.R.; Cho, K.R. High-Grade Serous Carcinomas Arise in the Mouse Oviduct via Defects Linked to the Human Disease. J. Pathol. 2018, 243, 16–25. [Google Scholar] [CrossRef]
- Perets, R.; Wyant, G.A.; Muto, K.W.; Bijron, J.G.; Poole, B.B.; Chin, K.T.; Chen, J.Y.H.; Ohman, A.W.; Stepule, C.D.; Kwak, S.; et al. Transformation of the Fallopian Tube Secretory Epithelium Leads to High-Grade Serous Ovarian Cancer in Brca;Tp53;Pten Models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, L.; Li, L.; Yang, B.; Kong, B.; Yao, G.; Zheng, W. Ovarian Serous Carcinogenesis from Tubal Secretory Cells. Histol. Histopathol. 2015, 30, 1295–1302. [Google Scholar]
- Erickson, B.K.; Conner, M.G.; Landen, C.N.J. The Role of the Fallopian Tube in the Origin of Ovarian Cancer. Am. J. Obstet. Gynecol. 2013, 209, 409–414. [Google Scholar] [CrossRef]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian Surface Epithelium: Biology, Endocrinology, and Pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar]
- Rabban, J.T.; Karnezis, A.N.; Zaloudek, C.J. Junctional Epithelial Zones of the Fallopian Tube: Cancer Hotspots? Int. J. Gynecol. Pathol. 2011, 30, 1–3. [Google Scholar] [CrossRef]
- Flesken-Nikitin, A.; Hwang, C.I.; Cheng, C.Y.; Michurina, T.V.; Enikolopov, G.; Nikitin, A.Y. Ovarian Surface Epithelium at the Junction Area Contains a Cancer-Prone Stem Cell Niche. Nature 2013, 495, 241–245. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Abedini, A.; Al-Hujaily, E.M.; Tang, Y.; Garson, K.; Collins, O.; Vanderhyden, B.C. PAX2 Maintains the Differentiation of Mouse Oviductal Epithelium and Inhibits the Transition to a Stem Cell-Like State. Oncotarget 2017, 8, 76881–76897. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Kafkova, S.; Leonhardt, K.; Kellner, C.; Einenkel, J. Serous Tubal In Situ Carcinoma (STIC) in Primary Peritoneal Serous Carcinomas. Int. J. Gynecol. Pathol. 2013, 32, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Yemelyanova, A.; Zaino, R.J.; Kurman, R.J. The Fallopian Tube-Peritoneal Junction: A Potential Site of Carcinogenesis. Int. J. Gynecol. Pathol. 2011, 30, 4–11. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, C.H.; Xia, W.; Yang, J.Y.; Xiong, Y.; Li, C.W.; Yu, W.H.; Rehman, S.K.; Hsu, J.L.; Lee, H.H.; et al. p53 Regulates Epithelial–Mesenchymal Transition and Stem Cell Properties Through Modulating miRNAs. Nat. Cell Biol. 2011, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- TeKippe, M.; Harrison, D.E.; Chen, J. Expansion of Hematopoietic Stem Cell Phenotype and Activity in Trp53-Null Mice. Exp. Hematol. 2003, 31, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Roberts, A.L.; Dunphy, K.A.; Bigelow, C.; Yan, H.; Jerry, D.J. Repression of Mammary Stem/Progenitor Cells by p53 Is Mediated by Notch and Separable from Apoptotic Activity. Stem Cells 2011, 29, 119–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCloskey, C.W.; Goldberg, R.L.; Carter, L.E.; Gamwell, L.F.; Al-Hujaily, E.M.; Collins, O.; Macdonald, E.A.; Garson, K.; Daneshmand, M.; Carmona, E.; et al. A New Spontaneously Transformed Syngeneic Model of High-Grade Serous Ovarian Cancer with a Tumor-Initiating Cell Population. Front. Oncol. 2014, 4, 53. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Aalmri, S.; Mashhour, M.; Ghandorah, S.; Alshangiti, A.; Azam, F.; Selwi, W.; Gharaibeh, L.; Alatawi, Y.; Alruwaii, Z.; et al. PD-L1 Is Highly Expressed in Ovarian Cancer and Associated with Cancer Stem Cells Populations Expressing CD44 and Other Stem Cell Markers. BMC Cancer 2023, 23, 13. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Alruwaii, Z.I.; Mashhour, M.; Almsned, F.M.; Asraf, R.; Alrsheedy, W.; Alessa, A.; Almohanna, H.; Selwi, W.; Azam, F. Dysgerminomas: Germ Cell Tumors Exhibit High Expression of PD-L1 and Associated with High TILs and Good Prognosis. Sci. Rep. 2024, 14, 24191. [Google Scholar] [CrossRef]
- Kar, K.; Ghosh, S.; Roy, A.K. A Study of CD44 Positive Cancer Cells in Epithelial Ovarian Cancer and Their Correlation with P53 and Ki67. J. Lab. Physicians 2021, 13, 50–57. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Zhao, L.; Zhao, S.; Cui, M. Overexpression of CD44 and EpCAM May Be Associated with the Initiation and Progression of Epithelial Ovarian Cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 4780–4786. [Google Scholar]
- Saifudeen, Z.; Liu, J.; Dipp, S.; Yao, X.; Li, Y.; McLaughlin, N.; Aboudehen, K.; El-Dahr, S.S. A p53-Pax2 Pathway in Kidney Development: Implications for Nephrogenesis. PLoS ONE 2012, 7, e44972. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High Grade Serous Ovarian Carcinomas Originate in the Fallopian Tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Al-Hujaily, E.; Alamri, S.; Ghandorah, S.; Garson, K.; Vanderhyden, B. PAX2 Induces Vascular-Like Structures in Normal Ovarian Cells and Ovarian Cancer. Exp. Ther. Med. 2022, 23, 412. [Google Scholar] [CrossRef]
- Allison, K.H.; Upson, K.; Reed, S.D.; Jordan, C.D.; Newton, K.M.; Doherty, J.; Swisher, E.M.; Garcia, R.L. PAX2 Loss by Immunohistochemistry Occurs Early and Often in Endometrial Hyperplasia. Int. J. Gynecol. Pathol. 2012, 31, 159–167. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, X.; Wu, X. High Expressions of Lgr5 and ALDH1 in Primary Epithelial Ovarian Cancer Correlate with Advanced Tumor Stage and Grade as Well as Poor Prognosis of the Patients. Gynecol. Obstet. Invest. 2016, 81, 162–168. [Google Scholar] [CrossRef]
- Carlson, J.W.; Miron, A.; Jarboe, E.A.; Parast, M.M.; Hirsch, M.S.; Lee, Y.; Muto, M.G.; Kindelberger, D.; Crum, C.P. Serous Tubal Intraepithelial Carcinoma: Its Potential Role in Primary Peritoneal Serous Carcinoma and Serous Cancer Prevention. J. Clin. Oncol. 2008, 26, 4160–4165. [Google Scholar] [CrossRef]
- Liu, S.; Ginestier, C.; Charafe-Jauffret, E.; Foco, H.; Kleer, C.G.; Merajver, S.D.; Dontu, G.; Wicha, M.S. BRCA1 Regulates Human Mammary Stem/Progenitor Cell Fate. Proc. Natl. Acad. Sci. USA 2008, 105, 1680–1685. [Google Scholar] [CrossRef]
- Carter, L.E.; Cook, D.P.; McCloskey, C.W.; Grondin, M.A.; Landry, D.A.; Dang, T.; Collins, O.; Gamwell, L.F.; Dempster, H.A.; Vanderhyden, B.C. Transcriptional Heterogeneity of Stemness Phenotypes in the Ovarian Epithelium. Commun. Biol. 2021, 4, 527. [Google Scholar] [CrossRef]
- Clark-Knowles, K.V.; Garson, K.; Jonkers, J.; Vanderhyden, B.C. Conditional Inactivation of Brca1 in the Mouse Ovarian Surface Epithelium Results in an Increase in Preneoplastic Changes. Exp. Cell Res. 2007, 313, 133–145. [Google Scholar] [CrossRef]
- Clark-Knowles, K.V.; Senterman, M.K.; Collins, O.; Vanderhyden, B.C. Conditional Inactivation of Brca1, p53 and Rb in Mouse Ovaries Results in the Development of Leiomyosarcomas. PLoS ONE 2009, 4, e8534. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Two Genetic Hits (More or Less) to Cancer. Nat. Rev. Cancer 2001, 1, 157–162. [Google Scholar] [CrossRef]
- Richardson, M.T.; Recouvreux, M.S.; Karlan, B.Y.; Walts, A.E.; Orsulic, S. Ciliated Cells in Ovarian Cancer Decrease with Increasing Tumor Grade and Disease Progression. Cells 2022, 11, 4009. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Lin, W.; Wang, Y.; Zhang, J.; Chambers, S.K.; Li, B.; Lea, J.; Wang, Y.; Wang, Y.; Zheng, W. Loss of Tubal Ciliated Cells as a Risk for “Ovarian” or Pelvic Serous Carcinoma. Am. J. Cancer Res. 2020, 10, 3815–3827. [Google Scholar] [PubMed]
- Easton, D.F.; Ford, D.; Bishop, D.T. Breast and Ovarian Cancer Incidence in BRCA1-Mutation Carriers. Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1995, 56, 265–271. [Google Scholar]
- Roh, M.H.; Kindelberger, D.; Crum, C.P. Serous Tubal Intraepithelial Carcinoma and the Dominant Ovarian Mass: Clues to Serous Tumor Origin? Am. J. Surg. Pathol. 2009, 33, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Kurman, R.J.; Vang, R.; Sehdev, A.S.; Han, G.; Soslow, R.; Wang, T.-L.; Shih, I.-M. TP53 Mutations in Serous Tubal Intraepithelial Carcinoma and Concurrent Pelvic High-Grade Serous Carcinoma—Evidence Supporting the Clonal Relationship of the Two Lesions. J. Pathol. 2012, 226, 421–426. [Google Scholar] [CrossRef]
- Leonhardt, K.; Einenkel, J.; Sohr, S.; Engeland, K.; Horn, L.C. p53 Signature and Serous Tubal In-Situ Carcinoma in Cases of Primary Tubal and Peritoneal Carcinomas and Serous Borderline Tumors of the Ovary. Int. J. Gynecol. Pathol. 2011, 30, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.M.J.; Dorsman, J.C.; Massuger, L.F.; Ansink, A.C.; Weegenaar, J.; Shvarts, A.; Kenemans, P.; Verheijen, R.H.M. BRCA1 and p53 Protein Expression in Cultured Ovarian Surface Epithelial Cells Derived from Women with and without a BRCA1 Germline Mutation. Arch. Gynecol. Obstet. 2006, 274, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ardighieri, L.; Mori, L.; Conzadori, S.; Bugatti, M.; Falchetti, M.; Donzelli, C.M.; Ravaggi, A.; Odicino, F.E.; Facchetti, F. Identical TP53 Mutations in Pelvic Carcinosarcomas and Associated Serous Tubal Intraepithelial Carcinomas Provide Evidence of Their Clonal Relationship. Virchows Arch. 2016, 469, 61–69. [Google Scholar] [CrossRef]
- Cao, L.; Li, W.; Kim, S.; Brodie, S.G.; Deng, C.X. Senescence, Aging, and Malignant Transformation Mediated by p53 in Mice Lacking the Brca1 Full-Length Isoform. Genes Dev. 2003, 17, 201–213. [Google Scholar] [CrossRef]
- Al-Hujaily, E.M.; Tang, Y.; Yao, D.S.; Carmona, E.; Garson, K.; Vanderhyden, B.C. Divergent Roles of PAX2 in the Etiology and Progression of Ovarian Cancer. Cancer Prev. Res. 2015, 8, 759–771. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwosaibai, K.; Vanderhyden, B.C.; Alsaffar, F.A.; Alamri, S.; Almotlak, A.A. P53 Mutation Induces Epithelial-to-Mesenchymal Transition (EMT) Associated with Stem Cell Properties and Tumorigenesis in Fallopian Tube Cells. Cancers 2025, 17, 3317. https://doi.org/10.3390/cancers17203317
Alwosaibai K, Vanderhyden BC, Alsaffar FA, Alamri S, Almotlak AA. P53 Mutation Induces Epithelial-to-Mesenchymal Transition (EMT) Associated with Stem Cell Properties and Tumorigenesis in Fallopian Tube Cells. Cancers. 2025; 17(20):3317. https://doi.org/10.3390/cancers17203317
Chicago/Turabian StyleAlwosaibai, Kholoud, Barbara C. Vanderhyden, Fatimah A. Alsaffar, Salma Alamri, and Abdulaziz A. Almotlak. 2025. "P53 Mutation Induces Epithelial-to-Mesenchymal Transition (EMT) Associated with Stem Cell Properties and Tumorigenesis in Fallopian Tube Cells" Cancers 17, no. 20: 3317. https://doi.org/10.3390/cancers17203317
APA StyleAlwosaibai, K., Vanderhyden, B. C., Alsaffar, F. A., Alamri, S., & Almotlak, A. A. (2025). P53 Mutation Induces Epithelial-to-Mesenchymal Transition (EMT) Associated with Stem Cell Properties and Tumorigenesis in Fallopian Tube Cells. Cancers, 17(20), 3317. https://doi.org/10.3390/cancers17203317






