Simple Summary
Regorafenib is an oral multikinase inhibitor approved for patients with metastatic colorectal cancer (mCRC) who have shown progression after standard treatment protocols. The standard initial dose of 160 mg per day often leads to toxicities that may limit treatment adherence. The phase II ReDOS trial indicated that initiating treatment with a lower dose and progressively increasing it enhances tolerability while maintaining efficacy. We performed a comprehensive sub-analysis of the Italian ReTrITA study, involving 729 patients who received treatment with regorafenib. Patients utilising a dose-escalation strategy demonstrated extended progression-free survival and a reduction in severe adverse events relative to those on fixed dosing, with overall survival remaining similar across both groups. The findings demonstrate that flexible dose escalation is a viable method to achieve a balance between efficacy and tolerability in refractory mCRC within a routine practice context, thereby endorsing its application as a practical strategy to enhance treatment outcomes.
Abstract
Background: Regorafenib is a recognised treatment for refractory metastatic colorectal cancer (mCRC). The phase II ReDOS trial indicated that a stepwise dose escalation approach could enhance tolerability and persistence while maintaining efficacy. The ReTrITA study, a significant multicentre real-world cohort in Italy, served as the foundation for this sub-analysis concentrating solely on patients treated with regorafenib. Methods: This retrospective analysis encompassed 713 patients treated at 17 Italian centres from 2012 to 2023. Patients were categorised into two groups: ReDOS-like escalation (n = 313) and fixed dosing (no-ReDOS) (n = 400). The endpoints assessed were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR), and safety. Survival was assessed using Kaplan–Meier and Cox models, accompanied by exploratory subgroup analyses. Results: The median overall survival (OS) was comparable between the escalation and fixed dosing groups, recorded at 7.4 months and 6.7 months, respectively (HR 1.00, 95% CI 0.85–1.18, p = 0.93). Progression-free survival (PFS) demonstrated a significant improvement with escalation, recording 3.1 months compared to 3.9 months (HR 0.76, 95% CI 0.65–0.89, p = 0.0007). Subgroup analyses demonstrated a consistent progression-free survival (PFS) benefit in patients aged ≥70 years (HR 0.71, p = 0.015), with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 (HR 0.76, p = 0.022), RAS wild-type tumours (HR 0.69, p = 0.026), and rectal primaries (HR 0.72, p = 0.043). The disease control rate (DCR) and objective response rate (ORR) were comparable, at 23.2% versus 25.3% and 2.0% compared 2.6%, respectively. Although not statistically significant, the fixed dose group’s duration of response (DoR) was numerically longer (15.4 months) than that of the variable dosing group (8.9 months). A lower percentage of patients experienced grade 3/4 adverse events with escalation (35.4% compared to 39.5%, p = 0.0042). Conclusions: This sub-analysis of the ReTrITA cohort demonstrates that regorafenib dose escalation is achievable in real-world settings, resulting in notable improvements in progression-free survival and enhanced tolerability, while not adversely affecting overall survival. These results support and improve the findings of the ReDOS study, showing that dosage escalation is possible and helpful in a diverse, unselected group of people, which is what is performed in routine oncology treatment. The findings are consistent with both randomised and observational studies, endorsing individualised dosing as a practical strategy in refractory mCRC.
1. Introduction
Colorectal cancer (CRC) is the fourth most commonly diagnosed malignancy globally and is the second leading cause of cancer-related deaths. The World Health Organisation (WHO) says that, in 2020, more than 1.9 million people were diagnosed with colorectal cancer (CRC) for the first time, and more than 930,000 people died from it around the world [1]. This makes it the second most prevalent type of cancer and the third leading cause of cancer-related deaths. Recent GLOBOCAN forecasts show that the number of people with colorectal cancer around the world will climb by between 63% and 73%, which means that by 2040 there will be 3.2 million more cases and 1.6 million more deaths [2].
Despite notable progress in screening, prevention, and multimodal therapies, metastatic colorectal cancer (mCRC) remains a significant clinical challenge, with around 50–60% of patients ultimately developing distant metastases, the majority of which are unresectable [3,4,5,6]. Although mortality rates among older populations have declined, there has been a paradoxical increase in incidence among younger adults. This highlights the ongoing need for innovative treatment strategies and optimised sequencing methods. These worrying trends show that CRC is becoming more common around the world and that we need to find better and more bearable ways to treat it, especially for those whose cancer has spread and is resistant to treatment.
In salvage scenarios, therapeutic options are limited [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Regorafenib, an oral multikinase inhibitor, received FDA approval in 2012 for the treatment of patients with mCRC previously treated with standard chemotherapy and targeted therapies. Subsequent approvals extended its indication to gastrointestinal stromal tumours (GIST) and hepatocellular carcinoma (HCC), confirming its role as an established therapeutic option in refractory solid tumours [23,24]. It has been recognised as a standard treatment for refractory mCRC, supported by key phase III trials. The CORRECT and CONCUR studies indicated a survival benefit of regorafenib compared to placebo, accompanied by manageable yet clinically significant toxicities such as hand–foot skin reaction, fatigue, hypertension, and hepatotoxicity [25,26]. Investigations in real-world settings, including CONSIGN, REBECCA, and CORRELATE, have substantiated the safety and efficacy of regorafenib across various populations [27,28,29]. The phase II ReDOS trial implemented a dose-escalation approach, demonstrating that gradual titration from 80 mg to 160 mg enhanced treatment adherence and tolerability relative to the standard 160 mg starting dose, thus supporting alternative dosing strategies [30].
The ReTrITA (Regorafenib and Trifluridine/Tipiracil in Italian Patients) study was developed as one of the largest real-world, multicentre retrospective analyses in Italy, building on this background. Between 2012 and 2023, 1156 patients with refractory metastatic colorectal cancer (mCRC) were enrolled across 17 oncology centres and treated with regorafenib and/or trifluridine/tipiracil, either as monotherapy or in sequential regimens. The study demonstrated comparable survival outcomes for the two drugs when used separately; however, it indicated significantly enhanced overall and progression-free survival when regorafenib was administered before trifluridine/tipiracil (R/T) compared to the reverse order (T/R). The R/T sequence resulted in a median overall survival (OS) of 16.6 months, compared to 12.6 months for T/R, and a median progression-free survival (PFS) of 11.5 months versus 8.5 months, respectively. The results were consistent across multiple clinically relevant subgroups, underscoring the significance of treatment sequence in the management of late-line mCRC [31].
The ReTrITA study adhered to the Declaration of Helsinki and obtained approval from the Ethics Committee of Area 4 Lazio, Rome, Italy (protocol number 29-2024; approval date 4 March 2024). Patient data were anonymised, and informed consent was exempted in compliance with national data protection regulations.
This sub-analysis examines patients treated with regorafenib in the ReTrITA cohort, comparing outcomes between those managed with the ReDOS escalation strategy and those receiving standard or modified non-ReDOS schedules. This study aims to elucidate the potential benefits of dose optimisation in routine clinical practice by integrating efficacy, safety, and subgroup analyses, thereby enhancing the evidence base for personalised regorafenib therapy in refractory mCRC. Figure 1 displays the study design.
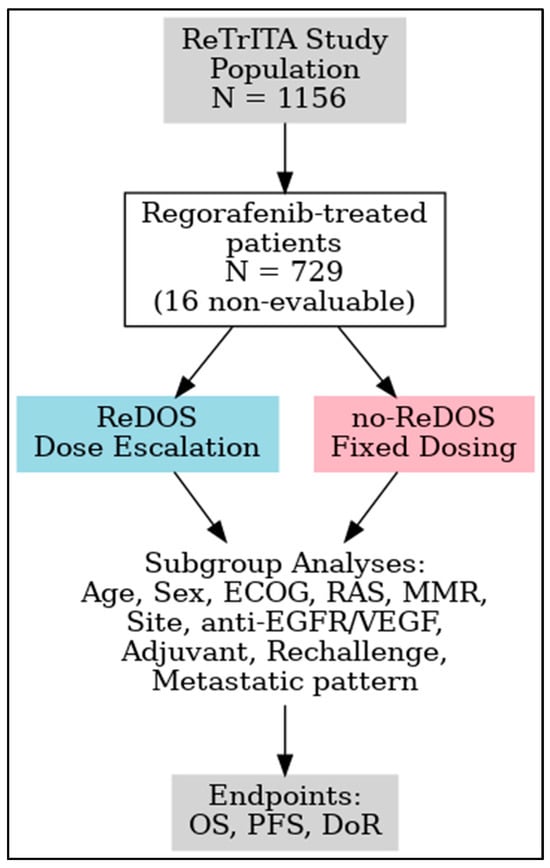
Figure 1.
Study design of the ReTrITA sub-analysis. Abbreviations: T, trifluridine/tipiracil; R, regorafenib.
2. Patients and Methods
2.1. Study Overview
The ReTrITA study was published on 18 June 2025. This retrospective, multicentre analysis encompassed 1156 patients with refractory metastatic colorectal cancer who were treated from 2012 to 2023 at 17 Italian cancer centres. Patients were assigned to receive either trifluridine/tipiracil (T) or regorafenib (R) as monotherapies, or in sequential treatments: T/R or R/T. The inclusion criteria included adult patients with metastatic colorectal cancer who were refractory to standard therapies and received treatment with regorafenib and/or trifluridine/tipiracil between 2012 and 2023 at participating centres [31].
Key findings indicated no significant differences in overall survival (OS) or progression-free survival (PFS) between R and T when administered as monotherapies (e.g., OS: ~5.0 vs. 5.9 months, PFS: ~3.2 vs. 3.3 months; non-significant). The R/T sequence demonstrated a significant enhancement in outcomes relative to T/R, with a median overall survival (OS) of 16.6 months compared to 12.6 months (p = 0.0004) and a median progression-free survival (PFS) of 11.5 months versus 8.5 months (p < 0.0001). The survival benefit was consistent across clinically relevant subgroups, including older patients, individuals with RAS mutations, and those with microsatellite-stable tumours. Toxicity profiles were consistent with expectations: haematologic toxicities were predominant with T, while non-hematologic toxicities were more common with R. The R/T sequence was linked to a higher incidence of grade 3/4 toxicities, yet exhibited a lower occurrence of neutropenia [31].
2.2. Outcome Parameters
This sub-analysis of the ReTrITA study concentrated on overall survival (OS) and progression-free survival (PFS) as the main endpoints. Overall survival (OS) is defined as the time from the initiation of regorafenib until death from any cause, whereas progression-free survival (PFS) is defined as the duration from the start of treatment to the first occurrence of clinically or/and radiologically confirmed disease progression or death. Secondary endpoints comprised the objective response rate (ORR), which is the percentage of patients achieving a complete response (CR) or partial response (PR), and the disease control rate (DCR), defined as CR, PR, or stable disease (SD), evaluated in accordance with RECIST v1.1. Furthermore, duration of response (DoR) was defined as the interval from the initiation of regorafenib treatment to disease progression or death in patients who attained complete response (CR) or partial response (PR). Safety outcomes comprised the incidence of treatment-related grade 3/4 adverse events (AEs), evaluated in accordance with NCI CTCAE v4.0 criteria.
2.3. Drug Administration
In this sub-analysis we focused exclusively on patients administered regorafenib, as illustrated in Figure 1 of the original study design. Regorafenib was taken orally once daily for 21 days within each 28-day cycle. The no-ReDOS group received an initial dose of 160 mg/day, with subsequent modifications determined by the clinician’s judgement. The ReDOS group executed a dose-escalation protocol, initiating with 80 mg/day in week 1, advancing to 120 mg/day in week 2, and reaching 160 mg/day in week 3, contingent upon tolerance, and thereafter remaining at the maximum tolerated dose of up to 160 mg/day. Both groups permitted dose reductions, interruptions, or delays contingent upon patient tolerance. Treatment persisted until disease progression, intolerable toxicity, decline in performance status, or withdrawal of consent occurred.
2.4. Statistical Analysis
Analyses were conducted utilising MedCalc v19.4 (MedCalc Software, Ostend, Belgium). Descriptive statistics provided a summary of demographic, clinical, and treatment characteristics. Overall survival (OS) and progression-free survival (PFS) were estimated using Kaplan–Meier methods, and survival comparisons between the ReDOS and non-ReDOS groups were conducted using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated employing Cox proportional hazards models. Comparisons of categorical variables, including adverse event incidence, utilised Fisher’s exact test or chi-square tests as deemed appropriate. DoR was presented with median values and ranges. A two-sided p-value of ≤0.05 was deemed statistically significant.
Exploratory subgroup analyses were performed across clinically relevant categories, including age, sex, ECOG performance status, RAS mutation status, MMR status, primary tumour location (right colon, left colon, rectum), prior exposure to anti-EGFR/VEGF agents, history of adjuvant treatment, use of rechallenge strategies, and metastatic disease pattern. Forest plots presented hazard ratios and 95% confidence intervals for OS and PFS across these subgroups to facilitate interpretation. All subgroup analyses were exploratory, and p-values were not adjusted for multiplicity; thus, these findings should be interpreted cautiously.
The analysis incorporated all patients administered regorafenib to reduce selection bias. The lead investigator, who also served as the data manager, had complete access to the database and conducted statistical analyses. Patient selection was performed by independent investigators who were blinded to clinical outcomes. All outcomes and definitions were established prior to the collection of data. However, due to the retrospective design, the results should be regarded as exploratory.
2.5. Ethical Approval
The ReTrITA study adhered to the Declaration of Helsinki and received approval from the Ethics Committee of Area 4 Lazio, Rome, Italy (protocol number 29-2024, approved on 4 March 2024). Patient data were anonymised to maintain confidentiality, and informed consent was waived in instances where patients were unreachable or declined to provide it, in compliance with Italian data protection regulations.
3. Results
3.1. Patient Characteristics
Table 1 summarises the baseline demographic and clinical characteristics of patients involved in this ReTrITA sub-study, we used bold for statistically significant numbers. This sub-analysis included 713 patients who were treated with regorafenib. Among them, 313 (43.9%) were treated following the ReDOS escalation strategy, whereas 400 (56.1%) were managed with no-ReDOS schedules (p = 0.0011).

Table 1.
Baseline demographic and clinical characteristics of patients included in the ReTrITA sub-study.
The median age was similar across groups (58 vs. 59 years, p = 0.9263), and the distribution of patients aged ≥ 70 years was also comparable (43.7% vs. 37.8%, p = 0.1042). The sex distribution was balanced, with males constituting 58.2% and females 60.8%, p = 0.4824. The prevalence of RAS mutations was similar across cohorts (56.0% vs. 58.8%, p = 0.6549). The distribution of primary tumour locations was similar; however, a higher percentage of right-sided primaries was observed in the no-ReDOS group (35.3% vs. 30.3%, p = 0.1948).
Significant variations were observed in mismatch repair (MMR) status. In the ReDOS group, a higher prevalence of pMMR disease was observed (78.0% compared to 37.8%). Conversely, the no-ReDOS cohort exhibited a significant proportion of missing or untested MMR status (60.5% versus 18.5%, p < 0.0001).
A notable difference in performance status was observed, with a higher percentage of ECOG 0 patients in the ReDOS cohort (37.4% vs. 28.3%) and a larger proportion of ECOG 2 patients in the no-ReDOS group (12.8% vs. 6.1%, p = 0.0017). Prior adjuvant therapy occurred more frequently in ReDOS patients (30.0% compared to 24.3%), but this difference was not statistically significant (p = 0.0838).
Liver-only involvement in metastatic disease was more prevalent in the ReDOS group (17.6% vs. 9.0%), while the no-ReDOS group showed a higher incidence of combined liver and extrahepatic disease (56.8% vs. 48.2%, p = 0.0019).
Disparities in chemotherapy administration were also noted. The ReDOS group consistently received intensive first-line and second-line regimens, including doublets or triplets, with minimal data missing. In contrast, the no-ReDOS group exhibited a significant percentage of patients with unknown treatment history (31.0% in first line; 35.3% in second line). Both differences exhibited statistical significance (p < 0.0001).
Exposure to biological agents demonstrated considerable variability. In first-line treatment, anti-EGFR therapy was more prevalent in the ReDOS cohort (31.6% vs. 20.0%), while a higher proportion of no-ReDOS patients had not received any targeted agent (40.0% vs. 17.2%; p < 0.0001). In the second-line setting, the utilisation of anti-VEGF was markedly higher in ReDOS patients (66.1% compared to 45.0%). Conversely, no-ReDOS patients exhibited a higher prevalence of no exposure to biological agents (51.3% versus 26.8%; p < 0.0001).
These findings indicate that while baseline demographic factors (age, sex, RAS status, tumour location) were largely comparable, significant imbalances were observed in ECOG performance status, metastatic distribution, MMR testing, prior chemotherapy intensity, and the use of biological agents. These differences must be taken into account when analysing survival and toxicity outcomes, as they may indicate variations in clinical practice, patient selection, and data completeness between the two treatment groups.
3.2. Survival Outcomes
The sub-analysis indicated that overall survival (OS) was not significantly different between the two groups. The median overall survival (OS) was 7.4 months (95% confidence interval [CI], 6.4–8.9) for the ReDOS group and 6.7 months (95% CI, 6.0–10.1) for the no-ReDOS cohort, with mean survival times being nearly equivalent at 14.2 months versus 14.3 months. The log-rank test indicated no statistically significant difference (χ2 = 0.008, DF = 1, p = 0.9298; HR 1.00, 95% CI 0.85–1.18) (Figure 2). The ReDOS escalation strategy significantly prolonged progression-free survival (PFS). The median progression-free survival (PFS) was 3.1 months (95% confidence interval [CI], 3.0–35.6) in the ReDOS group, in contrast to 3.9 months (95% CI, 3.5–66.2) in the no-ReDOS group. The log-rank test indicated a significant benefit for the ReDOS cohort (χ2 = 11.55, DF = 1, p = 0.0007; HR 0.76, 95% CI 0.65–0.89) (Figure 3).
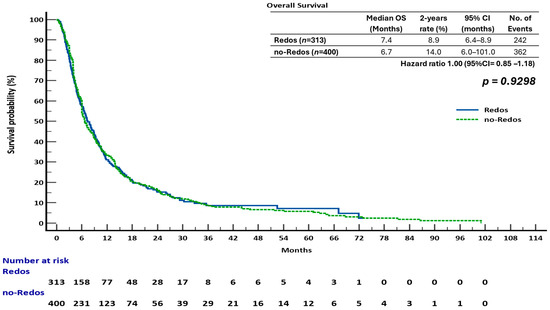
Figure 2.
Overall survival (OS) in patients treated with regorafenib according to dosing strategy.
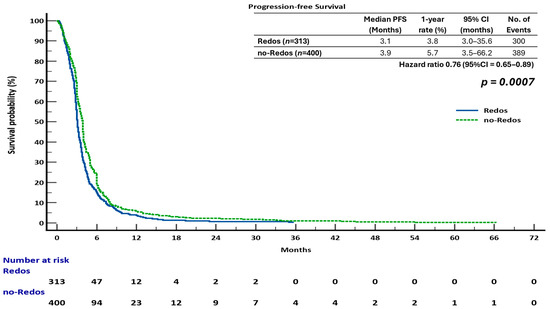
Figure 3.
Progression-free survival (PFS) in patients treated with regorafenib according to dosing strategy.
These findings suggest that while overall survival did not significantly differ between dosing strategies, progression-free survival improved in patients on the ReDOS escalation schedule, highlighting a potential benefit of gradual dose titration in delaying disease progression. The difference in overall survival and progression-free survival outcomes may indicate the influence of subsequent therapies, patient selection, or the retrospective nature of the study design. The statistically significant improvement in progression-free survival supports the hypothesis that the ReDOS strategy could help with early disease control while achieving overall survival rates similar to those of no-ReDOS regimens.
3.3. Summary of Efficacy Outcomes
The efficacy results of this study, as detailed in the preceding sections, are summarised in Table 2. The objective response rates (ORR) were low in both cohorts, with 2.0% for ReDOS and 2.6% for no-ReDOS (p = 0.4386). The disease control rate (DCR) was comparable, recorded at 23.2% and 25.3%, respectively (p = 0.6580). The duration of response (DoR) was greater in patients not receiving ReDOS (15.4 months compared to 8.9 months), though this difference did not achieve statistical significance (HR 1.68, 95% CI 0.35–7.94; p = 0.5944). The findings indicate similar overall survival across strategies, with the no-ReDOS group demonstrating a progression-free survival advantage, although response rates and disease control were constrained in both cohorts.

Table 2.
Efficacy outcomes in patients treated with regorafenib (ReDOS vs. no-ReDOS).
3.4. Comparative Analysis of Subgroups Survival
The comparative evaluation of regorafenib dose-escalation (ReDOS) versus fixed-dose (no-ReDOS) strategies across clinically relevant subgroups provides important insights into patient outcomes in refractory metastatic colorectal cancer.
3.4.1. Overall Survival (OS)
In the overall population, OS did not differ significantly between ReDOS and no-ReDOS, with hazard ratios (HRs) close to unity and confidence intervals crossing 1. Subgroup analyses confirmed this general finding, although selected signals emerged. Among patients aged ≥ 70 years, OS was 7.1 months under ReDOS compared with 6.6 months for no-ReDOS (HR 0.89, p = 0.378). In contrast, younger patients showed nearly identical outcomes (7.9 vs. 7.1 months; HR 0.91, p = 0.435). ECOG PS stratification revealed that patients with PS2 experienced worse survival with ReDOS (3.7 vs. 4.4 months; HR 1.74, p = 0.046), whereas outcomes were comparable in PS = 0–1.
Molecular subgroups, such as RAS wild-type and mutant, showed no significant differences, and frontline exposure to anti-EGFR or anti-VEGF did not alter OS outcomes. Patients with dMMR exhibited a longer OS with ReDOS (13.1 months compared to 10.0 months), although the results did not achieve statistical significance. Tumour sidedness suggested a trend: right-sided primaries had modestly better outcomes under ReDOS (8.1 vs. 6.1 months; HR 0.84), while rectal tumours favoured no-ReDOS (8.2 vs. 10.0 months; HR 1.38) (Figure 4).
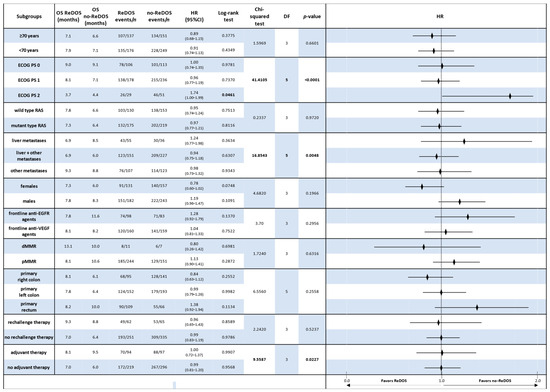
Figure 4.
Forest plot of overall survival (OS) by clinically relevant subgroups, comparing patients treated with regorafenib according to the ReDOS escalation strategy versus no-ReDOS dosing.
Overall, Chi-squared analyses supported the absence of significant OS differences across most subgroups, with the exception of ECOG PS2, where ReDOS was inferior.
3.4.2. Progression-Free Survival (PFS)
In contrast, subgroup analyses of PFS always showed that the no-ReDOS strategy was better. For people 70 years and older, the median PFS was 3.1 months with ReDOS and 3.7 months without it (HR 1.46, p = 0.0031). A comparable tendency was noted in younger patients (<70 years), where no-ReDOS resulted in an extended PFS (3.9 vs. 3.0 months; HR 1.22, p = 0.057). Performance level was a crucial factor: PS1 patients experienced 3.9 months without ReDOS compared to 3.1 months with ReDOS (HR 1.37, p = 0.0029), and PS2 patients also exhibited poorer outcomes with ReDOS (2.6 vs. 2.8 months; HR 1.83, p = 0.027) (Figure 5).
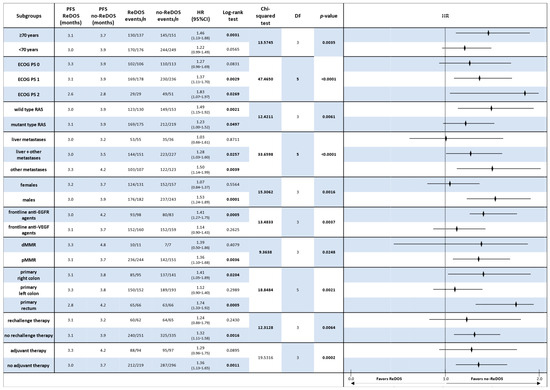
Figure 5.
Forest plot of progression-free survival (PFS) by clinically relevant subgroups, comparing patients treated with regorafenib according to the ReDOS escalation strategy versus no-ReDOS dosing.
These results were even stronger when genetic and clinical factors were used to group the data. Patients with RAS wild-type and mutant genotypes both experienced enhanced benefits from no-ReDOS, with hazard ratios of 1.49 and 1.23, respectively. Patients with pMMR also had longer PFS with no-ReDOS (3.7 months vs. 3.1 months; HR 1.36, p = 0.0036). Tumour location studies corroborated suboptimal outcomes with ReDOS in right-sided primary (HR 1.41, p = 0.020) and rectal tumours (HR 1.74, p = 0.001).
Further studies indicated that male patients and individuals with previous anti-EGFR exposure exhibited considerably enhanced disease management under no-ReDOS, but prior anti-VEGF medication did not significantly affect the comparison. Both the adjuvant and non-adjuvant groups had longer PFS with no-ReDOS, but only the non-adjuvant group reached significance (HR 1.36, p = 0.001). Global Chi-squared analysis corroborated considerable heterogeneity across ECOG PS (χ2 = 47.47, df = 5, p < 0.0001), metastatic site (χ2 = 33.66, df = 5, p < 0.0001), tumour location (χ2 = 18.85, df = 5, p = 0.0021), and adjuvant exposure (χ2 = 19.53, df = 3, p = 0.0002).
3.5. Survival Outcomes by Initial Regorafenib Dosing Strategy
In a supplementary analysis, patients were categorised into three groups based on initial regorafenib dosing: ReDOS escalation (n = 313), fixed reduced dose < 160 mg (n = 142), and standard 160 mg dose (n = 258). The median overall survival (OS) was 7.4 months (95% CI, 6.4–8.9) for the ReDOS group, 8.3 months (95% CI, 6.3–10.0) for the <160 mg group, and 6.1 months (95% CI, 6.0–10.1) for the 160 mg group, with no statistically significant differences observed among the groups (log-rank χ2 = 1.95, p = 0.3781). Pairwise hazard ratios indicated no overall survival benefit: HR 0.90 (95% CI, 0.72–1.11) for ReDOS compared to <160 mg and HR 1.05 (95% CI, 0.87–1.25) for ReDOS compared to 160 mg (Figure 6A).
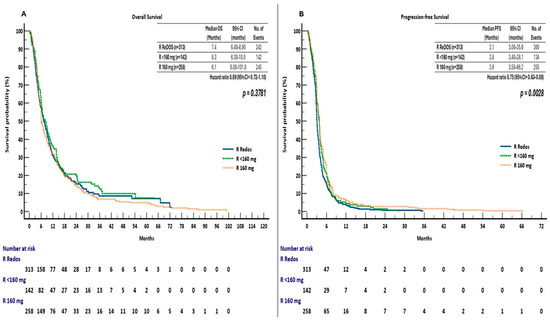
Figure 6.
(A) Overall survival (OS) and (B) Progression-free survival (PFS) by initial regorafenib dosing strategy in the ReTrITA sub-analysis (ReDOS escalation; fixed reduced dose < 160 mg; standard 160 mg).
Progression-free survival demonstrated significant differences among dosing groups (log-rank χ2 = 11.77, p = 0.0028). The median PFS was 3.1 months (95% confidence interval [CI], 3.0–35.6) for ReDOS, 3.8 months (95% CI, 3.4–28.1) for doses under 160 mg, and 3.9 months (95% CI, 3.5–66.2) for 160 mg doses. Hazard ratios indicated a decreased risk of progression or mortality with ReDOS compared to <160 mg (HR 0.83, 95% CI, 0.67–1.02) and 160 mg (HR 0.75, 95% CI, 0.63–0.89) (Figure 6B).
The findings indicate that while OS was not influenced by the initial dose, PFS outcomes were enhanced by the ReDOS escalation strategy, implying that gradual dose titration could enhance early disease management without negatively impacting overall survival.
4. Safety
This subanalysis of the ReTrITA study examined real-world regorafenib administration patterns in metastatic colorectal cancer, systematically comparing the incidence of treatment-related grade 3/4 adverse events (AEs) between patients receiving regorafenib via the ReDOS escalation strategy and those treated with no-ReDOS regimens (Table 3).

Table 3.
Grade 3/4 adverse events in patients treated with regorafenib according to ReDOS versus no-ReDOS dosing strategies in the ReTrITA sub-analysis.
Overall, grade 3/4 adverse events were found in both groups, yet their distribution exhibited significant differences. The total number of grade 3/4 events was similar (141 in the ReDOS group compared to 203 in the no-ReDOS group, p = 0.0008). However, the proportion of patients experiencing at least one grade 3/4 toxicity was significantly lower in the ReDOS cohort (35.4% versus 39.5%, p = 0.0042). This indicates that stepwise dose escalation may enhance tolerability, even with comparable overall event counts.
In the analysis of toxicities, haematologic grade 3/4 events were infrequent and showed no significant difference between the groups (7.8% vs. 10.3%, p = 0.0771). Non-hematologic toxicities predominated the safety profile, constituting more than 90% of high-grade events in both groups. The relative frequency was notably elevated in the ReDOS group (92.2% versus 89.7%, p = 0.0032), indicating a shift in the toxicity profile associated with the dosing method used.
Anaemia emerged as the predominant grade 3/4 haematologic toxicity, affecting 63.6% of patients in the ReDOS group and 61.9% in the no-ReDOS group. The occurrence of neutropenia, febrile neutropenia, and thrombocytopenia was low and uniformly distributed across cohorts, underscoring the limited haematologic effects of regorafenib in this setting.
Non-hematologic toxicities displayed increased variability. Fatigue was the most common severe adverse event, affecting 39.0% of patients with Grade 3/4 toxicities in the ReDOS group, in contrast to 23.6% in the no-ReDOS group. The hand-foot skin reaction was notable, with a higher incidence in no-ReDOS patients (21.2% versus 16.9%). Hypertension and liver dysfunctions were observed at similar rates among cohorts, whereas diarrhoea and skin disorders were more prevalent in the no-ReDOS group. The “other” category, which encompassed various less frequent toxicities, was also more represented in the no-ReDOS cohort (27.1% vs. 20.3%).
The findings suggest that regorafenib toxicity in the ReTrITA study aligned with the known safety profile of the drug; however, the dosing strategy affected the pattern and distribution of adverse events. The ReDOS escalation approach correlated with a reduced incidence of severe toxicities, especially regarding cumulative burden; however, specific non-hematologic toxicities, including fatigue, persisted as significant concerns. This suggests that personalised dose optimisation strategies could improve the tolerability of regorafenib while maintaining its overall safety profile.
5. Discussion
This sub-analysis of the ReTrITA study presents a substantial real-world dataset concentrated on regorafenib dosing strategies in refractory metastatic colorectal cancer (mCRC). The study compares outcomes of patients treated with a ReDOS-like dose escalation against standard or modified fixed dosing (no-ReDOS), providing insights into how titration strategies can optimise the balance between efficacy and tolerability in clinical practice. The findings support the concept that the efficacy of regorafenib can be maintained and, in certain instances, enhanced when the drug is administered via a flexible escalation strategy that emphasises tolerability and continuity of treatment.
In the context of previous real-world observational studies (Table 4), notable similarities and differences are observed. The current ReTrITA sub-analysis, focussing on regorafenib-treated patients (n = 729), indicated a median overall survival (OS) of 7.8 to 8.9 months across groups, with progression-free survival (PFS) ranging from 2.8 to 4.0 months, demonstrating favourable outcomes compared to other observational cohorts.

Table 4.
Comparison of survival outcomes across the ReTrITA sub-analysis and selected real-world studies of regorafenib in refractory metastatic colorectal cancer (mCRC).
By contrast, the Spanish RE-SEARCH study (n = 242, 15 centres) primarily investigated real-world dosing patterns [32]. Most patients (63.8%) initiated regorafenib at <160 mg, and dose-escalation from reduced starting doses became increasingly frequent over time, especially after 2019. The median PFS was 3.0 months, with no significant differences in efficacy observed across dose patterns. Patients initiating treatment at 160 mg, followed by dose reductions, exhibited the highest incidence of grade 3–4 toxicities, recorded at 40.6%. ReTrITA demonstrates the clinical advantages of dose-escalation regarding PFS and tolerability, whereas RE-SEARCH illustrates the gradual adoption of reduced starting doses followed by escalation by Spanish oncologists to enhance treatment feasibility. Both studies support the idea that flexible dosing strategies can preserve efficacy and improve safety in the routine management of refractory metastatic colorectal cancer.
Nakashima et al. (n = 2530) assessed dose appropriateness in Japan, considering body weight as a factor. Lighter patients demonstrated tolerance to low-dose regorafenib (≤120 mg), achieving outcomes similar to heavier patients on full doses, with overall survival (OS) ranging from 7.5 to 8.0 months across strata [33]. The results of ReTrITA corroborate this evidence, indicating that increasing the dosage from 80 mg does not negatively impact survival rates but rather improves tolerability, thereby underscoring the significance of personalised treatment approaches.
Yan et al. conducted an analysis of 77 patients in China, reporting an overall survival (OS) of 17.8 months and progression-free survival (PFS) of 4.6 months, which are longer than those observed in most Western cohorts. The inclusion of patients in earlier treatment lines and those receiving combinations with immunotherapy impacts the comparability of these findings [34]. However, their observation that intermediate doses (120 mg) resulted in the longest overall survival aligns with the ReTrITA finding that escalation strategies, as opposed to a standard 160 mg initiation, may provide the most favourable therapeutic index.
When compared with the large US real-world retrospective analysis by Bekaii-Saab et al. (703 patients), notable similarities and differences emerge. In the US cohort, the adoption of flexible dosing increased markedly after inclusion of the ReDOS strategy in the NCCN Guidelines (from 21% to 45%), with a corresponding improvement in treatment persistence, as reflected by a higher proportion of patients reaching the third cycle of therapy (36.5% pre-ReDOS vs. 45.5% post-ReDOS). Although survival outcomes were not the primary focus of that study, these data support the feasibility and increasing uptake of dose individualization in everyday practice [35]. Compared with the ReTrITA findings, the US study provides complementary evidence that dose escalation is being translated into clinical benefit through longer treatment duration.
The CORRELATE Taiwan analysis (128 patients) offers additional insights from an Asian setting. In this population, 71.9% of patients started regorafenib at a dose lower than 160 mg/day, and the median OS was 11.6 months with a PFS of 2.2 months [36]. This OS is numerically longer than reported in both the global CORRELATE population and in our ReTrITA sub-analysis, although cross-study comparisons should be made with caution due to potential differences in baseline characteristics, mutation prevalence, and subsequent therapies. Of note, the Taiwanese patients demonstrated a higher prevalence of RAS and BRAF mutations compared to other populations, which may have influenced outcomes.
In elderly cohorts, such as the Japanese multicentre study (Hatori et al.), reduced-dose regorafenib demonstrated sustained efficacy while reducing toxicity, with overall survival (OS) around 7.0 months [37]. This finding aligns with the ReTrITA subgroup aged ≥ 70 years, where dose escalation resulted in a median OS nearing 8.0 months.
The analysis by Peeters et al. (2024) [38], conducted in France, Italy, and Belgium with a sample of 355 patients, primarily examined treatment persistence rather than survival endpoints. Patients were categorised into three groups: ReDOS-like initiation at <160 mg/day, dose-adjusted initiation at 160 mg/day with subsequent reductions, and standard fixed-dose at 160 mg/day. The median duration of treatment (DoT) was longer in the dose-adjusted group (1.9 months) compared with both the ReDOS-like (1.4 months) and standard (1.0 months) groups. Moreover, the proportion of patients reaching at least three cycles of therapy was substantially higher in the flexible dosing groups (93% in dose-adjusted, 67% in ReDOS-like) compared with 64% in the standard arm. Patients in the flexible groups exhibited poorer baseline prognostic factors, such as ECOG PS ≥ 2, multiple metastatic sites, and greater rates of RAS mutations, yet they achieved longer treatment persistence [38]. ReTrITA and Peeters offer complementary insights when considered collectively. ReTrITA establishes that dose escalation improves disease control without compromising overall survival. Peeters emphasises that flexible regimens, including escalation or dose adjustment, enhance treatment adherence and persistence, even in patients with less favourable characteristics. Both studies converge on the conclusion that rigid adherence to the 160 mg daily starting dose is rarely feasible in clinical practice and that tailoring regorafenib dosing represents a pragmatic strategy to optimise both tolerability and continuity of care in refractory metastatic colorectal cancer.
Collectively, these real-world studies all point toward the same conclusion: regorafenib is rarely administered in real life at the rigid 160 mg starting dose used in pivotal trials. Instead, physicians routinely adapt the dose according to patient characteristics, tolerability, and guidelines. While outcomes vary slightly across regions, the consistency of PFS (~3 months) underscores the reproducibility of regorafenib activity when managed flexibly.
From a clinical perspective, this real-world sub-analysis illustrates the significance of administering the appropriate dosage of regorafenib to patients with refractory mCRC. The ReDOS experiment was the first to test the method of dosage escalation. Then, the ReTrITA group showed that flexible titration works in the real world and might help patients take the drug as directed without making it less effective. This way, doctors can change a patient’s treatment based on how well they are doing overall and how well it is working for them. In the late-line setting, where there are not many therapy alternatives and the goal is to remain up with life, this is really important. The findings corroborate recent observational studies indicating that adaptive dosing effectively balances efficacy and tolerability. Patients undergoing progressive escalation may experience prolonged exposure to the therapy, yielding cumulative benefits over time due to their increased adherence and reduced likelihood of discontinuation. The ReTrITA results support the hypothesis that regorafenib can be a helpful aspect of tailored treatment programmes.
Taken together, the strengths of the ReTrITA sub-analysis include a large, homogeneous cohort of regorafenib-treated patients, a systematic comparison between escalation and fixed dosing, and detailed subgroup analyses with forest plots that confirm the consistency of progression-free survival benefits across clinically relevant categories. It is essential to recognise the limitations. The retrospective design presents potential biases, such as non-standardised dose assignment and variability in clinical practice. Baseline imbalances were significant: variations in ECOG performance status, metastatic patterns, the extent of missing or untested MMR, prior chemotherapy intensity, and prior biologic exposure—especially the greater proportion of unknown histories in no-ReDOS—may affect both tolerability and outcomes. The presence of missing data and variability in prior treatments among different centres restricts comparability. Furthermore, although the advantages in PFS were statistically significant, the differences in OS were modest, indicating both the refractory characteristics of the population and the confounding influence of subsequent therapies. All subgroup signals should be regarded as generating hypotheses. In contrast to certain Asian studies, pharmacogenomic and body-weight-adjusted analyses were not accessible. The observational, retrospective nature of this analysis, combined with non-random allocation to dosing strategies, introduces the potential for confounding by indication.
It is important to recall that the groups were different at the outset of the study, especially when it came to ECOG performance status, MMR profile, and previous use of biologic drugs. These variations may have made the treatment less effective. These differences show how unpredictable real-world clinical practice may be. This is because treatment decisions are based on things like the patient’s health, the biology of the disease, and past treatments, not strict study inclusion requirements. So, even though there is always a chance of confounding, the general direction and size of the connections we identified support the conclusions, as we used a non-randomised, retrospective strategy.
Subgroup survival analysis reveals significant variations in overall survival and progression-free survival patterns. Excluding individuals with ECOG PS2, who exhibited suboptimal performance with ReDOS, overall survival outcomes were generally similar across all dosing regimens. Trials assessing progression-free survival consistently indicated that the fixed-dose strategy outperformed the alternative approach across nearly all clinically significant categories. This discrepancy indicates that the method of drug administration may not significantly influence survival; however, PFS suggests that in specific patient populations, a fixed dose could improve disease management.
The data demonstrate that no-ReDOS dosage consistently improves PFS, especially in patients with a history of anti-EGFR therapy, older adults, individuals with right-sided or rectal primaries, or RAS wild-type tumours. Personalised treatment approaches are essential, as ReDOS may be ineffective and potentially harmful in frailer or high-risk subgroups.
The dose-escalation strategy worked in real life and had a solid safety record. It also made patients more likely to persist with therapy without diminishing their odds of surviving. The fixed-dose group had a longer DoR than the dose-escalation group, which is interesting because the dose-escalation group tended to have higher PFS. There are several possible reasons for this. These include bias in deciding on patients in the real world, where healthier patients may have been more likely to be able to handle starting with a fixed dose; an imbalance in the number of patients in each group; and possible differences in how drugs work or how they move through the body that could change the timing and consolidation of tumour response. These findings necessitate further examination in subsequent research that include pharmacokinetic monitoring and a standardised method for assessing responsiveness.
In aggregate, ReTrITA endorses a pragmatic escalation strategy that focusses on optimising early tolerability to maintain treatment and enhance PFS, while acknowledging that OS in refractory metastatic colorectal cancer (mCRC) is significantly affected by subsequent therapies and patient frailty. The conclusions align with randomised evidence and current real-world practices that implement flexible dosing. Prospective studies ought to (i) validate escalation compared to fixed starts with standardised toxicity management, (ii) incorporate exposure metrics such as early cumulative dose and metabolite levels, and (iii) refine the selection of individuals for whom escalation is most beneficial (e.g., older adults, ECOG 1–2), ideally within a learning health system framework.
6. Conclusions
This analysis demonstrates that regorafenib dose escalation is a feasible and well-tolerated strategy for patients with refractory mCRC, enhancing treatment persistence and optimising patient management while maintaining overall survival rates. The findings endorse individualised dosing as an effective approach to improve tolerability and sustain clinical benefit in standard practice. In comparison to other extensive real-world studies, including CORRELATE, RE-SEARCH, and the Japanese and U.S. cohorts, our findings reinforce the emerging consensus that individualised dosing, as opposed to standard full-dose initiation, optimises both efficacy and tolerability. The strengths of this study include a substantial Italian multicentre dataset, thorough subgroup analyses, and relevance to real-world applications. The retrospective design and lack of prospective validation highlight the necessity for confirmatory trials. In the future, the integration of escalation protocols with the biomarker-driven selection and optimisation of supportive care may enhance regorafenib’s role in the continuum of care for mCRC.
Author Contributions
Conceptualization, C.S.; methodology, C.S.; software, C.S.; validation, C.S.; formal analysis, C.S.; investigation: C.S., M.B., M.A.C., A.A. (Annunziato Anghelone), A.P., C.G., A.B., J.L., L.A., E.D.G., I.V.Z., C.M., E.D., A.A. (Adele Artemi), D.G., D.C.C., A.E., M.R., F.M., G.A., F.Z., M.S., F.S. (Francesco Schietroma), M.G.M., F.S. (Fiorenza Santamaria), M.D., A.C., R.S., A.M., E.L.-C. and M.G.C.; resources, C.S.; data curation, C.S.; Writing—Original draft preparation, C.S.; Writing—Review and editing, C.S.; visualisation, C.S.; supervision, C.S.; project administration, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Area 4 Lazio, Rome, Italy, (protocol number 29-2024, 4 March 2024).
Informed Consent Statement
In accordance with the retrospective nature of the study, the requirement for informed consent was waived in cases where the patient was unreachable and/or unable to provide consent.
Data Availability Statement
The data to support the results reported in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Colorectal Cancer—WHO Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 12 October 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer Version 4.2025—27 June 2025. Available online: www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 19 September 2025).
- National Cancer Institute. Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 19 September 2025).
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casadei-Gardini, A.; Vagheggini, A.; Gelsomino, F.; Spallanzani, A.; Ulivi, P.; Orsi, G.; Rovesti, G.; Andrikou, K.; Tamburini, E.; Scartozzi, M.; et al. Is There an Optimal Choice in Refractory Colorectal Cancer? A Network Meta-Analysis. Clin. Colorectal Cancer 2020, 19, 82–90.e9. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Lum, C.; Latham, S.; Tipping Smith, S.; Prenen, H.; Segelov, E. Refractory Metastatic Colorectal Cancer: Current Challenges and Future Prospects. Cancer Manag. Res. 2020, 12, 5819–5830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parmar, A.; Chan, K.K.W.; Ko, Y.J. Metastatic colorectal cancer: Therapeutic options for treating refractory disease. Curr. Oncol. 2019, 26 (Suppl. 1), S24–S32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cherri, S.; Libertini, M.; Noventa, S.; Oneda, E.; Meriggi, F.; Zaniboni, A. What Is Next for Refractory Colorectal Cancer CRC? Looking Beyond SUNLIGHT, FRESCO2, RECURSE and CORRECT. Int. J. Mol. Sci. 2025, 26, 2522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Signorelli, C.; Calegari, M.A.; Basso, M.; Anghelone, A.; Lucchetti, J.; Minelli, A.; Angotti, L.; Zurlo, I.V.; Schirripa, M.; Chilelli, M.G.; et al. Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study. Curr. Oncol. 2023, 30, 5456–5469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekaii-Saab, T.; Sruti, I.; Shi, J.; Dai, W.; Patton, G.; Appukkuttan, S.; Hocum, B.; Katta, A.; Babajanyan, S.; Cosgrove, D. Real-world treatment patterns and outcomes among patients initiating sequential regorafenib and trifluridine/tipiracil ± bevacizumab in patients with metastatic colorectal cancer in a US community setting (SEQRT2). Front. Oncol. 2025, 15, 1591245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bazarbashi, S.; Alkhatib, R.; Aseafan, M.; Tuleimat, Y.; Abdel-Aziz, N.; Mahrous, M.; Elsamany, S.; Elhassan, T.; Alghamdi, M. Efficacy of Chemotherapy Rechallenge Versus Regorafenib or Trifluridine/Tipiracil in Third-Line Setting of Metastatic Colorectal Cancer: A Multicenter Retrospective Comparative Study. JCO Glob. Oncol. 2024, 10, e2300461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Signorelli, C.; Calegari, M.A.; Anghelone, A.; Passardi, A.; Frassineti, G.L.; Bittoni, A.; Lucchetti, J.; Angotti, L.; Di Giacomo, E.; Zurlo, I.V.; et al. Survival Outcomes with Regorafenib and/or Trifluridine/Tipiracil Sequencing to Rechallenge with Third-Line Regimens in Metastatic Colorectal Cancer: A Multicenter Retrospective Real-World Subgroup Comparison from the ReTrITA Study. Curr. Oncol. 2024, 31, 7793–7808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wildtype, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746, Erratum in Lancet Oncol. 2016, 17, e420. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Fountzilas, C.; Kardosh, A.; Lenz, H.J.; Elez, E.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theik, N.W.Y.; Muminovic, M.; Alvarez-Pinzon, A.M.; Shoreibah, A.; Hussein, A.M.; Raez, L.E. NTRK Therapy among Different Types of Cancers, Review and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 2366. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Di Nicolantonio, F.; Schrock, A.B.; Lee, J.; Morano, F.; Fucà, G.; Nikolinakos, P.; Drilon, A.; Hechtman, J.F.; Christiansen, J. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann. Oncol. 2018, 29, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- U.S. Food and Drug Administration. Regorafenib Prescribing Information. Bayer HealthCare Pharmaceuticals Inc. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2025/203085Orig1s016ltr.pdf (accessed on 12 October 2025).
- Aljubran, A.; Elshenawy, M.A.; Kandil, M.; Zahir, M.N.; Shaheen, A.; Gad, A.; Alshaer, O.; Alzahrani, A.; Eldali, A.; Bazarbashi, S. Efficacy of Regorafenib in Metastatic Colorectal Cancer: A Multi-institutional Retrospective Study. Clin. Med. Insights Oncol. 2019, 13, 1179554918825447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Belum, V.R.; Wu, S.; Lacouture, M.E. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: A meta-analysis. Investig. New Drugs 2013, 31, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Martinelli, E.; Cascinu, S.; Sobrero, A.; Banzi, M.; Seitz, J.F.; Barone, C.; Ychou, M.; Peeters, M.; Brenner, B.; et al. Regorafenib for Patients with Metastatic Colorectal Cancer Who Progressed After Standard Therapy: Results of the Large, Single-Arm, Open-Label Phase IIIb CONSIGN Study. Oncologist 2019, 24, 185–192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adenis, A.; de la Fouchardiere, C.; Paule, B.; Burtin, P.; Tougeron, D.; Wallet, J.; Dourthe, L.M.; Etienne, P.L.; Mineur, L.; Clisant, S.; et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016, 16, 412, Erratum in BMC Cancer 2016, 16, 518. https://doi.org/10.1186/s12885-016-2559-8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ducreux, M.; Petersen, L.N.; Öhler, L.; Bergamo, F.; Metges, J.P.; de Groot, J.W.; Wang, J.Y.; García Paredes, B.; Dochy, E.; Fiala-Buskies, S.; et al. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer in routine clinical practice in the prospective, observational CORRELATE study. Eur. J. Cancer 2019, 123, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Ou, F.S.; Ahn, D.H.; Boland, P.M.; Ciombor, K.K.; Heying, E.N.; Dockter, T.J.; Jacobs, N.L.; Pasche, B.C.; Cleary, J.M.; et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): A randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019, 20, 1070–1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Signorelli, C.; Calegari, M.A.; Anghelone, A.; Passardi, A.; Gallio, C.; Bittoni, A.; Lucchetti, J.; Angotti, L.; Di Giacomo, E.; Zurlo, I.V.; et al. Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study. Cancers 2025, 17, 2037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López Muñoz, A.M.; González Flores, E.; Carral Maseda, A.; Pimentel Cáceres, P.; Afonso Gómez, R.; López López, C.; Jimeno Maté, R.; Reina Zoilo, J.J.; Castañón López, C.; Salgado Fernández, M.; et al. Real-world dosing patterns of regorafenib for patients with metastatic colorectal cancer in Spain: The RE-SEARCH study. Clin. Transl. Oncol. 2025, 27, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Li, K.; Chen, Q.; de Silva, S.; Li, H.; Kawakami, K.; Wei, Q.; Luo, S.; Zhao, H. Appropriate dose of regorafenib based on body weight of colorectal cancer patients: A retrospective cohort study. BMC Cancer 2023, 23, 1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, H.; Liu, J.; Zhang, Y.; Chen, S.; Xu, J.; Gao, D.; Li, H.; Fang, X.; Wang, Y.; Wang, H.; et al. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: A retrospective cohort study. J. Gastrointest. Oncol. 2024, 15, 987–1001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekaii-Saab, T.; Khan, N.; Ostojic, H.; Jiao, X.; Chen, G.; Lin, W.; Bruno, A. Real-world dosing of regorafenib and outcomes among patients with metastatic colorectal cancer: A retrospective analysis using US claims data. BMC Cancer 2024, 24, 939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, K.H.; Yang, T.S.; Hsu, T.C.; Tzu-Liang Chen, W.; Chen, H.H.; Teng, H.W.; Lin, B.W.; Kuan, F.C.; Chiang, F.F.; Duann, C.W.; et al. Real-world evidence of the safety and effectiveness of regorafenib in Taiwanese patients with metastatic colorectal cancer: CORRELATE Taiwan. J. Formos. Med. Assoc. 2021, 120, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Kawakami, K.; Wakatsuki, T.; Shinozaki, E.; Kobayashi, K.; Aoyama, T.; Nakano, Y.; Suzuki, K.; Yamaguchi, K.; Hama, T. Association Between Regorafenib Dose and Efficacy Against Metastatic Colorectal Cancer in a Real-World Setting. Dose Response 2021, 19, 15593258211047658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peeters, M.; Chibaudel, B.; Barzi, A.; Ostojic, H.; Pan, X.; Yasuda, M.; Chen, C.-C.; Multani, J.; Casey, V.; Vassilev, Z. Real world (RW) use and outcomes of regorafenib (REG) flexible dosing in patients (pts) with metastatic colorectal cancer (mCRC) in Europe. J. Clin. Oncol. 2024, 42, 47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).