Comparative Evaluation of Risk Assessment Models for Predicting Venous Thromboembolic Events in Cancer Patients with Implanted Central Venous Access Devices
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
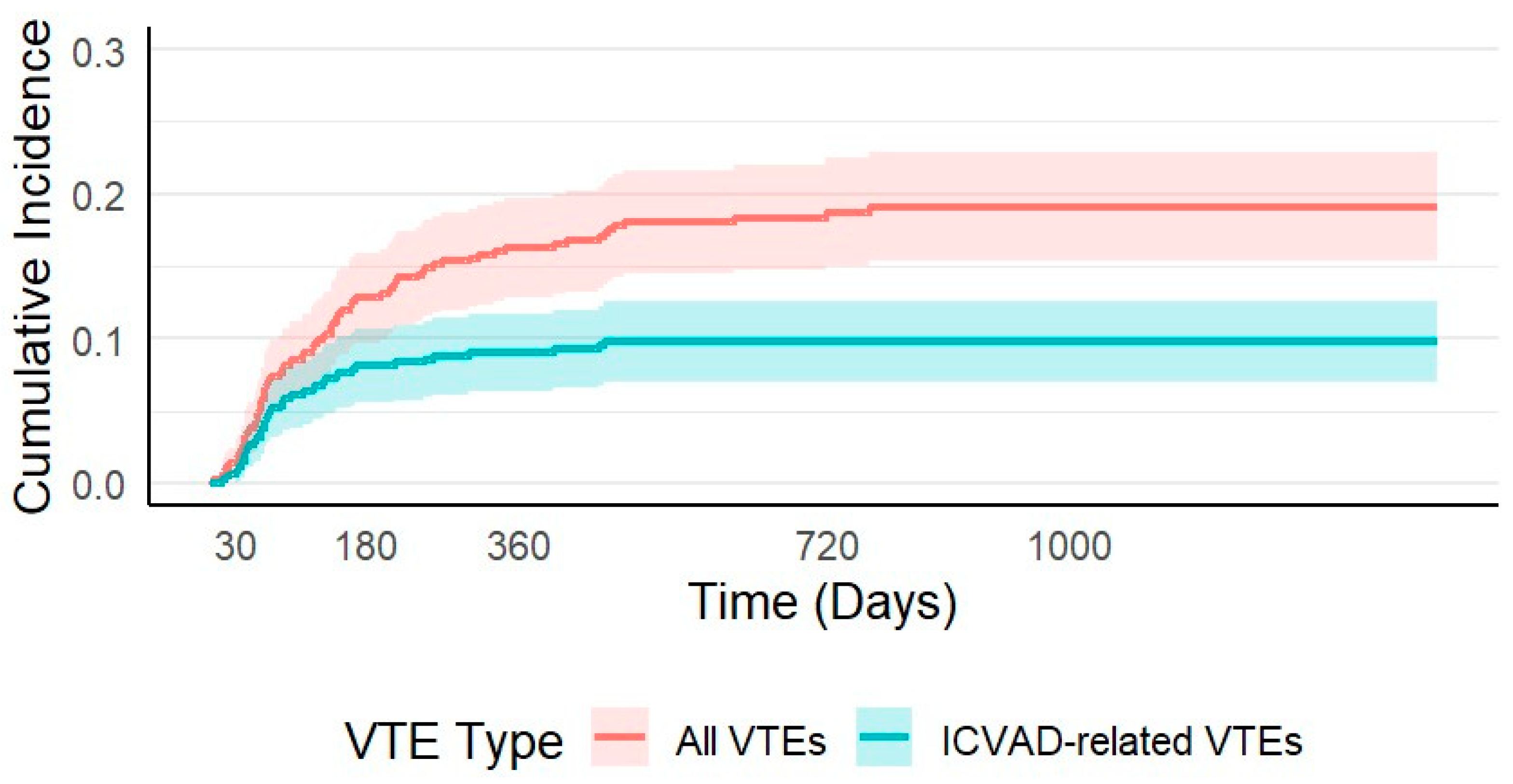
3.2. Venous Thromboembolism
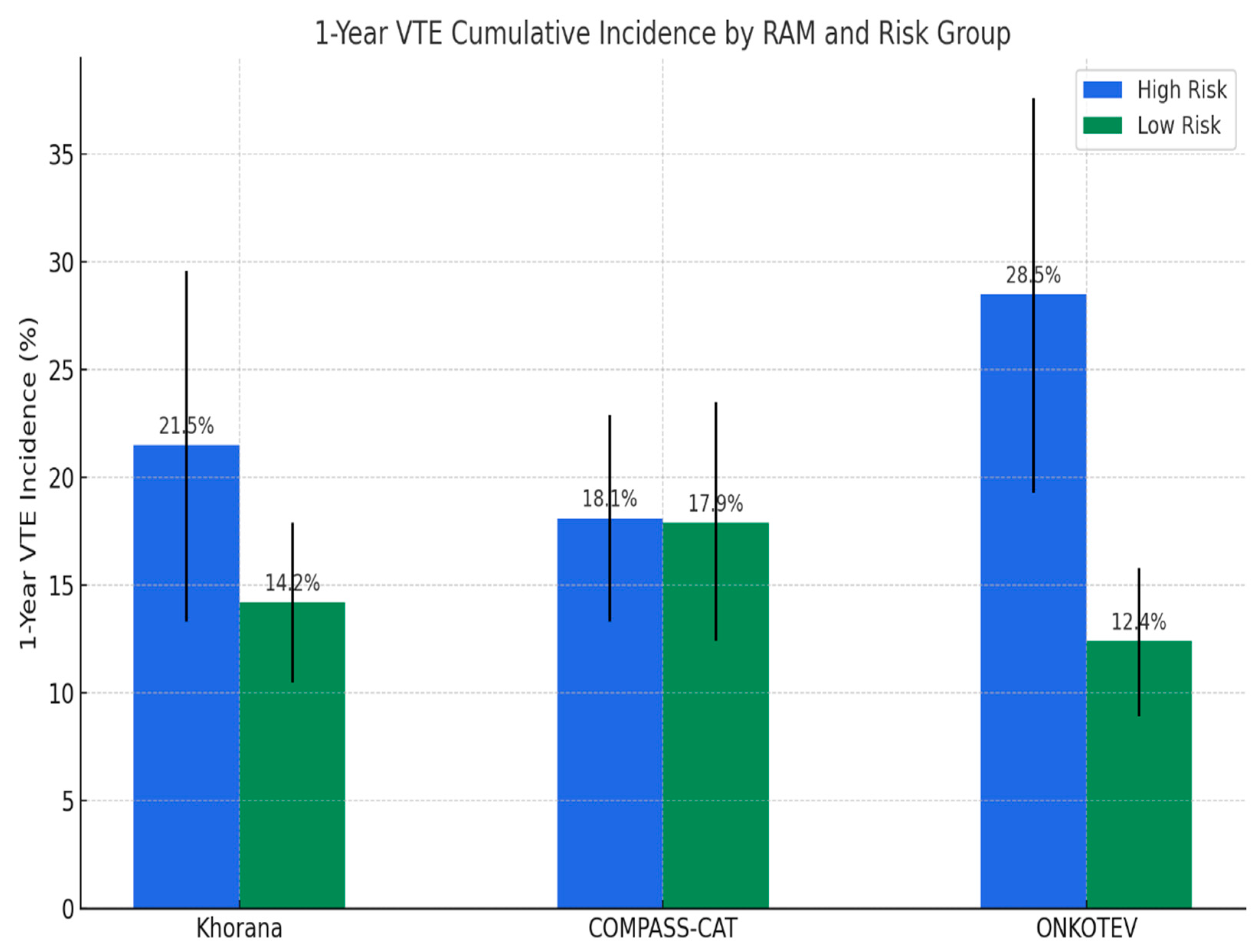
3.3. Risk Assessment Models
3.3.1. All VTEs
3.3.2. ICVAD Related VTEs
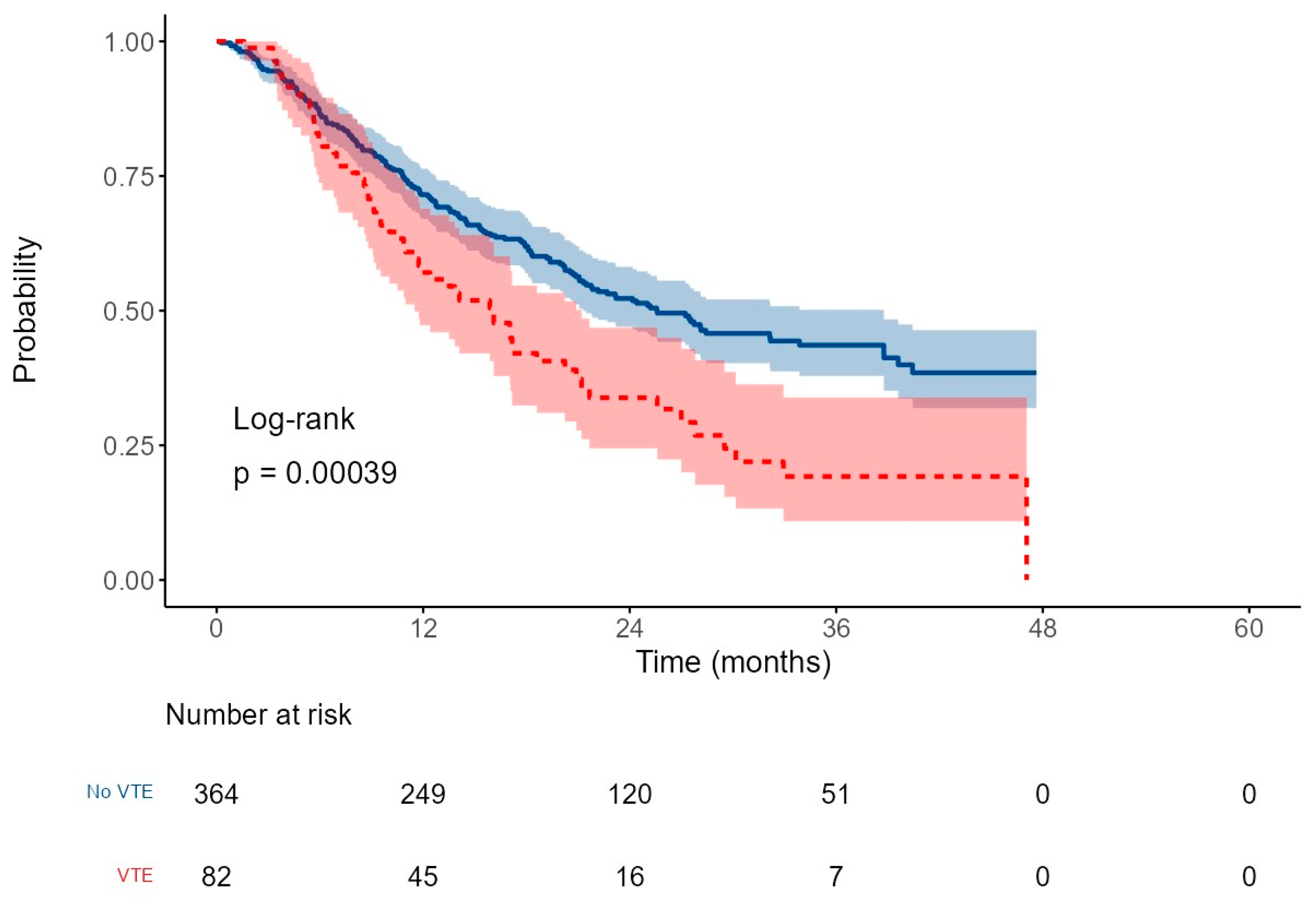
3.4. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the receiver operating characteristic curve |
| BMI | Body mass index |
| CI | Cumulative incidence |
| CVC | Central venous catheter |
| DOAC | Direct oral anticoagulant |
| DVT | Deep vein thrombosis |
| Hb | Hemoglobin |
| HR | Hazard ratio |
| ICVAD | Implanted central venous access device |
| LMWH | Low-molecular-weight heparin |
| NPV | Negative predictive value |
| OS | Overall survival |
| PE | Pulmonary embolism |
| PPV | Positive predictive value |
| RAM | Risk assessment model |
| ROC | Receiver operating characteristic |
| VTE | Venous thromboembolic event(s) |
| WBC | White blood cell count |
References
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Doggen, C.J.M.; Osanto, S.; Rosendaal, F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J. Clin. Oncol. 2003, 21, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Anand, S.; Hickner, A.; Buist, M.; Rogers, M.A.; Saint, S.; Flanders, S.A. Risk of venous thromboembolism associated with peripherally inserted central catheters: A systematic review and meta-analysis. Lancet 2013, 382, 311–325, Erratum in Lancet 2013, 382, 1328. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, I.; Pulvirenti, E.; Mannino, M.; Toro, A. Increased use of percutaneous technique for totally implantable venous access devices. Is it real progress? A 27-year comprehensive review on early complications. Ann. Surg. Oncol. 2010, 17, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Soh, K.L.; Ying, Y.; Liu, Y.; Huang, X.; Huang, J. Risk of VTE associated with PORTs and PICCs in cancer patients: A systematic review and meta-analysis. Thromb. Res. 2022, 213, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Campello, E. Venous thromboembolism in cancer patients undergoing chemotherapy: Risk factors and prevention. Semin. Thromb. Hemost. 2021, 47, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Decousus, H.; Bourmaud, A.; Fournel, P.; Bertoletti, L.; Labruyère, C.; Presles, E.; Merah, A.; Laporte, S.; Stefani, L.; Del Piano, F.; et al. ONCOCIP Investigators. Cancer-associated thrombosis in patients with implanted ports: A prospective multicenter French cohort study (ONCOCIP). Blood 2018, 132, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Moinat, C.H.; Périard, D.; Grueber, A.; Hayoz, D.; Magnin, J.-L.; André, P.; Kung, M.; Betticher, D.C. Predictors of venous thromboembolic events associated with central venous port insertion in cancer patients. J. Oncol. 2014, 2014, 743181. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khorana, A.A.; McCrae, K.R.; Milentijevic, D.; Fortier, J.; Nelson, W.W.; Laliberté, F.; Crivera, C.; Lefebvre, P. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res. Pract. Thromb. Haemost. 2017, 1, 14–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; A Khorana, A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymoss, S.; et al. International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019, 20, e566–e581. [Google Scholar] [CrossRef] [PubMed]
- van Es, N.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Otten, H.-M.; Mahé, I.; Wilts, I.T.; Twint, D.C.; Porreca, E.; Arrieta, O.; et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica 2017, 102, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I. COMPASS–CAT Working Group. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: The prospective COMPASS-cancer-associated thrombosis study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Chen, F.; Deng, L.; Lin, K.; Shi, X.; Zhaoliang, S.; Wang, Y. Febuxostat attenuates paroxysmal atrial fibrillation-induced regional endothelial dysfunction. Thromb. Res. 2017, 149, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, M.; Kretzschmar, A.; Kröning, H.; Biakhov, M.; Irwin, D.; Marschner, N.; Slabber, C.; Fountzilas, G.; Garin, A.; Abecasis, N.G.F.; et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: Final results of a double-blind, placebo-controlled phase III trial. Ann. Oncol. 2006, 17, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Bertoglio, S.; Di Somma, F.C.; Paoletti, F.; Ageno, W.; Bazzan, M.; Parise, P.; Quintavalla, R.; Naglieri, E.; et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: A double-blind, placebo-controlled, randomized study in cancer patients. J. Clin. Oncol. 2005, 23, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Brandt, W.; Brown, C.; Wang, T.-F.; Tagalakis, V.; Shivakumar, S.; Ciuffini, L.A.; Mallick, R.; Wells, P.S.; Carrier, M. Efficacy and safety of apixaban for primary prevention of thromboembolism in patients with cancer and a central venous catheter: A subgroup analysis of the AVERT trial. Thromb. Res. 2022, 216, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Bull, L.; Kinzie, M.; Andresen, M. Central catheter-associated deep vein thrombosis in cancer: Clinical course, prophylaxis, treatment. BMJ Support. Palliat. Care 2021, 11, 371–380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piran, S.; Ngo, V.; McDiarmid, S.; Le Gal, G.; Petrcich, W.; Carrier, M. Incidence and risk factors of symptomatic venous thromboembolism related to implanted ports in cancer patients. Thromb. Res. 2014, 133, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Jordan, K.; Buerke, M.; Arnold, D.; John, E.; Spielmann, R.-P.; Behrmann, C. Port catheter insufficiency: Incidence and clinical-radiological correlations. Onkologie 2008, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Knebel, P.; Lopez-Benitez, R.; Fischer, L.; Radeleff, B.A.; Stampfl, U.; Bruckner, T.; Hennes, R.; Kieser, M.; Kauczor, H.-U.; Büchler, M.W.; et al. Insertion of totally implantable venous access devices: An expertise-based, randomized, controlled trial (NCT00600444). Ann. Surg. 2011, 253, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Büller, H.R.; van Es, N. CAT-prediction collaborators. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Overvad, T.F.; Ording, A.G.; Nielsen, P.B.; Skjøth, F.; Albertsen, I.E.; Noble, S.; Vistisen, A.K.; Gade, I.L.; Severinsen, M.T.; Piazza, G.; et al. Validation of the Khorana score for predicting venous thromboembolism in 40,218 patients with cancer initiating chemotherapy. Blood Adv. 2022, 6, 2967–2976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spyropoulos, A.C.; Eldredge, J.B.; Anand, L.N.; Zhang, M.; Qiu, M.; Nourabadi, S.; Rosenberg, D.J. External validation of a venous thromboembolic risk score for cancer outpatients with solid tumors: The COMPASS-CAT venous thromboembolism risk assessment model. Oncologist 2020, 25, e1083–e1090. [Google Scholar] [CrossRef] [PubMed]
- Vladić, N.; Englisch, C.; Berger, J.M.; Moik, F.; Berghoff, A.S.; Preusser, M.; Pabinger, I.; Ay, C. Validation of risk assessment models for venous thromboembolism in patients with cancer receiving systemic therapies. Blood Adv. 2025, 9, 3340–3349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cella, C.A.; Knoedler, M.; Hall, M.; Arcopinto, M.; Bagnardi, V.; Gervaso, L.; Pellicori, S.; Spada, F.; Zampino, M.G.; Ravenda, P.S.; et al. Validation of the ONKOTEV risk prediction model for venous thromboembolism in outpatients with cancer. JAMA Netw. Open 2023, 6, e230010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Godinho, J.; Casa-Nova, M.; Moreira-Pinto, J.; Simões, P.; Branco, F.P.; Leal-Costa, L.L.; Faria, A.; Lopes, F.; Teixeira, J.A.; Passos-Coelho, J.L. ONKOTEV score as a predictive tool for thromboembolic events in pancreatic cancer: A retrospective analysis. Oncologist 2020, 25, e284–e290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cella, C.A.; Djulbegovic, B.; Hozo, I.; Lordick, F.; Bagnardi, V.; Frassoni, S.; Gervaso, L.; Fazio, N. Comparison of Khorana vs. ONKOTEV predictive score to individualize anticoagulant prophylaxis in outpatients with cancer. Eur. J. Cancer. 2024, 209, 114234. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crobach, M.J.T.; Anijs, R.J.S.; Brækkan, S.K.; Severinsen, M.T.; Hammerstrøm, J.; Skille, H.; Kristensen, S.R.; Paulsen, B.; Tjønneland, A.; Versteeg, H.H.; et al. Survival after cancer-related venous thrombosis: The Scandinavian Thrombosis and Cancer Study. Blood Adv. 2023, 7, 4072–4079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, U.T.; Walker, A.J.; Baig, S.; Card, T.R.; Kirwan, C.C.; Grainge, M.J. Venous thromboembolism and mortality in breast cancer: Cohort study with systematic review and meta-analysis. BMC Cancer 2017, 17, 747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, K.; Duan, R.; Wu, Y. Prognostic value of venous thromboembolism in patients with advanced pancreatic cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1331706. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Zhang, L.; Zhong, D. Chemotherapy-induced thrombocytopenia: Literature review. Discov. Oncol. 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number (%) | 12-Month VTE CI (95% CI) | HR (95% CI) | p-Value |
|---|---|---|---|---|
| Age ≥ 60 years | 124 (34%) | 18.8% (12.6–25.0%) | 1.24 (0.80–1.93) | 0.33 |
| Sex | ||||
| Female | 235(52.7%) | 15.3% (10.4–20.2%) | 1.00 (0.65–1.54) | 0.995 |
| Male | 211 (47.3%) | 16.3% (11.5–21.0%) | ||
| Current smoker | 138 (31%) | 18.9% (12.3–25.4%) | 1.34 (0.83–2.17) | 0.23 |
| BMI ≥ 30 kg/m2 | 118 (26.5%) | 18.3% (11.0–26.1%) | 0.85 (0.43–1.70) | 0.65 |
| Antiplatelet | 49 (11%) | 18.7% (7.5–29.8%) | 1.13 (0.59–2.14) | 0.7 |
| Time from cancer diagnosis to ICVAD insertion <6 months | 253 (79.1%) | 16.6% (12.7–20.5%) | 1.07 (0.59–2.14) | 0.33 |
| Comorbidities | ||||
| Diabetes | 122 (27.4%) | 20.7% (13.4–27.9%) | 1.70 (1.09–2.64) | 0.02 |
| Hypertension | 153 (34.4%) | 17.7% (11.6–23.8%) | 1.19 (0.77–1.87) | 0.43 |
| Peripheral vascular disease | 6 (1.3%) | 0% (0–0%) | 1.43 (0.43–4.78) | 0.24 |
| Stroke | 4 (0.9%) | 0% (0–0%) | 1.27 (0.21–7.67) | 0.82 |
| Coronary artery disease | 33 (7.4%) | 18.2% (4.8–31.6%) | 1.17 (0.55–2.50) | 0.68 |
| Hyperlipidemia | 56 (12.6%) | 12.5 (3.8–21.2%) | 0.85 (0.43–1.70) | 0.69 |
| ≥2 comorbidities | 49 (11%) | 16.4% (10.3–22.6%) | 1.01 (0.93–1.11) | 0.76 |
| Cancer site | ||||
| Gastric | 116 (26%) | 19.3% (12.0–26.6%) | 1.14 (0.71–1.84) | 0.58 |
| Pancreatic | 82 (18.4%) | 18.4% (9.9–26.9%) | 1.22 (0.71–2.10) | 0.44 |
| Colorectal | 132 (29.6%) | 16.7% (10.3–23.1%) | 1.32 (0.84–2.07) | 0.23 |
| Breast | 62 (13.9%) | 8.1% (1.2–14.9%) | 0.45 (0.20–1.04) | 0.063 |
| Head and neck | 19 (4.3%) | 0% (0–0%) | 0 (0–0) | <0.001 |
| Esophageal | 22 (4.9%) | 15.9% (12.4–19.4%) | 1.28 (0.51–3.21) | 0.6 |
| Others * | 13 (2.9%) | 0% (0–0%) | 0.74 (0.21–2.68) | 0.65 |
| High risk sites according to Khorana RAM | 198 (44%) | 19.4% (13.9–25.0%) | 1.26 (0.82–1.94) | 0.28 |
| Localized disease | 199(44.6%) | 12.6% (8.0–17.3%) | 1.71 (1.08–2.72) | 0.023 |
| Metastatic disease | 247 (55.4%) | 18.4% (13.5–23.2%) | ||
| Localized | 136(30.5%) | 13.3% (7.5–19.0%) | 1.18 (0.92–1.52) | 0.2 |
| Locally advanced or metastatic | 310 (69.5%) | 16.9% (12.7–21.1%) | ||
| Vascular or lymphatic compression | 71 (15.9%) | 29.9% (19.1–40.8%) | 2.44 (1.47–4.06) | <0.001 |
| No vascular or lymphatic compression | 375 (84.1%) | 13.1% (9.7–16.6%) | ||
| WBC > 11 × 109/L | 42 (9.4%) | 29.1% (15.0–43.2%) | 2.19 (1.24–3.86) | 0.007 |
| Hb < 10 g/dL | 69 (15.5%) | 17.6% (8.5–26.8%) | 1.27 (0.73–2.21) | 0.4 |
| Platelets ≥ 350 × 103/µL | 111 (24.9%) | 15.3% (8.6–22.1%) | 0.98 (0.60–1.61) | 0.93 |
| Hospitalization | 109 (24.4%) | 13.0% (6.6–19.4%) | 0.82 (0.48–1.40) | 0.46 |
| Anthracycline or hormonal therapy | 29 (6.5%) | 6.9% (0–16.3%) | 0.89 (0.74–1.08) | 0.23 |
| Novel therapy | 84 (18.8%) | 14.5% (6.8–22.1%) | 0.97 (0.55–1.69) | 0.9 |
| Line infection | 27 (8.3%) | 13.5% (2.3–24.7%) | 1.31 (0.68–2.52) | 0.42 |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| WBC > 11 × 109/L | 1.72 (0.95–3.11) | 0.073 |
| Diabetes | 1.44 (0.92–2.27) | 0.110 |
| Metastatic disease | 1.37 (0.85–2.20) | 0.190 |
| Vascular compression | 2.10 (1.27–3.48) | 0.0038 |
| Metric | Khorana % (95% CI) | ONCOTEV % (95% CI) | COMPASS-CAT % (95% CI) |
|---|---|---|---|
| All VTEs | |||
| Sensitivity | 28.0 (19.5–38.6) | 39.0 (29.2–49.8) | 57.3 (46.5–67.5) |
| Specificity | 79.1 (74.7–83.0) | 82.4 (78.2–86.0) | 43.4 (38.4–48.5) |
| PPV | 23.2 (16.0–32.5) | 33.3 (24.7–43.2) | 18.6 (14.3–23.8) |
| NPV | 83.0 (78.7–86.6) | 85.7 (81.7–89.0) | 81.9 (75.8–86.7) |
| Accuracy | 69.7 (65.3–73.8) | 74.4 (70.2–78.3) | 46.0 (41.4–50.6) |
| AUC | 0.536 | 0.607 | 0.504 |
| ICVAD-related VTEs | |||
| Sensitivity | 23.3 (12.0–39.0) | 30.2 (17.0–46.0) | 53.5 (38.0–69.0) |
| Specificity | 77.9 (74.0–82.0) | 79.4 (75.0–83.0) | 42.9 (38.0–48.0) |
| PPV | 10.1 (5.0–18.0) | 13.5 (7.0–22.0) | 9.1 (6.0–13.0) |
| NPV | 90.5 (87.0–93.0) | 91.4 (88.0–94.0) | 89.6 (84.0–94.0) |
| Accuracy | 72.6 (68.0–77.0) | 74.7 (70.0–79.0) | 43.9 (39.0–49.0) |
| AUC | 0.506 | 0.548 | 0.482 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma’koseh, M.; Farfoura, H.; Abunasser, M.; El-Atrash, M.; Zayed, A.; Hamdan-Mansour, R.; Abdel Rahman, Z.; Ghatasheh, T.; Alshobaki, M.; Al-Jaghbeer, M.J.; et al. Comparative Evaluation of Risk Assessment Models for Predicting Venous Thromboembolic Events in Cancer Patients with Implanted Central Venous Access Devices. Cancers 2025, 17, 3308. https://doi.org/10.3390/cancers17203308
Ma’koseh M, Farfoura H, Abunasser M, El-Atrash M, Zayed A, Hamdan-Mansour R, Abdel Rahman Z, Ghatasheh T, Alshobaki M, Al-Jaghbeer MJ, et al. Comparative Evaluation of Risk Assessment Models for Predicting Venous Thromboembolic Events in Cancer Patients with Implanted Central Venous Access Devices. Cancers. 2025; 17(20):3308. https://doi.org/10.3390/cancers17203308
Chicago/Turabian StyleMa’koseh, Mohammad, Heba Farfoura, Mahmoud Abunasser, Maryam El-Atrash, Anas Zayed, Renad Hamdan-Mansour, Zaid Abdel Rahman, Tala Ghatasheh, Mohammad Alshobaki, Mohammed J. Al-Jaghbeer, and et al. 2025. "Comparative Evaluation of Risk Assessment Models for Predicting Venous Thromboembolic Events in Cancer Patients with Implanted Central Venous Access Devices" Cancers 17, no. 20: 3308. https://doi.org/10.3390/cancers17203308
APA StyleMa’koseh, M., Farfoura, H., Abunasser, M., El-Atrash, M., Zayed, A., Hamdan-Mansour, R., Abdel Rahman, Z., Ghatasheh, T., Alshobaki, M., Al-Jaghbeer, M. J., & Abdel-Razeq, H. (2025). Comparative Evaluation of Risk Assessment Models for Predicting Venous Thromboembolic Events in Cancer Patients with Implanted Central Venous Access Devices. Cancers, 17(20), 3308. https://doi.org/10.3390/cancers17203308







