The Urokinase-Type Plasminogen Activator Receptor (uPAR) as a Mediator of Physiological and Pathological Processes: Potential Therapeutic Strategies
Simple Summary
Abstract
1. Introduction
Role of uPAR in Physiology and Pathology
2. Molecular Structure of uPAR
2.1. Structural Domains and Ligand Binding
2.2. uPAR in Cell Signaling Pathways
| Ligand/Interacting Partner | Binding Domain on uPAR | Functional Effects | Reference |
|---|---|---|---|
| uPA (urokinase-type plasminogen activator) | DI | Activates plasminogen, leading to ECM degradation, cell migration, and invasion | [82] |
| Vitronectin | DI, DII, DIII | Stabilizes the uPA-uPAR complex, enhances cell adhesion and migration | [61,83] |
| Integrins (e.g., α5β1, αvβ3) | DI, DII, DIII | Promotes focal adhesion formation, facilitates cell migration, signaling | [63,84,85] |
| Factor XII | DII | Initiates the intrinsic coagulation pathway and contributes to fibrinolysis and inflammatory responses | [86,87] |
| High molecular weight kinin-free kininogen (HKa) | DI-DII | Regulates inflammation and coagulation processes | [88] |
| Factor VIIa | DII | Involved in the extrinsic coagulation pathway, influences cell signaling | [86] |
| Tissue Factor Pathway Inhibitor (TFPI) | - | Regulate coagulation by inhibiting the TF-FVIIa complex | [89] |
| Thrombospondin-1 (TSP-1) | - | Inhibits angiogenesis, modulates uPAR-dependent signaling | [90,91] |
| Urokinase receptor-associated protein (uPARAP/Endo180) | DI-DIII | Facilitates collagen degradation, linked to ECM remodeling and tumor progression | [92] |
| Plasminogen | DI | Precursor of plasmin, plays a role in fibrinolysis, linked to cell migration | [93,94] |
| SRPX2 | DI-DII-DIII | SRPX2 binds to uPAR, facilitating angiogenesis and tumor cell migration | [95] |
| Amino-terminal fragment (ATF) | DI | ATF binds to uPAR, promoting proteolysis, cell migration, and cancer progression | [45,88] |
| Cathepsin B | DI-DIII | Protease that can degrade ECM, involved in tumor progression and metastasis | [96] |
| Streptococcal surface dehydrogenase (SDH) | DI | SDH binds TO uPAR, aiding bacterial adherence and contributing to Group A Streptococcus infection | [97] |
| RAGE | - | RAGE interacts with uPAR through integrin αVβ3, promoting ROS production and tumor cell migration | [98] |
| uPAR-associated GPI-anchored proteins (GPI-APs) | GPI-anchor region | uPAR interacts with various GPI-APs, contributing to signal transduction and cell migration | [99] |
| α2-Macroglobulin | DI | Binds uPAR-uPA complex, involved in cell signaling and tissue remodeling | [100] |
| Fibroblast Activation Protein (FAP) | - | FAP interacts with uPAR through FAK-Src-JAK2 signaling, promoting tumor invasion and immune suppression | [101,102,103] |
| Complement component C3a | - | Involved in immune response modulation, enhances inflammation | [104] |
| gp130 | - | gp130 interacts with uPAR via the JAK/STAT3 pathway, promoting tumor cell proliferation and immune modulation | [78] |
| EGFR (Epidermal Growth Factor Receptor) | DII-DIII | uPAR-EGFR interaction enhances EGFR signaling, leading to increased cell proliferation, migration, and survival | [52,105] |
| GPCRs (e.g., FPRL1/LXA4R) | Variable | Facilitates chemotaxis, immune response modulation, and cancer cell invasion | [52,106] |
3. uPAR and Cancer
3.1. Involvement in Tumor Progression
3.2. Invasion and Metastasis
3.3. uPAR in Bone Metastasis and Skeletal Remodeling
3.4. Regulation of Angiogenesis
3.5. Modulation of the Tumor Microenvironment
4. Clinical Significance of uPAR as a Biomarker in Cancer
4.1. Diagnostic and Prognostic Implications
4.2. Therapeutic Targeting of uPAR in Cancer
4.2.1. Small Molecule and Peptide-Based Inhibitors
4.2.2. Monoclonal Antibodies
4.2.3. RNA-Based Therapies
4.2.4. Combination Therapies Involving Immune Checkpoint Inhibitors
4.2.5. Role of PAI-1 in uPAR-Targeted Therapy
| Therapeutic Approach | Therapeutic Agents | Mechanism of Action | References |
|---|---|---|---|
| Monoclonal Antibodies (mAbs) | huATN-658-Humanized mAb targeting uPAR DII–DIII (MNPR-101) | Blocks uPAR-uPA interaction, reducing tumor growth and metastasis | [149,159,177,178] |
| 2G10-Anti-uPAR mAb | Inhibits uPAR-α5β1 integrin interaction, reducing migration and invasion | [161,162,162,179] | |
| VH Domains Targeting uPAR | Human antibody VH domains targeting uPAR for reducing metastasis and tumor growth | [180] | |
| 4G10-Anti-uPAR mAb | Targets uPAR-integrin interaction, reducing metastasis | [181,182] | |
| 8B12 | Blocks uPA-uPAR interaction, reducing ECM degradation and cancer cell invasion | [182,183,184] | |
| Small Molecule Inhibitors | Upamostat (WX-671)-Oral uPAR inhibitor | Blocks uPA binding to uPAR, inhibiting plasminogen activation and tumor invasion | [45,153,185] |
| IPR-456 | Inhibits uPAR, blocking cell invasion and metastasis | [186] | |
| IPR-803 | Inhibits uPAR-mediated signaling, reducing tumor cell invasion and metastasis | [187,188] | |
| ARM-U2 | Inhibits uPAR-uPA interaction, reducing metastasis and tumor growth | [189] | |
| LLL fsi | Blocks uPAR signaling, reducing cancer cell invasion and metastasis | [190] | |
| Compound 6 | Blocks cell adhesion to Vn Block cell adhesion to vitronectin and impair FPR-dependent cell migration | [191] | |
| Compound 37 | |||
| 2-(Pyridin-2-ylamino)-quinolin-8-ol | Inhibits uPAR, impairing cell migration and reducing metastasis | [105] | |
| 2,2′-(methylimino)di (8-quinolinol) | Inhibits uPAR, blocking cell migration and tumor progression | ||
| Therapeutic Peptides | WX-360 | Inhibits uPAR, reducing tumor cell migration and invasion | [192,193] |
| AE105 | Binds to uPAR, blocking its interaction with ligands, reducing tumor invasion | [194] | |
| AE120 | Targets uPAR, inhibiting cancer cell migration and metastasis | ||
| pERERY-NH2 | Interferes with uPAR-mediated signaling, impairing tumor cell invasion | [195] | |
| RERF | Inhibits uPAR, blocking cancer cell adhesion and migration | [196] | |
| UPARANT (cenupatide) | Blocks uPAR activation, reducing tumor angiogenesis and metastasis | [192,193,194,195,196,197,198,199,200,201,202,203] | |
| RI-3 | Inhibits uPAR-uPA interaction, impairing cancer progression | [204] | |
| Cyclized SRSRY | Inhibits uPAR, blocking integrin interaction, reducing cell migration | [205] | |
| SRS(P)RY | Disrupts uPAR signaling, impairing cell migration and invasion | [206] | |
| P25 | Binds uPAR, blocking interactions with ligands, reducing metastasis | [68,207] | |
| M25 | Impairs β1-integrindependent spreading and migration | [208] | |
| α325 | Blocks filopodia formation and matrix invasion in vitro | [209,210] | |
| m.P243-251 | Binds to uPAR, impairing cancer cell invasion and metastasis | [211] | |
| Nanodrugs | AE147 Peptide-Conjugated Nanocarriers | Targets uPAR-overexpressing cancer cells, delivering therapeutic agents and reducing metastasis | [176,212] |
5. uPAR in Other Diseases
5.1. uPAR in Cardiovascular Diseases
5.2. uPAR in Infectious Diseases
5.3. uPAR in Neurological Disorders
| Disease Category | Role of uPAR | Mechanism/Process Involved | Implications | References |
|---|---|---|---|---|
| Cardiovascular Diseases | Atherosclerosis, Thrombosis, Cardiac Remodeling | uPAR promotes smooth muscle cell migration, ECM degradation and inflammation | Increases risk of cardiovascular events (e.g., MI, heart failure) | [40,122,246] |
| Infectious Diseases | Bacterial Infections (e.g., Streptococcus), Viral Infections (e.g., COVID-19) | uPAR regulates immune cell recruitment; suPAR elevated in severe cases | Biomarker for disease severity, impacts infection outcomes | [16,226,247,248,249] |
| Lung Fibrosis | Promotes fibroblast activation, immune cell recruitment, and ECM remodeling | uPAR/uPA axis drives TGF-β signaling, myofibroblast differentiation, collagen deposition, and chronic inflammation | Contributes to ARDS, post-viral fibrosis (e.g., COVID-19), and idiopathic pulmonary fibrosis (IPF) | [230,231,232,233] |
| Neurological Disorders | Neurodegeneration (e.g., Alzheimer’s, Parkinson’s), MS | uPAR mediates BBB breakdown, ECM degradation, neuroinflammation | Contributes to neurodegeneration, demyelination, cognitive decline | [18,250,251,252,253] |
| Autoimmune Diseases | Rheumatoid Arthritis, Lupus | uPAR involved in joint inflammation, ECM remodeling, immune response | Promotes chronic inflammation, joint destruction | [254,255,256,257,258] |
| Ocular Diseases | Age-Related Macular Degeneration (AMD) | uPAR involved in abnormal blood vessel growth (neovascularization) | Linked to progression of AMD and other retinal diseases | [259,260] |
| Pulmonary Diseases | Chronic Obstructive Pulmonary Disease (COPD), Asthma | uPAR involved in airway remodeling and inflammation | Correlates with disease severity and lung function decline | [44,261,262] |
| Renal Diseases | Glomerulonephritis, Chronic Kidney Disease (CKD) | uPAR regulates podocyte dysfunction, immune responses | Associated with kidney damage, proteinuria | [263,264,265] |
| Gastrointestinal Diseases | Inflammatory Bowel Disease (IBD), Crohn’s Disease | uPAR promotes mucosal inflammation, immune cell migration | Associated with worsened inflammation and disease severity | [265,266,267] |
| Cancer Metastasis | Various Cancers | uPAR mediates ECM degradation, cell migration, invasion | Associated with cancer progression, metastasis | [34,36,268,269,270,271] |
6. Novel Insights into uPAR Function and Regulation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stahl, A.; Mueller, B.M. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J. Cell Biol. 1995, 129, 335–344. [Google Scholar] [CrossRef]
- Zhai, B.-T.; Tian, H.; Sun, J.; Zou, J.-B.; Zhang, X.-F.; Cheng, J.-X.; Shi, Y.-J.; Fan, Y.; Guo, D.-Y. Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer. J. Transl. Med. 2022, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Carmeliet, P. uPAR: A versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 2002, 3, 932–943. [Google Scholar] [CrossRef]
- Mahmood, N.; Rabbani, S.A. Fibrinolytic system and cancer: Diagnostic and therapeutic applications. Int. J. Mol. Sci. 2021, 22, 4358. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): Diagnostic, prognostic, and therapeutic applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Leurer, C.; Rabbani, S.A. Plasminogen Activator System–Diagnostic, Prognosis and Therapeutic Implications in Breast Cancer. In A Concise Review of Molecular Pathology of Breast Cancer; IntechOpen: London, UK, 2015. [Google Scholar]
- Rabbani, S.A.; Mazar, A.P. The role of the plasminogen activation system in angiogenesis and metastasis. Surg. Oncol. Clin. N. Am. 2001, 10, 393–415. [Google Scholar] [CrossRef]
- Shetty, S.; Idell, S. Urokinase/urokinase receptor-mediated signaling in cancer. In Apoptosis, Cell Signaling, and Human Diseases; Molecular Mechanisms; Humana Press: Totowa, NJ, USA, 2007; Volume 2, pp. 167–179. [Google Scholar]
- Zhang, H.; Xie, Z.; Xie, S.; Wu, J.; Khan, M.; Gao, P.; Li, J. Targeting Urokinase-type plasminogen activator receptor (uPAR) in cancer therapy: Insights from the development of small-molecule inhibitors. Bioorg. Chem. 2025, 163, 108773. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Wu, C.-Z.; Cheng, C.-W.; Chen, J.-S.; Chang, L.-C. Redrawing Urokinase Receptor (uPAR) Signaling with Cancer Driver Genes for Exploring Possible Anti-Cancer Targets and Drugs. Pharmaceuticals 2023, 16, 1435. [Google Scholar] [CrossRef]
- Vincenza Carriero, M.; Patrizia Stoppelli, M. The urokinase-type plasminogen activator and the generation of inhibitors of urokinase activity and signaling. Curr. Pharm. Des. 2011, 17, 1944–1961. [Google Scholar] [CrossRef] [PubMed]
- Roldan, A.; Cubellis, M.V.; Masucci, M.; Behrendt, N.; Lund, L.; Danø, K.; Appella, E.; Blasi, F. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J. 1990, 9, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Laurenzana, A.; Margheri, F.; Biagioni, A.; Chillà, A.; Pimpinelli, N.; Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Calorini, L.; Serratì, S. EGFR/uPAR interaction as druggable target to overcome vemurafenib acquired resistance in melanoma cells. eBioMedicine 2019, 39, 194–206. [Google Scholar] [CrossRef]
- Huai, Q.; Mazar, A.P.; Kuo, A.; Parry, G.C.; Shaw, D.E.; Callahan, J.; Li, Y.; Yuan, C.; Bian, C.; Chen, L. Structure of human urokinase plasminogen activator in complex with its receptor. Science 2006, 311, 656–659. [Google Scholar] [CrossRef]
- Mondino, A.; Blasi, F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004, 25, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yepes, M. The uPA/uPAR system in astrocytic wound healing. Neural Regen. Res. 2022, 17, 2404–2406. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Diaz, A.; Yepes, M. Urokinase-type plasminogen activator (uPA) and its receptor (uPAR) promote neurorepair in the ischemic brain. Recept. Clin. Investig. 2017, 4, e1552. [Google Scholar]
- Montuori, N.; Ragno, P. Role of uPA/uPAR in the modulation of angiogenesis. In Angiogenesis, Lymphangiogenesis and Clinical Implications; Chemical Immunology and Allergy; S. Karger AG: Basel, Switzerland, 2014; Volume 99, pp. 105–122. [Google Scholar]
- Breuss, J.M.; Uhrin, P. VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adhes. Migr. 2012, 6, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.D.; Margheri, F.; Serratì, S.; Chillà, A.; Laurenzana, A.; Fibbi, G. The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Curr. Pharm. Des. 2011, 17, 1924–1943. [Google Scholar] [CrossRef]
- Alfano, D.; Franco, P.; Stoppelli, M.P. Modulation of cellular function by the urokinase receptor signalling: A mechanistic view. Front. Cell Dev. Biol. 2022, 10, 818616. [Google Scholar] [CrossRef]
- Heissig, B.; Dhahri, D.; Eiamboonsert, S.; Salama, Y.; Shimazu, H.; Munakata, S.; Hattori, K. Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell. Mol. Life Sci. 2015, 72, 4759–4770. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (uPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Baart, V. Lighting up Cancer Aggressiveness: Targeting the Urokinase Plasminogen Activator Receptor for Intraoperative Optical Imaging; Universiteit Leiden: Leiden, The Netherlands, 2023. [Google Scholar]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The plasminogen–activator plasmin system in physiological and pathophysiological angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, V.A.; Plekhanova, O.S.; Parfyonova, Y.V. Regulation of arterial remodeling and angiogenesis by urokinase-type plasminogen activator. Can. J. Physiol. Pharmacol. 2009, 87, 231–251. [Google Scholar] [CrossRef]
- Kretschmer, M.; Rüdiger, D.; Zahler, S. Mechanical aspects of angiogenesis. Cancers 2021, 13, 4987. [Google Scholar] [CrossRef]
- Huber, M.C.; Mall, R.; Braselmann, H.; Feuchtinger, A.; Molatore, S.; Lindner, K.; Walch, A.; Gross, E.; Schmitt, M.; Falkenberg, N. uPAR enhances malignant potential of triple-negative breast cancer by directly interacting with uPA and IGF1R. BMC Cancer 2016, 16, 615. [Google Scholar] [CrossRef]
- Meng, S.; Tripathy, D.; Shete, S.; Ashfaq, R.; Saboorian, H.; Haley, B.; Frenkel, E.; Euhus, D.; Leitch, M.; Osborne, C. uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc. Natl. Acad. Sci. USA 2006, 103, 17361–17365. [Google Scholar] [CrossRef] [PubMed]
- Lester, R.D.; Jo, M.; Montel, V.; Takimoto, S.; Gonias, S.L. uPAR induces epithelial–mesenchymal transition in hypoxic breast cancer cells. J. Cell Biol. 2007, 178, 425–436. [Google Scholar] [CrossRef]
- Hugdahl, E.; Bachmann, I.M.; Schuster, C.; Ladstein, R.G.; Akslen, L.A. Prognostic value of uPAR expression and angiogenesis in primary and metastatic melanoma. PLoS ONE 2019, 14, e0210399. [Google Scholar] [CrossRef]
- Laurenzana, A.; Chillà, A.; Luciani, C.; Peppicelli, S.; Biagioni, A.; Bianchini, F.; Tenedini, E.; Torre, E.; Mocali, A.; Calorini, L. uPA/uPAR system activation drives a glycolytic phenotype in melanoma cells. Int. J. Cancer 2017, 141, 1190–1200. [Google Scholar] [CrossRef]
- Rabbani, S.A.; Xing, R.H. Role of urokinase (uPA) and its receptor (uPAR) in invasion and metastasis of hormone-dependent malignancies. Int. J. Oncol. 1998, 12, 911–931. [Google Scholar] [CrossRef]
- Wach, S.; Brandl, M.; Borchardt, H.; Weigelt, K.; Lukat, S.; Nolte, E.; Al-Janabi, O.; Hart, M.; Grässer, F.; Giedl, J. Exploring the MIR143-UPAR axis for the inhibition of human prostate cancer cells in vitro and in vivo. Mol. Ther. Nucleic Acids 2019, 16, 272–283. [Google Scholar] [CrossRef]
- Li, Y.; Cozzi, P. Targeting uPA/uPAR in prostate cancer. Cancer Treat. Rev. 2007, 33, 521–527. [Google Scholar] [CrossRef]
- Eefsen, R.L.; Engelholm, L.; Alpizar-Alpizar, W.; Van den Eynden, G.G.; Vermeulen, P.B.; Christensen, I.J.; Laerum, O.D.; Rolff, H.C.; Høyer-Hansen, G.; Vainer, B. Inflammation and uPAR-expression in colorectal liver metastases in relation to growth pattern and neo-adjuvant therapy. Cancer Microenviron. 2015, 8, 93–100. [Google Scholar] [CrossRef]
- Boonstra, M.C.; Verbeek, F.P.; Mazar, A.P.; Prevoo, H.A.; Kuppen, P.J.; Van de Velde, C.J.; Vahrmeijer, A.L.; Sier, C.F. Expression of uPAR in tumor-associated stromal cells is associated with colorectal cancer patient prognosis: A TMA study. BMC Cancer 2014, 14, 269. [Google Scholar] [CrossRef]
- Illemann, M.; Laerum, O.D.; Hasselby, J.P.; Thurison, T.; Høyer-Hansen, G.; Nielsen, H.J.; Danish Study Group on Early Detection of Colorectal Cancer; Christensen, I.J. Urokinase-type plasminogen activator receptor (uPAR) on tumor-associated macrophages is a marker of poor prognosis in colorectal cancer. Cancer Med. 2014, 3, 855–864. [Google Scholar] [CrossRef]
- Ismail, A.; Hayek, S.S. Role of Soluble Urokinase-Type Plasminogen Activator Receptor in Cardiovascular Disease. Curr. Cardiol. Rep. 2023, 25, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Buraczewska-Buczko, A.; Pawlak, D.; Mysliwiec, M. Hyperfibrinolysis, uPA/suPAR system, kynurenines, and the prevalence of cardiovascular disease in patients with chronic renal failure on conservative treatment. Am. J. Med. Sci. 2010, 339, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble urokinase plasminogen activator receptor (suPAR) as a biomarker of systemic chronic inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef]
- Desmedt, S.; Desmedt, V.; Delanghe, J.; Speeckaert, R.; Speeckaert, M. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit. Rev. Clin. Lab. Sci. 2017, 54, 117–133. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Zhang, Y.; Zhang, Y.; Xiao, W. The role of uPAR in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir. Res. 2013, 14, 67. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Di Carluccio, G.; Motti, M.L.; Carriero, M.V. Therapeutic strategies targeting urokinase and its receptor in cancer. Cancers 2022, 14, 498. [Google Scholar] [CrossRef]
- Casey, J.R.; Petranka, J.G.; Kottra, J.; Fleenor, D.E.; Rosse, W.F. The structure of the urokinase-type plasminogen activator receptor gene. Blood 1994, 84, 1151–1156. [Google Scholar] [CrossRef]
- Yu, S.; Sui, Y.; Wang, J.; Li, Y.; Li, H.; Cao, Y.; Chen, L.; Jiang, L.; Yuan, C.; Huang, M. Crystal structure and cellular functions of uPAR dimer. Nat. Commun. 2022, 13, 1665. [Google Scholar] [CrossRef]
- Xu, X.; Gårdsvoll, H.; Yuan, C.; Lin, L.; Ploug, M.; Huang, M. Crystal structure of the urokinase receptor in a ligand-free form. J. Mol. Biol. 2012, 416, 629–641. [Google Scholar] [CrossRef]
- Mertens, H.D.; Kjaergaard, M.; Mysling, S.; Gårdsvoll, H.; Jørgensen, T.J.; Svergun, D.I.; Ploug, M. A flexible multidomain structure drives the function of the urokinase-type plasminogen activator receptor (uPAR). J. Biol. Chem. 2012, 287, 34304–34315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gandhi, S.; Yuan, C.; Luo, Z.; Li, R.; Gårdsvoll, H.; de Lorenzi, V.; Sidenius, N.; Huang, M.; Ploug, M. Mapping the topographic epitope landscape on the urokinase plasminogen activator receptor (uPAR) by surface plasmon resonance and X-ray crystallography. Data Brief 2015, 5, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Hansen, L.V.; Jacobsen, B.; Gardsvoll, H.; Ploug, M. Structure and ligand interactions of the urokinase receptor (uPAR). Front. Biosci. 2008, 13, 5441–5461. [Google Scholar] [CrossRef] [PubMed]
- Li Santi, A.; Napolitano, F.; Montuori, N.; Ragno, P. The urokinase receptor: A multifunctional receptor in cancer cell biology. Therapeutic implications. Int. J. Mol. Sci. 2021, 22, 4111. [Google Scholar] [CrossRef]
- Ploug, M. Structure-function relationships in the interaction between the urokinase-type plasminogen activator and its receptor. Curr. Pharm. Des. 2003, 9, 1499–1528. [Google Scholar] [CrossRef]
- Llinas, P.; Helene Le Du, M.; Gårdsvoll, H.; Danø, K.; Ploug, M.; Gilquin, B.; Stura, E.A.; Ménez, A. Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide. EMBO J. 2005, 24, 1655–1663. [Google Scholar] [CrossRef]
- Ploug, M.; Rahbek-Nielsen, H.; Ellis, V.; Roepstorff, P.; Dano, K. Chemical modification of the urokinase-type plasminogen activator and its receptor using tetranitromethane. Evidence for the involvement of specific tyrosine residues in both molecules during receptor-ligand interaction. Biochemistry 1995, 34, 12524–12534. [Google Scholar] [CrossRef]
- Minopoli, M.; Polo, A.; Ragone, C.; Ingangi, V.; Ciliberto, G.; Pessi, A.; Sarno, S.; Budillon, A.; Costantini, S.; Carriero, M.V. Structure-function relationship of an Urokinase Receptor-derived peptide which inhibits the Formyl Peptide Receptor type 1 activity. Sci. Rep. 2019, 9, 12169. [Google Scholar] [CrossRef]
- Behrendt, N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): Membrane proteins engaged in matrix turnover during tissue remodeling. Biol. Chem. 2004, 385, 103–136. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, V.; Ploug, M.; Engelholm, L.H. The urokinase receptor (uPAR) as a “Trojan Horse” in targeted cancer therapy: Challenges and opportunities. Cancers 2021, 13, 5376. [Google Scholar] [CrossRef]
- Belvederi, F.; Leggeri, S.; Urbani, A.; Baroni, S. suPAR as a biomarker of support in different clinical settings. Clin. Chim. Acta 2025, 573, 120303. [Google Scholar] [CrossRef]
- Nusshag, C.; Wei, C.; Hahm, E.; Hayek, S.S.; Li, J.; Samelko, B.; Rupp, C.; Szudarek, R.; Speer, C.; Kälble, F. suPAR links a dysregulated immune response to tissue inflammation and sepsis-induced acute kidney injury. JCI Insight 2023, 8, e165740. [Google Scholar] [CrossRef]
- Liu, M.; Lin, L.; Høyer-Hansen, G.; Ploug, M.; Li, H.; Jiang, L.; Yuan, C.; Li, J.; Huang, M. Crystal structure of the unoccupied murine urokinase-type plasminogen activator receptor (uPAR) reveals a tightly packed DII–DIII unit. FEBS Lett. 2019, 593, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, A.; Li Santi, A.; Amodio, G.; Alfano, D.; Remondelli, P.; Montuori, N.; Ragno, P. The urokinase receptor takes control of cell migration by recruiting integrins and FPR1 on the cell surface. PLoS ONE 2014, 9, e86352. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, L.; Aguirre-Ghiso, J.A. Urokinase receptor and integrin partnership: Coordination of signaling for cell adhesion, migration and growth. Curr. Opin. Cell Biol. 2000, 12, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Rysenkova, K.; Klimovich, P.; Shmakova, A.; Karagyaur, M.; Ivanova, K.; Aleksandrushkina, N.; Tkachuk, V.; Rubina, K.; Semina, E. Urokinase receptor deficiency results in EGFR-mediated failure to transmit signals for cell survival and neurite formation in mouse neuroblastoma cells. Cell. Signal. 2020, 75, 109741. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef]
- Degryse, B. The urokinase receptor and integrins constitute a cell migration signalosome. In The Cancer Degradome: Proteases and Cancer Biology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 451–474. [Google Scholar]
- van der Pluijm, G.; Sijmons, B.; Vloedgraven, H.; van der Bent, C.; Drijfhout, J.-W.; Verheijen, J.; Quax, P.; Karperien, M.; Papapoulos, S.; Löwik, C. Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am. J. Pathol. 2001, 159, 971–982. [Google Scholar] [CrossRef]
- Kugler, M.C.; Wei, Y.; Chapman, H.A. Urokinase receptor and integrin interactions. Curr. Pharm. Des. 2003, 9, 1565–1574. [Google Scholar] [CrossRef]
- Liu, D.; Ghiso, J.A.A.; Estrada, Y.; Ossowski, L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 2002, 1, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trejo, D.; Aguiñiga-Sanchez, I.; Ledesma-Martínez, E.; Weiss-Steider, B.; Sierra-Mondragón, E.; Santiago-Osorio, E. Anti-cancer potential of casein and its derivatives: Novel strategies for cancer treatment. Med. Oncol. 2024, 41, 200. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.F.; Xue, Y.-Q.; Fang, L.-K.; Guo, X.-H.; Guo, X.; Liu, M.; Mo, B.-Y.; Yang, M.-R.; Liu, F. uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cell. Mol. Immunol. 2018, 15, 171–181. [Google Scholar] [CrossRef]
- Gondi, C.S.; Kandhukuri, N.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int. J. Oncol. 2007, 31, 19–27. [Google Scholar] [CrossRef]
- Downward, J. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 2004, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.G.; Wagner, A.J.; Conzen, S.D.; Jordan, J.; Bellacosa, A.; Tsichlis, P.N.; Hay, N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997, 11, 701–713. [Google Scholar] [CrossRef]
- Nowicki, T.S.; Zhao, H.; Darzynkiewicz, Z.; Moscatello, A.; Shin, E.; Schantz, S.; Tiwari, R.K.; Geliebter, J. Downregulation of uPAR inhibits migration, invasion, proliferation, FAK/PI3K/Akt signaling and induces senescence in papillary thyroid carcinoma cells. Cell Cycle 2011, 10, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lu, K.; He, Q.; Tang, Y.; Li, H.; Pazo, E.E.; Hu, L.; Wei, R. INOS ablation promotes corneal wound healing via activation of Akt signaling. Exp. Eye Res. 2024, 243, 109886. [Google Scholar] [CrossRef]
- Shushakova, N.; Tkachuk, N.; Dangers, M.; Tkachuk, S.; Park, J.-K.; Hashimoto, K.; Haller, H.; Dumler, I. Urokinase-induced activation of the gp130/Tyk2/Stat3 pathway mediates a pro-inflammatory effect in human mesangial cells via expression of the anaphylatoxin C5a receptor. J. Cell Sci. 2005, 118, 2743–2753. [Google Scholar] [CrossRef]
- Dumler, I.; Kopmann, A.; Weis, A.; Mayboroda, O.A.; Wagner, K.; Gulba, D.C.; Haller, H. Urokinase activates the Jak/Stat signal transduction pathway in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 290–297. [Google Scholar] [CrossRef]
- Thornton, S.; Raghu, H.; Cruz, C.; Frederick, M.D.; Palumbo, J.S.; Mullins, E.S.; Almholt, K.; Usher, P.A.; Flick, M.J. Urokinase plasminogen activator and receptor promote collagen-induced arthritis through expression in hematopoietic cells. Blood Adv. 2017, 1, 545–556. [Google Scholar] [CrossRef]
- Dumler, I.; Weis, A.; Mayboroda, O.A.; Maasch, C.; Jerke, U.; Haller, H.; Gulba, D.C. The Jak/Stat pathway and urokinase receptor signaling in human aortic vascular smooth muscle cells. J. Biol. Chem. 1998, 273, 315–321. [Google Scholar] [CrossRef]
- Sidenius, N.; Sier, C.F.; Blasi, F. Shedding and cleavage of the urokinase receptor (uPAR): Identification and characterisation of uPAR fragments in vitro and in vivo. FEBS Lett. 2000, 475, 52–56. [Google Scholar] [CrossRef]
- Sidenius, N.; Blasi, F. Domain 1 of the urokinase receptor (uPAR) is required for uPAR-mediated cell binding to vitronectin. FEBS Lett. 2000, 470, 40–46. [Google Scholar] [CrossRef]
- Ahn, S.B.; Mohamedali, A.; Anand, S.; Cheruku, H.R.; Birch, D.; Sowmya, G.; Cantor, D.; Ranganathan, S.; Inglis, D.W.; Frank, R. Characterization of the interaction between heterodimeric αvβ6 integrin and urokinase plasminogen activator receptor (uPAR) using functional proteomics. J. Proteome Res. 2014, 13, 5956–5964. [Google Scholar] [CrossRef]
- Tang, C.-H.; Wei, Y. The urokinase receptor and integrins in cancer progression. Cell. Mol. Life Sci. 2008, 65, 1916–1932. [Google Scholar] [CrossRef]
- LaRusch, G.A.; Mahdi, F.; Shariat-Madar, Z.; Adams, G.; Sitrin, R.G.; Zhang, W.M.; McCrae, K.R.; Schmaier, A.H. Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood J. Am. Soc. Hematol. 2010, 115, 5111–5120. [Google Scholar] [CrossRef]
- Stavrou, E.X.; Fang, C.; Bane, K.L.; Long, A.T.; Naudin, C.; Kucukal, E.; Gandhi, A.; Brett-Morris, A.; Mumaw, M.M.; Izadmehr, S. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Investig. 2018, 128, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Eden, G.; Archinti, M.; Furlan, F.; Murphy, R.; Degryse, B. The urokinase receptor interactome. Curr. Pharm. Des. 2011, 17, 1874–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Li, X.; Sun, D.; He, Z. Clinical research on the changes of plasma TFPI and uPA system in malignant tumor. Chin. Ger. J. Clin. Oncol. 2004, 3, 78–80. [Google Scholar] [CrossRef]
- Albo, D.; Tuszynski, G. Thrombospondin-1 up-regulates tumor cell invasion through the urokinase plasminogen activator receptor in head and neck cancer cells. J. Surg. Res. 2004, 120, 21–26. [Google Scholar] [CrossRef]
- Huang, T.; Sun, L.; Yuan, X.; Qiu, H. Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget 2017, 8, 84546. [Google Scholar] [CrossRef]
- Gucciardo, F.; Pirson, S.; Baudin, L.; Lebeau, A.; Noël, A. uPARAP/Endo180: A multifaceted protein of mesenchymal cells. Cell. Mol. Life Sci. 2022, 79, 255. [Google Scholar] [CrossRef]
- Binder, B.R.; Mihaly, J.; Prager, G.W. uPAR–uPA–PAI-1 interactions and signaling: A vascular biologist’s view. Thromb. Haemost. 2007, 97, 336–342. [Google Scholar]
- Engelholm, L.H.; List, K.; Netzel-Arnett, S.; Cukierman, E.; Mitola, D.J.; Aaronson, H.; Kjøller, L.; Larsen, J.K.; Yamada, K.M.; Strickland, D.K. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J. Cell Biol. 2003, 160, 1009–1015. [Google Scholar] [CrossRef]
- Royer-Zemmour, B.; Ponsole-Lenfant, M.; Gara, H.; Roll, P.; Lévêque, C.; Massacrier, A.; Ferracci, G.; Cillario, J.; Robaglia-Schlupp, A.; Vincentelli, R. Epileptic and developmental disorders of the speech cortex: Ligand/receptor interaction of wild-type and mutant SRPX2 with the plasminogen activator receptor uPAR. Hum. Mol. Genet. 2008, 17, 3617–3630. [Google Scholar] [CrossRef]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef]
- Jin, H.; Song, Y.P.; Boel, G.; Kochar, J.; Pancholi, V. Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells. J. Mol. Biol. 2005, 350, 27–41. [Google Scholar] [CrossRef]
- Curran, C.S.; Kopp, J.B. RAGE pathway activation and function in chronic kidney disease and COVID-19. Front. Med. 2022, 9, 970423. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Sun, P. uPAR, beyond regulating physiological functions, has orchestrated roles in cancer. Int. J. Oncol. 2022, 61, 151. [Google Scholar] [CrossRef]
- Rajagopal, V.; Kreitman, R.J. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the α2-macroglobulin receptor. J. Biol. Chem. 2000, 275, 7566–7573. [Google Scholar] [CrossRef] [PubMed]
- Basalova, N.; Alexandrushkina, N.; Grigorieva, O.; Kulebyakina, M.; Efimenko, A. Fibroblast Activation Protein Alpha (FAPα) in Fibrosis: Beyond a Perspective Marker for Activated Stromal Cells? Biomolecules 2023, 13, 1718. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Chang, L.; Pure, E. Fibroblast activation protein in remodeling tissues. Curr. Mol. Med. 2012, 12, 1220–1243. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3–CCL2 signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef]
- Han, R.; Hu, S.; Qin, W.; Shi, J.; Hou, Q.; Wang, X.; Xu, X.; Zhang, M.; Zeng, C.; Liu, Z. C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis. JCI Insight 2019, 4, e122912. [Google Scholar] [CrossRef]
- Chaurasia, P.; Mezei, M.; Zhou, M.-M.; Ossowski, L. Computer aided identification of small molecules disrupting uPAR/α5β1-integrin interaction: A new paradigm for metastasis prevention. PLoS ONE 2009, 4, e4617. [Google Scholar] [CrossRef]
- Degryse, B. Is uPAR the centre of a sensing system involved in the regulation of inflammation? Curr. Med. Chem. Anti-Inflamm. Anti-Allergy Agents 2003, 2, 237–259. [Google Scholar] [CrossRef]
- Hu, J.; Jo, M.; Eastman, B.M.; Gilder, A.S.; Bui, J.D.; Gonias, S.L. uPAR induces expression of transforming growth factor β and interleukin-4 in cancer cells to promote tumor-permissive conditioning of macrophages. Am. J. Pathol. 2014, 184, 3384–3393. [Google Scholar] [CrossRef] [PubMed]
- Campelo, J.; Gomes, F.; Alcântara, A.; Nascimento, M.; Tavares, I.; Silva, J.; Fraiji, N.; Albuquerque, S.; Gonçalves, M.; Neto, J. PLASMINOGEN ACTIVATING RECEPTOR UROKINASE (U-PAR): INTERACTION AND IMPLICATIONS IN TUMOR MICROENVIRONMENT IN LEUKEMIAS. Hematol. Transfus. Cell Ther. 2024, 46, S145. [Google Scholar] [CrossRef]
- Heidari, Z.; Naeimzadeh, Y.; Fallahi, J.; Savardashtaki, A.; Razban, V.; Khajeh, S. The role of tissue factor in signaling pathways of pathological conditions and angiogenesis. Curr. Mol. Med. 2024, 24, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Zhao, Y.; Jiang, X.; Yuan, H.; Wang, H.; Cui, X.; Xu, J.; Zhao, J.; Wang, J. uPAR: An essential factor for tumor development. J. Cancer 2021, 12, 7026. [Google Scholar] [CrossRef]
- Mueller, S.C.; Artym, V.V.; Kelly, T. Invadopodia: Interface for invasion. In The Cancer Degradome: Proteases and Cancer Biology; Springer: New York, NY, USA, 2008; pp. 403–431. [Google Scholar]
- Masi, I.; Caprara, V.; Bagnato, A.; Rosanò, L. Tumor cellular and microenvironmental cues controlling invadopodia formation. Front. Cell Dev. Biol. 2020, 8, 584181. [Google Scholar] [CrossRef]
- Annis, M.G.; Ouellet, V.; Rennhack, J.P.; L’Esperance, S.; Rancourt, C.; Mes-Masson, A.-M.; Andrechek, E.R.; Siegel, P.M. Integrin-uPAR signaling leads to FRA-1 phosphorylation and enhanced breast cancer invasion. Breast Cancer Res. 2018, 20, 9. [Google Scholar] [CrossRef]
- Venetis, K.; Piciotti, R.; Sajjadi, E.; Invernizzi, M.; Morganti, S.; Criscitiello, C.; Fusco, N. Breast cancer with bone metastasis: Molecular insights and clinical management. Cells 2021, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Elaasser, B.; Arakil, N.; Mohammad, K.S. Bridging the gap in understanding bone metastasis: A multifaceted perspective. Int. J. Mol. Sci. 2024, 25, 2846. [Google Scholar] [CrossRef]
- Kanno, Y.; Ishisaki, A.; Miyashita, M.; Matsuo, O. The blocking of uPAR suppresses lipopolysaccharide-induced inflammatory osteoclastogenesis and the resultant bone loss through attenuation of integrin β3/Akt pathway. Immun. Inflamm. Dis. 2016, 4, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Baart, V.; Houvast, R.; de Geus-Oei, L.; Quax, P.; Kuppen, P.; Vahrmeijer, A.; Sier, C. Molecular imaging of the urokinase plasminogen activator receptor: Opportunities beyond cancer. EJNMMI Res. 2020, 10, 87. [Google Scholar] [CrossRef]
- Nakamura, I.; Duong, L.T.; Rodan, S.B.; Rodan, G.A. Involvement of αvβ3 integrins in osteoclast function. J. Bone Miner. Metab. 2007, 25, 337–344. [Google Scholar] [CrossRef]
- Mahmood, N.; Arakelian, A.; Khan, H.A.; Tanvir, I.; Mazar, A.P.; Rabbani, S.A. uPAR antibody (huATN-658) and Zometa reduce breast cancer growth and skeletal lesions. Bone Res. 2020, 8, 18. [Google Scholar] [CrossRef]
- Rabbani, S.A.; Ateeq, B.; Arakelian, A.; Valentino, M.L.; Shaw, D.E.; Dauffenbach, L.M.; Kerfoot, C.A.; Mazar, A.P. An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia 2010, 12, 778–788. [Google Scholar] [CrossRef]
- Biagioni, A.; Laurenzana, A.; Menicacci, B.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Guasti, D.; Paoli, P.; Serratì, S.; Mocali, A. uPAR-expressing melanoma exosomes promote angiogenesis by VE-Cadherin, EGFR and uPAR overexpression and rise of ERK1, 2 signaling in endothelial cells. Cell. Mol. Life Sci. 2021, 78, 3057–3072. [Google Scholar] [CrossRef]
- Uhrin, P.; Breuss, J.M. uPAR: A modulator of VEGF-induced angiogenesis. Cell Adhes. Migr. 2013, 7, 23–26. [Google Scholar] [CrossRef]
- Rao, J.; Gujrati, M.; Chetty, C. Tumor-associated soluble uPAR-directed endothelial cell motility and tumor angiogenesis. Oncogenesis 2013, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Unseld, M.; Chilla, A.; Pausz, C.; Mawas, R.; Breuss, J.; Zielinski, C.; Schabbauer, G.; Prager, G.W. PTEN expression in endothelial cells is down-regulated by uPAR to promote angiogenesis. Thromb. Haemost. 2015, 114, 379–389. [Google Scholar] [CrossRef]
- Herkenne, S.; Paques, C.; Nivelles, O.; Lion, M.; Bajou, K.; Pollenus, T.; Fontaine, M.; Carmeliet, P.; Martial, J.A.; Nguyen, N.-Q.-N. The interaction of uPAR with VEGFR2 promotes VEGF-induced angiogenesis. Sci. Signal. 2015, 8, ra117. [Google Scholar] [CrossRef]
- Carriero, M.V.; Bifulco, K.; Minopoli, M.; Lista, L.; Maglio, O.; Mele, L.; Di Carluccio, G.; De Rosa, M.; Pavone, V. UPARANT: A Urokinase receptor–derived peptide inhibitor of VEGF-driven angiogenesis with enhanced stability and in vitro and in vivo potency. Mol. Cancer Ther. 2014, 13, 1092–1104. [Google Scholar] [CrossRef]
- Ungefroren, H.; Sebens, S.; Seidl, D.; Lehnert, H.; Hass, R. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011, 9, 18. [Google Scholar] [CrossRef]
- Bharadwaj, A.G.; Holloway, R.W.; Miller, V.A.; Waisman, D.M. Plasmin and plasminogen system in the tumor microenvironment: Implications for cancer diagnosis, prognosis, and therapy. Cancers 2021, 13, 1838. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Seiwert, T.Y. The role of macrophages in the tumor microenvironment and tumor metabolism. Semin. Immunopathol. 2023, 45, 187–201. [Google Scholar] [CrossRef]
- Lindsten, T.; Hedbrant, A.; Ramberg, A.; Wijkander, J.; Solterbeck, A.; Eriksson, M.; Delbro, D.; Erlandsson, A. Effect of macrophages on breast cancer cell proliferation, and on expression of hormone receptors, uPAR and HER-2. Int. J. Oncol. 2017, 51, 104–114. [Google Scholar] [CrossRef]
- Poettler, M.; Unseld, M.; Mihaly-Bison, J.; Uhrin, P.; Koban, F.; Binder, B.R.; Zielinski, C.C.; Prager, G.W. The urokinase receptor (CD87) represents a central mediator of growth factor-induced endothelial cell migration. Thromb. Haemost. 2012, 108, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sater, M.S.; AlDehaini, D.M.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. Plasma IL-6, TREM1, uPAR, and IL6/IL8 biomarkers increment further witnessing the chronic inflammation in type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2023, 44, 259–269. [Google Scholar] [CrossRef]
- Gussen, H.; Hohlstein, P.; Bartneck, M.; Warzecha, K.T.; Buendgens, L.; Luedde, T.; Trautwein, C.; Koch, A.; Tacke, F. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J. Intensive Care 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, V.; Crowley, V.M.; Weckman, A.M.; Kain, K.C. suPAR to risk-stratify patients with malaria. Front. Immunol. 2022, 13, 931321. [Google Scholar] [CrossRef]
- Memarzadeh, S.; Kozak, K.R.; Chang, L.; Natarajan, S.; Shintaku, P.; Reddy, S.T.; Farias-Eisner, R. Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 10647–10652. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Friedman, A. Serum upar as biomarker in breast cancer recurrence: A mathematical model. PLoS ONE 2016, 11, e0153508. [Google Scholar] [CrossRef]
- Bruun, S.B.; Madsen, J.B.; Brasen, C.L. Establishing reference intervals for soluble urokinase plasminogen activator receptor in Northern European adults. Pract. Lab. Med. 2024, 39, e00371. [Google Scholar] [CrossRef]
- Langkilde, A.; Hansen, T.W.; Ladelund, S.; Linneberg, A.; Andersen, O.; Haugaard, S.B.; Jeppesen, J.; Eugen-Olsen, J. Increased plasma soluble uPAR level is a risk marker of respiratory cancer in initially cancer-free individuals. Cancer Epidemiol. Biomark. Prev. 2011, 20, 609–618. [Google Scholar] [CrossRef]
- Huang, M.; Li, L.; Shen, J.; Wang, Y.; Wang, R.; Yuan, C.; Huang, M.; Jiang, L. Plasma levels of the active form of suPAR are associated with COVID-19 severity. Crit. Care 2020, 24, 704. [Google Scholar] [CrossRef]
- Andres, S.A.; Edwards, A.B.; Wittliff, J.L. Expression of urokinase-type plasminogen activator (uPA), its receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas and their clinical relevance. J. Clin. Lab. Anal. 2012, 26, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Soydinc, H.O.; Duranyildiz, D.; Guney, N.; Derin, D.; Yasasever, V. Utility of serum and urine uPAR levels for diagnosis of breast cancer. Asian Pac. J. Cancer Prev. 2012, 13, 2887–2889. [Google Scholar] [CrossRef]
- Lund, I.K.; Illemann, M.; Thurison, T.; J Christensen, I.; Hoyer-Hansen, G. uPAR as anti-cancer target: Evaluation of biomarker potential, histological localization, and antibody-based therapy. Curr. Drug Targets 2011, 12, 1744–1760. [Google Scholar] [CrossRef]
- Thurison, T.; Lomholt, A.F.; Rasch, M.G.; Lund, I.K.; Nielsen, H.J.; Christensen, I.J.; Høyer-Hansen, G. A new assay for measurement of the liberated domain I of the urokinase receptor in plasma improves the prediction of survival in colorectal cancer. Clin. Chem. 2010, 56, 1636–1640. [Google Scholar] [CrossRef]
- Blumenschein, G.R., Jr.; Reck, M.; Fossella, F.; Stewart, D.J.; Lathia, C.; Peña, C. Plasma biomarkers correlating with clinical outcome in a phase II study of sorafenib in advanced NSCLC. Cancer Biomark. 2012, 10, 287–298. [Google Scholar] [CrossRef]
- Lu, J.-J.; Guo, H.; Gao, B.; Zhang, Y.; Lin, Q.-L.; Shi, J.; Liu, J.-J.; Liu, J. Prognostic value of urokinase plasminogen activator system in non-small cell lung cancer: A systematic review and meta-analysis. Mol. Clin. Oncol. 2018, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, H.; Qian, D.; Wang, Y.; Guan, Y.; Er, P.; Song, Y.; Liu, N.; Wang, J.; Zhao, L. Predictive value of EGF and uPAR for chemoradiotherapy response and survival in patients with esophageal squamous cell carcinoma. Ann. Transl. Med. 2020, 8, 1152. [Google Scholar] [CrossRef]
- Pavón, M.A.; Arroyo-Solera, I.; Céspedes, M.V.; Casanova, I.; León, X.; Mangues, R. uPA/uPAR and SERPINE1 in head and neck cancer: Role in tumor resistance, metastasis, prognosis and therapy. Oncotarget 2016, 7, 57351. [Google Scholar]
- Porcelli, L.; Guida, M.; De Summa, S.; Di Fonte, R.; De Risi, I.; Garofoli, M.; Caputo, M.; Negri, A.; Strippoli, S.; Serratì, S. uPAR+ extracellular vesicles: A robust biomarker of resistance to checkpoint inhibitor immunotherapy in metastatic melanoma patients. J. Immunother. Cancer 2021, 9, e002372. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cai, Y.; Wei, Y.; Donate, F.; Juarez, J.; Parry, G.; Chen, L.; Meehan, E.J.; Ahn, R.W.; Ugolkov, A. Identification of a new epitope in uPAR as a target for the cancer therapeutic monoclonal antibody ATN-658, a structural homolog of the uPAR binding integrin CD11b (αM). PLoS ONE 2014, 9, e85349. [Google Scholar] [CrossRef] [PubMed]
- Hazan, K.; Cirmirakis, A.; Rothman, T. RedHill Biopharma Ltd.: Strategic Expansion from R&D to Sales of Drugs in the US; Initiation of Coverage; Research & Consulting Ltd.: Nottingham, UK, 2017. [Google Scholar]
- Kumar, A.A.; Buckley, B.J.; Ranson, M. The urokinase plasminogen activation system in pancreatic cancer: Prospective diagnostic and therapeutic targets. Biomolecules 2022, 12, 152. [Google Scholar] [CrossRef]
- Lai, X.; Cheng, D.; Xu, H.; Wang, J.; Lv, X.; Yao, H.; Li, L.; Wu, J.; Ye, S.; Li, Z. Phase I Trial of Upamostat Combined with Gemcitabine in Locally Unresectable or Metastatic Pancreatic Cancer: Safety and Preliminary Efficacy Assessment. Cancer Med. 2025, 14, e70550. [Google Scholar] [CrossRef]
- Duffy, M.J.; McGowan, P.M.; Harbeck, N.; Thomssen, C.; Schmitt, M. uPA and PAI-1 as biomarkers in breast cancer: Validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014, 16, 428. [Google Scholar] [CrossRef]
- Towle, M.J.; Lee, A.; Maduakor, E.C.; Schwartz, C.E.; Bridges, A.J.; Littlefield, B.A. Inhibition of urokinase by 4-substituted benzo [b] thiophene-2-carboxamidines: An important new class of selective synthetic urokinase inhibitor. Cancer Res. 1993, 53, 2553–2559. [Google Scholar]
- Skovgaard, D.; Persson, M.; Kjaer, A. PET imaging of urokinase-type plasminogen activator receptor (uPAR) in prostate cancer: Current status and future perspectives. Clin. Transl. Imaging 2016, 4, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, R.S.; Damaj, B.B.; Hachicha, M.; Incardona, F.; Howell, S.B.; Finlayson, M. A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol. Cancer Ther. 2011, 10, 2072–2082. [Google Scholar] [CrossRef]
- Finlayson, M. Modulation of CD44 activity by A6-peptide. Front. Immunol. 2015, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.A.; Loft, M.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Knigge, U.; Kjaer, A. Prospective phase II trial of prognostication by 68Ga-NOTA-AE105 uPAR PET in patients with neuroendocrine neoplasms: Implications for uPAR-targeted therapy. J. Nucl. Med. 2022, 63, 1371–1377. [Google Scholar] [CrossRef]
- Ateeq, B.; Rabbani, S.; Mazar, A.; Graham Parry, G. A selective anti-urokinase receptor (uPAR) antibody (ATN-658) blocks prostate cancer growth, migration, invasion and skeletal metastasis in vitro and in vivo. Cancer Res. 2007, 67, 4094. [Google Scholar]
- Nair, R.; Cittadine, A.; Kawamoto, E.; Kelly, A.; Robinson, C. Preclinical Evaluation of Anti-uPAR Antibody as a Radiolabeled PET Imaging Candidate in Solid Tumors. J. Nucl. Med. 2024, 65 (Suppl. S2), 241463. [Google Scholar]
- Duriseti, S. Discovery and Characterization of Human Recombinant Anti-uPAR Antibodies; University of California San Francisco: San Francisco, CA, USA, 2012. [Google Scholar]
- Duriseti, S.; Goetz, D.H.; Hostetter, D.R.; LeBeau, A.M.; Wei, Y.; Craik, C.S. Antagonistic anti-urokinase plasminogen activator receptor (uPAR) antibodies significantly inhibit uPAR-mediated cellular signaling and migration. J. Biol. Chem. 2010, 285, 26878–26888. [Google Scholar] [CrossRef]
- Tummalapalli, P.; Gondi, C.S.; Dinh, D.H.; Gujrati, M.; Rao, J.S. RNA interference-mediated targeting of urokinase plasminogen activator receptor and matrix metalloproteinase-9 gene expression in the IOMM-lee malignant meningioma cell line inhibits tumor growth, tumor cell invasion and angiogenesis. Int. J. Oncol. 2007, 31, 5–17. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Wang, S.; Gao, C.; Chen, J.; Zhang, L.; Zhao, Y.; Wang, J. Silencing of uPAR via RNA interference inhibits invasion and migration of oral tongue squamous cell carcinoma. Oncol. Lett. 2018, 16, 3983–3991. [Google Scholar] [CrossRef]
- Li, C.; Cao, S.; Liu, Z.; Ye, X.; Chen, L.; Meng, S. RNAi-mediated downregulation of uPAR synergizes with targeting of HER2 through the ERK pathway in breast cancer cells. Int. J. Cancer 2010, 127, 1507–1516. [Google Scholar] [CrossRef]
- Alapati, K.; Kesanakurti, D.; Rao, J.S.; Dasari, V.R. uPAR and cathepsin B-mediated compartmentalization of JNK regulates the migration of glioma-initiating cells. Stem Cell Res. 2014, 12, 716–729. [Google Scholar]
- Liang, X.; Yang, X.; Tang, Y.; Zhou, H.; Liu, X.; Xiao, L.; Gao, J.; Mao, Z. RNAi-mediated downregulation of urokinase plasminogen activator receptor inhibits proliferation, adhesion, migration and invasion in oral cancer cells. Oral Oncol. 2008, 44, 1172–1180. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Fu, X.; Wu, H.; Ye, X.; Huang, X.; Cui, Y.; Qian, C.-N.; Lu, Y.; Zhang, J. Urokinase-type plasminogen activator deficiency enhances CD8+ T cell infiltration and anti-PD-1 therapy efficacy in prostate cancer. Front. Immunol. 2025, 16, 1625226. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, L.; Zhang, J.; Zhou, H.; Yang, Z.; Wang, Y.; Cai, W.; Wen, F.; Jiang, X.; Zhang, T. Therapeutic strategies targeting uPAR potentiate anti–PD-1 efficacy in diffuse-type gastric cancer. Sci. Adv. 2022, 8, eabn3774. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Merhi, M.; Ahmad, F.; Taib, N.; Inchakalody, V.; Uddin, S.; Shablak, A.; Dermime, S. The complex network of transcription factors, immune checkpoint inhibitors and stemness features in colorectal cancer: A recent update. Semin. Cancer Biol. 2023, 89, 1–17. [Google Scholar] [CrossRef]
- Ilkovitch, D.; Carrio, R.; Lopez, D.M. uPA and uPA-receptor are involved in cancer-associated myeloid-derived suppressor cell accumulation. Anticancer Res. 2012, 32, 4263–4270. [Google Scholar]
- Cheng, W.; Kang, K.; Zhao, A.; Wu, Y. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J. Hematol. Oncol. 2024, 17, 54. [Google Scholar] [CrossRef]
- Cao, C.; Lawrence, D.A.; Li, Y.; Von Arnim, C.A.; Herz, J.; Su, E.J.; Makarova, A.; Hyman, B.T.; Strickland, D.K.; Zhang, L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006, 25, 1860–1870. [Google Scholar] [CrossRef]
- Placencio, V.R.; DeClerck, Y.A. Plasminogen activator inhibitor-1 in cancer: Rationale and insight for future therapeutic testing. Cancer Res. 2015, 75, 2969–2974. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, Y.; Won, W.R.; Lim, C.; Kim, J.C.; Kang, K.; Husni, P.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of AE147 peptide-conjugated nanocarriers for targeting uPAR-overexpressing cancer cells. Int. J. Nanomed. 2021, 16, 5437–5449. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.A.; Beck, I.; Bauer, T.W.; Ellis, L.M.; Mazar, A.P.; Parry, G.C. In vitro and in vivo characterization of a monoclonal antibody, ATN-658, targeting the uPA system. Cancer Res. 2005, 65, 1454–1455. [Google Scholar]
- Kenny, H.; Jagadeeswaran, S.; Mazar, A.; Lengyel, E. Treatment with an uPAR (ATN 658) antibody inhibits ovarian cancer metastasis. Mol. Cancer Ther. 2007, 6, C31. [Google Scholar]
- LeBeau, A.M.; Duriseti, S.; Murphy, S.T.; Pepin, F.; Hann, B.; Gray, J.W.; VanBrocklin, H.F.; Craik, C.S. Targeting uPAR with antagonistic recombinant human antibodies in aggressive breast cancer. Cancer Res. 2013, 73, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Li, W.; Hines, M.G.; Lyakhov, I.; Mellors, J.W.; Dimitrov, D.S. Human antibody VH domains targeting uPAR as candidate therapeutics for cancers. Front. Oncol. 2023, 13, 1194972. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, X.; Liu, Q.; Wilkins, J.A.; Chapman, H.A. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 1999, 144, 1285–1294. [Google Scholar] [CrossRef]
- Koshelnick, Y.; Ehart, M.; Hufnagl, P.; Heinrich, P.C.; Binder, B.R. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J. Biol. Chem. 1997, 272, 28563–28567. [Google Scholar] [CrossRef]
- Zhao, B.; Gandhi, S.; Yuan, C.; Luo, Z.; Li, R.; Gårdsvoll, H.; de Lorenzi, V.; Sidenius, N.; Huang, M.; Ploug, M. Stabilizing a flexible interdomain hinge region harboring the SMB binding site drives uPAR into its closed conformation. J. Mol. Biol. 2015, 427, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Sidenius, N.; Gandhi, S. Constitutively Active uPAR Variants and Their Use for the Generation and Isolation of Inhibitory Antibodies. Patent WO2013020898A1, 14 February 2013. [Google Scholar]
- Heinemann, V.; Ebert, M.; Laubender, R.P.; Bevan, P.; Mala, C.; Boeck, S. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br. J. Cancer 2013, 108, 766–770. [Google Scholar] [CrossRef]
- Khanna, M.; Wang, F.; Jo, I.; Knabe, W.E.; Wilson, S.M.; Li, L.; Bum-Erdene, K.; Li, J.; Sledge, W.G., Jr.; Khanna, R. Targeting multiple conformations leads to small molecule inhibitors of the uPAR· uPA protein–protein interaction that block cancer cell invasion. ACS Chem. Biol. 2011, 6, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Mani, T.; Wang, F.; Knabe, W.E.; Sinn, A.L.; Khanna, M.; Jo, I.; Sandusky, G.E.; Sledge, G.W., Jr.; Jones, D.R.; Khanna, R. Small-molecule inhibition of the uPAR· uPA interaction: Synthesis, biochemical, cellular, in vivo pharmacokinetics and efficacy studies in breast cancer metastasis. Bioorg. Med. Chem. 2013, 21, 2145–2155. [Google Scholar] [CrossRef]
- Wang, F.; Knabe, W.E.; Li, L.; Jo, I.; Mani, T.; Roehm, H.; Oh, K.; Li, J.; Khanna, M.; Meroueh, S.O. Design, synthesis, biochemical studies, cellular characterization, and structure-based computational studies of small molecules targeting the urokinase receptor. Bioorg. Med. Chem. 2012, 20, 4760–4773. [Google Scholar] [CrossRef]
- Rullo, A.F.; Fitzgerald, K.J.; Muthusamy, V.; Liu, M.; Yuan, C.; Huang, M.; Kim, M.; Cho, A.E.; Spiegel, D.A. Re-engineering the immune response to metastatic cancer: Antibody-recruiting small molecules targeting the urokinase receptor. Angew. Chem. 2016, 128, 3706–3710. [Google Scholar] [CrossRef]
- Lin, C.M.; Arancillo, M.; Whisenant, J.; Burgess, K. Unconventional Secondary Structure Mimics: Ladder-Rungs. Angew. Chem. 2020, 132, 9484–9488. [Google Scholar] [CrossRef]
- Rea, V.E.A.; Lavecchia, A.; Di Giovanni, C.; Rossi, F.W.; Gorrasi, A.; Pesapane, A.; de Paulis, A.; Ragno, P.; Montuori, N. Discovery of new small molecules targeting the vitronectin-binding site of the urokinase receptor that block cancer cell invasion. Mol. Cancer Ther. 2013, 12, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kopitz, C.; Schmalix, W.A.; Muehlenweg, B.; Kessler, H.; Schmitt, M.; Krüger, A.; Magdolen, V. High-affinity urokinase-derived cyclic peptides inhibiting urokinase/urokinase receptor-interaction: Effects on tumor growth and spread. FEBS Lett. 2002, 528, 212–216. [Google Scholar] [CrossRef]
- Schmiedeberg, N.; Schmitt, M.; Rölz, C.; Truffault, V.; Sukopp, M.; Bürgle, M.; Wilhelm, O.G.; Schmalix, W.; Magdolen, V.; Kessler, H. Synthesis, solution structure, and biological evaluation of urokinase type plasminogen activator (uPA)-derived receptor binding domain mimetics. J. Med. Chem. 2002, 45, 4984–4994. [Google Scholar] [CrossRef]
- Blood, C.H.; Neustadt, B.R.; Smith, E.M. Derivatives of Aminobenzoic and Aminobiphenylcarboxylic Acids Useful as Anti-Cancer Agents. U.S. Patent US6228985B1, 8 May 2001. [Google Scholar]
- Bifulco, K.; Longanesi-Cattani, I.; Gargiulo, L.; Maglio, O.; Cataldi, M.; De Rosa, M.; Stoppelli, M.P.; Pavone, V.; Carriero, M.V. An urokinase receptor antagonist that inhibits cell migration by blocking the formyl peptide receptor. FEBS Lett. 2008, 582, 1141–1146. [Google Scholar] [CrossRef]
- Carriero, M.V.; Longanesi-Cattani, I.; Bifulco, K.; Maglio, O.; Lista, L.; Barbieri, A.; Votta, G.; Masucci, M.T.; Arra, C.; Franco, R. Structure-based design of an urokinase-type plasminogen activator receptor–derived peptide inhibiting cell migration and lung metastasis. Mol. Cancer Ther. 2009, 8, 2708–2717. [Google Scholar] [CrossRef]
- Locri, F.; Pesce, N.A.; Aronsson, M.; Cammalleri, M.; De Rosa, M.; Pavone, V.; Bagnoli, P.; Kvanta, A.; Dal Monte, M.; André, H. Gaining insight on mitigation of rubeosis iridis by UPARANT in a mouse model associated with proliferative retinopathy. J. Mol. Med. 2020, 98, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Pecci, V.; De Rosa, M.; Pavone, V.; Bagnoli, P. The urokinase-type plasminogen activator system as drug target in retinitis pigmentosa: New pre-clinical evidence in the rd10 mouse model. J. Cell. Mol. Med. 2019, 23, 5176–5192. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Pecci, V.; Carmosino, M.; Procino, G.; Pini, A.; De Rosa, M.; Pavone, V.; Svelto, M.; Bagnoli, P. Inhibiting the urokinase-type plasminogen activator receptor system recovers STZ-induced diabetic nephropathy. J. Cell. Mol. Med. 2019, 23, 1034–1049. [Google Scholar] [CrossRef]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Marsili, S.; Lista, L.; De Rosa, M.; Pavone, V.; Rusciano, D.; Bagnoli, P. Diabetic retinopathy in the spontaneously diabetic Torii rat: Pathogenetic mechanisms and preventive efficacy of inhibiting the urokinase-type plasminogen activator receptor system. J. Diabetes Res. 2017, 2017, 2904150. [Google Scholar] [CrossRef]
- Boccella, S.; Panza, E.; Lista, L.; Belardo, C.; Ianaro, A.; De Rosa, M.; de Novellis, V.; Pavone, V. Preclinical evaluation of the urokinase receptor-derived peptide UPARANT as an anti-inflammatory drug. Inflamm. Res. 2017, 66, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Lista, L.; Aronsson, M.; Kvanta, A.; Rusciano, D.; De Rosa, M.; Pavone, V.; André, H. The urokinase receptor-derived peptide UPARANT mitigates angiogenesis in a mouse model of laser-induced choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2600–2611. [Google Scholar] [CrossRef]
- Dal Monte, M.; Rezzola, S.; Cammalleri, M.; Belleri, M.; Locri, F.; Morbidelli, L.; Corsini, M.; Paganini, G.; Semeraro, F.; Cancarini, A. Antiangiogenic effectiveness of the urokinase receptor-derived peptide UPARANT in a model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2392–2407. [Google Scholar] [CrossRef] [PubMed]
- Carriero, M.V.; Bifulco, K.; Ingangi, V.; Costantini, S.; Botti, G.; Ragone, C.; Minopoli, M.; Motti, M.L.; Rea, D.; Scognamiglio, G. Retro-inverso urokinase receptor antagonists for the treatment of metastatic sarcomas. Sci. Rep. 2017, 7, 1312. [Google Scholar] [CrossRef]
- Yousif, A.M.; Minopoli, M.; Bifulco, K.; Ingangi, V.; Di Carluccio, G.; Merlino, F.; Motti, M.L.; Grieco, P.; Carriero, M.V. Cyclization of the urokinase receptor-derived ser-arg-ser-arg-tyr peptide generates a potent inhibitor of trans-endothelial migration of monocytes. PLoS ONE 2015, 10, e0126172. [Google Scholar] [CrossRef]
- Yousif, A.M.; Ingangi, V.; Merlino, F.; Brancaccio, D.; Minopoli, M.; Bellavita, R.; Novellino, E.; Carriero, M.V.; Carotenuto, A.; Grieco, P. Urokinase receptor derived peptides as potent inhibitors of the formyl peptide receptor type 1-triggered cell migration. Eur. J. Med. Chem. 2018, 143, 348–360. [Google Scholar] [CrossRef]
- Goodson, R.J.; Doyle, M.V.; Kaufman, S.E.; Rosenberg, S. High-affinity urokinase receptor antagonists identified with bacteriophage peptide display. Proc. Natl. Acad. Sci. USA 1994, 91, 7129–7133. [Google Scholar] [CrossRef]
- Di, S. Identification of a urokinase receptor-integrin interaction site. Promiscuous Regul. Integrin function. J. Biol. Chem. 2000, 275, 10228–10234. [Google Scholar]
- Ghosh, S.; Johnson, J.J.; Sen, R.; Mukhopadhyay, S.; Liu, Y.; Zhang, F.; Wei, Y.; Chapman, H.A.; Stack, M.S. Functional relevance of urinary-type plasminogen activator receptor-α3β1 integrin association in proteinase regulatory pathways. J. Biol. Chem. 2006, 281, 13021–13029. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Eble, J.A.; Wang, Z.; Kreidberg, J.A.; Chapman, H.A. Urokinase receptors promote β1 integrin function through interactions with integrin α3β1. Mol. Biol. Cell 2001, 12, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.A.; Prager, G.W.; Mihaly-Bison, J.; Uhrin, P.; Sunzenauer, S.; Binder, B.R.; Schütz, G.J.; Freissmuth, M.; Breuss, J.M. VEGF-induced endothelial cell migration requires urokinase receptor (uPAR)-dependent integrin redistribution. Cardiovasc. Res. 2012, 94, 125–135. [Google Scholar]
- Yang, S.; Banik, N.; Han, B.; Lee, D.; Park, J. Peptide-Based Bioconjugates and Therapeutics for Targeted Anticancer Therapy. Pharmaceutics 2022, 14, 1378. [Google Scholar] [CrossRef]
- Fuhrman, B. The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis 2012, 222, 8–14. [Google Scholar] [CrossRef]
- Preissner, K.; Kanse, S.; Chavakis, T.; May, A. The dual role of the urokinase receptor system in pericellular proteolysis and cell adhesion: Implications for cardiovascular function. Basic Res. Cardiol. 1999, 94, 315–321. [Google Scholar] [CrossRef]
- Pawlak, K.; Ulazka, B.; Mysliwiec, M.; Pawlak, D. Vascular endothelial growth factor and uPA/suPAR system in early and advanced chronic kidney disease patients: A new link between angiogenesis and hyperfibrinolysis? Transl. Res. 2012, 160, 346–354. [Google Scholar] [CrossRef]
- Tkachuk, V.; Stepanova, V.; Little, P.J.; Bobik, A. Regulation and role of urokinase plasminogen activator in vascular remodelling. Clin. Exp. Pharmacol. Physiol. 1996, 23, 759–765. [Google Scholar] [CrossRef]
- Larmann, J.; Jurk, K.; Janssen, H.; Müller, M.; Herzog, C.; Lorenz, A.; Schmitz, M.; Nofer, J.-R.; Theilmeier, G. Hepatic overexpression of soluble urokinase receptor (uPAR) suppresses diet-induced atherosclerosis in low-density lipoprotein receptor-deficient (LDLR-/-) mice. PLoS ONE 2015, 10, e0131854. [Google Scholar] [CrossRef] [PubMed]
- Edsfeldt, A.; Nitulescu, M.; Grufman, H.; Grönberg, C.; Persson, A.; Nilsson, M.; Persson, M.; Björkbacka, H.; Gonçalves, I. Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke 2012, 43, 3305–3312. [Google Scholar] [CrossRef]
- Kanno, Y. The uPA/uPAR system orchestrates the inflammatory response, vascular homeostasis, and immune system in fibrosis progression. Int. J. Mol. Sci. 2023, 24, 1796. [Google Scholar] [CrossRef]
- Hodges, G.W.; Bang, C.N.; Eugen-Olsen, J.; Olsen, M.H.; Boman, K.; Ray, S.; Gohlke-Bärwolf, C.; Kesäniemi, Y.A.; Jeppesen, J.L.; Wachtell, K. SuPAR predicts cardiovascular events and mortality in patients with asymptomatic aortic stenosis. Can. J. Cardiol. 2016, 32, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Tyrrell, D.J.; Vasbinder, A.; Wei, C.; Presswalla, F.; Wang, H.; Blakely, P.; Ozel, A.B.; Graham, S.; Holton, G.H. Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis. J. Clin. Investig. 2022, 132, e158788. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.W.; Bang, C.N.; Wachtell, K.; Eugen-Olsen, J.; Jeppesen, J.L. suPAR: A new biomarker for cardiovascular disease? Can. J. Cardiol. 2015, 31, 1293–1302. [Google Scholar] [CrossRef]
- Goodchild, T.T.; Li, Z.; Lefer, D.J. Soluble urokinase plasminogen activator receptor: From biomarker to active participant in atherosclerosis and cardiovascular disease. J. Clin. Investig. 2022, 132, e165868. [Google Scholar] [CrossRef]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef]
- de Nooijer, A.H.; Pickkers, P.; Netea, M.G.; Kox, M. Inflammatory biomarkers to predict the prognosis of acute bacterial and viral infections. J. Crit. Care 2023, 78, 154360. [Google Scholar] [CrossRef]
- D’Alonzo, D.; De Fenza, M.; Pavone, V. COVID-19 and pneumonia: A role for the uPA/uPAR system. Drug Discov. Today 2020, 25, 1528–1534. [Google Scholar] [CrossRef]
- Yatsenko, T.; Rios, R.; Nogueira, T.; Salama, Y.; Takahashi, S.; Tabe, Y.; Naito, T.; Takahashi, K.; Hattori, K.; Heissig, B. Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 complex as a serum biomarker for COVID-19. Front. Immunol. 2024, 14, 1299792. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Panagopoulos, P.; Metallidis, S.; Dalekos, G.N.; Poulakou, G.; Gatselis, N.; Karakike, E.; Saridaki, M.; Loli, G.; Stefos, A. An open label trial of anakinra to prevent respiratory failure in COVID-19. eLife 2021, 10, e66125. [Google Scholar] [CrossRef]
- Uzunçakmak, S.K. SARS-CoV-2 Infection and Candidate Biomarkers. Eurasian J. Med. 2022, 54, S16. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Popov, V.S.; Romanov, I.P.; Khabibullin, N.R.; Sabitova, N.R.; Karpukhina, A.A.; Kozhevnikova, Y.A.; Kurilina, E.V.; Tsokolaeva, Z.I.; Klimovich, P.S. Urokinase system in pathogenesis of pulmonary fibrosis: A hidden threat of COVID-19. Int. J. Mol. Sci. 2023, 24, 1382. [Google Scholar] [CrossRef]
- Nekrasova, L.A.; Shmakova, A.A.; Samokhodskaya, L.M.; Kirillova, K.I.; Stoyanova, S.S.; Mershina, E.A.; Nazarova, G.B.; Rubina, K.A.; Semina, E.V.; Kamalov, A.A. The association of PLAUR genotype and soluble suPAR serum level with COVID-19-related lung damage severity. Int. J. Mol. Sci. 2022, 23, 16210. [Google Scholar] [CrossRef]
- Horowitz, J.C.; Tschumperlin, D.J.; Kim, K.K.; Osterholzer, J.J.; Subbotina, N.; Ajayi, I.O.; Teitz-Tennenbaum, S.; Virk, A.; Dotson, M.; Liu, F. Urokinase plasminogen activator overexpression reverses established lung fibrosis. Thromb. Haemost. 2019, 119, 1968–1980. [Google Scholar] [CrossRef]
- Oliveira, C.L.; Bates, J.H.; Suki, B. A network model of correlated growth of tissue stiffening in pulmonary fibrosis. New J. Phys. 2014, 16, 065022. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.-Y.; Gorin, F.A.; Lein, P.J. Review of evidence implicating the plasminogen activator system in blood-brain barrier dysfunction associated with Alzheimer’s disease. Ageing Neurodegener. Dis. 2022, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhao, Y.; Zhai, Z.; Zheng, J.; Zhou, Y.; Zhai, Q.; Cao, X.; Tian, J.; Zhao, L. Role of plasminogen activator inhibitor-1 in the diagnosis and prognosis of patients with Parkinson’s disease. Exp. Ther. Med. 2018, 15, 5517–5522. [Google Scholar] [CrossRef]
- Angelucci, F.; Nedelska, Z.; Napravnikova Manová, B.; Katonová, A.b.; Jurášová, V.; Svetlikova, D.; Vyhnálek, M.; Hort, J. Dementia with Lewy Bodies (DLB), Parkinson’s Disease (PD), and Multiple System Atrophy (MSA) Are Synucleopathies Characterized by Increased Serum Levels of Plasminogen Activator Inhibitor-1 (PAI-1). ACS Omega 2025, 10, 24194–24199. [Google Scholar] [CrossRef] [PubMed]
- Gveric, D.; Hanemaaijer, R.; Newcombe, J.; van Lent, N.A.; Sier, C.F.; Cuzner, M.L. Plasminogen activators in multiple sclerosis lesions: Implications for the inflammatory response and axonal damage. Brain 2001, 124, 1978–1988. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.; Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef]
- Quesnel, A.; Karagiannis, G.S.; Filippou, P.S. Extracellular proteolysis in glioblastoma progression and therapeutics. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188428. [Google Scholar] [CrossRef]
- Scarisbrick, I. The multiple sclerosis degradome: Enzymatic cascades in development and progression of central nervous system inflammatory disease. In Advances in multiple Sclerosis and Experimental Demyelinating Diseases; Springer: Berlin/Heidelberg, Germany, 2008; pp. 133–175. [Google Scholar]
- Lorenzano, S.; Inglese, M.; Koudriavtseva, T. Role of coagulation pathways in neurological diseases. Front. Neurol. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Yepes, M.; Woo, Y.; Martin-Jimenez, C. Plasminogen activators in neurovascular and neurodegenerative disorders. Int. J. Mol. Sci. 2021, 22, 4380. [Google Scholar] [CrossRef]
- Dong, W.; Huang, Y. Common Genetic Factors and Pathways in Alzheimer’s Disease and Ischemic Stroke: Evidences from GWAS. Genes 2023, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Eagleson, K.L.; Gravielle, M.C.; McFadyen-Ketchum, L.S.; Russek, S.J.; Farb, D.H.; Levitt, P. Genetic disruption of the autism spectrum disorder risk gene PLAUR induces GABAA receptor subunit changes. Neuroscience 2010, 168, 797–810. [Google Scholar] [CrossRef]
- Kyyriäinen, J.; Bolkvadze, T.; Koivisto, H.; Lipponen, A.; Pérez, L.O.; Ndode-Ekane, X.E.; Tanila, H.; Pitkänen, A. Deficiency of urokinase-type plasminogen activator and its receptor affects social behavior and increases seizure susceptibility. Epilepsy Res. 2019, 151, 67–74. [Google Scholar] [CrossRef]
- Stempien-Otero, A.; Plawman, A.; Meznarich, J.; Dyamenahalli, T.; Otsuka, G.; Dichek, D.A. Mechanisms of cardiac fibrosis induced by urokinase plasminogen activator. J. Biol. Chem. 2006, 281, 15345–15351. [Google Scholar] [CrossRef]
- Montuori, N.; Selleri, C.; Ragno, P. The urokinase-receptor in infectious diseases. Infez Med 2012, 20, 13–18. [Google Scholar] [PubMed]
- Hovius, J.W.; Bijlsma, M.F.; van der Windt, G.J.; Wiersinga, W.J.; Boukens, B.J.; Coumou, J.; Oei, A.; de Beer, R.; de Vos, A.F.; Veer, C.v.t. The urokinase receptor (uPAR) facilitates clearance of Borrelia burgdorferi. PLoS Pathog. 2009, 5, e1000447. [Google Scholar] [CrossRef]
- Yu, J.; Murthy, V.; Liu, S.-L. Relating GPI-anchored Ly6 proteins uPAR and CD59 to viral infection. Viruses 2019, 11, 1060. [Google Scholar] [CrossRef]
- Bruneau, N.; Szepetowski, P. The role of the urokinase receptor in epilepsy, in disorders of language, cognition, communication and behavior, and in the central nervous system. Curr. Pharm. Des. 2011, 17, 1914–1923. [Google Scholar] [CrossRef]
- Garcia-Monco, J.C.; Coleman, J.L.; Benach, J.L. Soluble urokinase receptor (uPAR, CD 87) is present in serum and cerebrospinal fluid in patients with neurologic diseases. J. Neuroimmunol. 2002, 129, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Archinti, M.; Britto, M.; Eden, G.; Furlan, F.; Murphy, R.; Degryse, B. The urokinase receptor in the central nervous system. CNS Neurol. Disord. Drug Targets 2011, 10, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Cinque, P.; Nebuloni, M.; Santovito, M.L.; Price, R.W.; Gisslen, M.; Hagberg, L.; Bestetti, A.; Vago, G.; Lazzarin, A.; Blasi, F. The urokinase receptor is overexpressed in the AIDS dementia complex and other neurological manifestations. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2004, 55, 687–694. [Google Scholar] [CrossRef]
- Welin, A.; Wirestam, L.; Karlsson, J.; Wetterö, J.; Enocsson, H.; Sjöwall, C. P25 Leukocyte cell surface expression of urokinase plasminogen activator receptor (uPAR) in patients with systemic lupus erythematosus. In Proceedings of the 14th European Lupus Meeting, Bruges, Belgium, 19–22 March 2024. [Google Scholar]
- Qin, D.; Song, D.; Huang, J.; Yu, F.; Zhao, M. Plasma-soluble urokinase-type plasminogen activator receptor levels are associated with clinical and pathological activities in lupus nephritis: A large cohort study from China. Lupus 2015, 24, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Enocsson, H.; Wirestam, L.; Dahle, C.; Padyukov, L.; Jönsen, A.; Urowitz, M.B.; Gladman, D.D.; Romero-Diaz, J.; Bae, S.-C.; Fortin, P.R. Soluble urokinase plasminogen activator receptor (suPAR) levels predict damage accrual in patients with recent-onset systemic lupus erythematosus. J. Autoimmun. 2020, 106, 102340. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Van Hoovels, L.; Benucci, M.; De Luca, R.; Coccia, C.; Bernardini, P.; Russo, E.; Amedei, A.; Guiducci, S.; Grossi, V. Soluble Urokinase Plasminogen Activator Receptor (suPAR) in Autoimmune Rheumatic and Non Rheumatic Diseases. J. Pers. Med. 2023, 13, 688. [Google Scholar] [CrossRef]
- Vasarhelyi, B.; Toldi, G.; Balog, A. The clinical value of soluble urokinase plasminogen activator receptor (suPAR) levels in autoimmune connective tissue disorders. EJIFCC 2016, 27, 122. [Google Scholar]
- Filippo, L. UPARANT, a Novel Multitarget Drug for Neovascular and Inflammatory Ocular Diseases; Università degli Studi di Siena: Siena, Italy, 2019. [Google Scholar]
- Cammalleri, M.; Dal Monte, M.; Pavone, V.; De Rosa, M.; Rusciano, D.; Bagnoli, P. The uPAR system as a potential therapeutic target in the diseased eye. Cells 2019, 8, 925. [Google Scholar] [CrossRef] [PubMed]
- Can, Ü. Soluble urokinase-type plasminogen activator receptor (suPAR) in multiple respiratory diseases. Recept. Clin. Investig. 2015, 2, 10. [Google Scholar]
- Portelli, M.A.; Bhaker, S.; Pang, V.; Bates, D.O.; Johnson, S.R.; Mazar, A.P.; Shaw, D.; Brightling, C.; Sayers, I. Elevated PLAUR is observed in the airway epithelium of asthma patients and blocking improves barrier integrity. Clin. Transl. Allergy 2023, 13, e12293. [Google Scholar] [CrossRef]
- Wei, C.; Li, J.; Adair, B.D.; Zhu, K.; Cai, J.; Merchant, M.; Samelko, B.; Liao, Z.; Koh, K.H.; Tardi, N.J. uPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J. Clin. Investig. 2019, 129, 1946–1959. [Google Scholar] [CrossRef]
- da Silva, C.A.; Araújo, L.S.; dos Reis Monteiro, M.L.G.; de Morais Pereira, L.H.; da Silva, M.V.; Castellano, L.R.C.; Corrêa, R.R.M.; Dos Reis, M.A.; Machado, J.R. Evaluation of the Diagnostic Potential of uPAR as a Biomarker in Renal Biopsies of Patients with FSGS. Dis. Markers 2019, 2019, 1070495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Eddy, A.A. Urokinase and its receptors in chronic kidney disease. Front. Biosci. A J. Virtual Libr. 2008, 13, 5462–5478. [Google Scholar] [CrossRef] [PubMed]
- Genua, M.; D’Alessio, S.; Cibella, J.; Gandelli, A.; Sala, E.; Correale, C.; Spinelli, A.; Arena, V.; Malesci, A.; Rutella, S. The urokinase plasminogen activator receptor (uPAR) controls macrophage phagocytosis in intestinal inflammation. Gut 2015, 64, 589–600. [Google Scholar] [CrossRef]
- Cheng, Y.; Hall, T.R.; Xu, X.; Yung, I.; Souza, D.; Zheng, J.; Schiele, F.; Hoffmann, M.; Mbow, M.L.; Garnett, J.P. Targeting uPA-uPAR interaction to improve intestinal epithelial barrier integrity in inflammatory bowel disease. EBioMedicine 2022, 75, 103758. [Google Scholar] [CrossRef]
- Mazar, A.P. The urokinase plasminogen activator receptor (uPAR) as a target for the diagnosis and therapy of cancer. Anti-Cancer Drugs 2001, 12, 387–400. [Google Scholar] [CrossRef]
- Dass, K.; Ahmad, A.; Azmi, A.S.; Sarkar, S.H.; Sarkar, F.H. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 2008, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Montuori, N.; Pesapane, A.; Rossi, F.W.; Giudice, V.; De Paulis, A.; Selleri, C.; Ragno, P. Urokinase type plasminogen activator receptor (uPAR) as a new therapeutic target in cancer. Transl. Med. UniSa 2016, 15, 15. [Google Scholar] [PubMed]
- de Bock, C.E.; Wang, Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med. Res. Rev. 2004, 24, 13–39. [Google Scholar] [CrossRef]
- Pakneshan, P.; Szyf, M.; Rabbani, S.A. Hypomethylation of urokinase (uPA) promoter in breast and prostate cancer: Prognostic and therapeutic implications. Curr. Cancer Drug Targets 2005, 5, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Pakneshan, P.; Xing, R.H.; Rabbani, S.A. Methylation status of uPA promoter as a molecular mechanism regulating prostate cancer invasion and growth in vitro and in vivo. FASEB J. 2003, 17, 1081–1088. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lu, C.-S.; Zhang, S.; Makra, L. Alteration of the Methylation Status of Urokinase Plasminogen Activator (uPA) is involved in Proliferation and Invasion of Nasopharyngeal Cancer Cells. Arch. Otolaryngol. Rhinol. 2015, 1, 023–027. [Google Scholar] [CrossRef]
- Pakneshan, P. Hypomethylation of Urokinase (uPA) Promoter in Hormone-Dependent Malignancies: Prognostic and Therapeutic Implications; McGill University: Montreal, QC, Canada, 2004. [Google Scholar]
- Huo, Z.; Li, X.; Zhou, J.; Fan, Y.; Wang, Z.; Zhang, Z. Hypomethylation and downregulation of miR-23b-3p are associated with upregulated PLAU: A diagnostic and prognostic biomarker in head and neck squamous cell carcinoma. Cancer Cell Int. 2021, 21, 564. [Google Scholar] [CrossRef]
- Grossi, I.; Salvi, A.; Baiocchi, G.; Portolani, N.; De Petro, G. Functional role of microRNA-23b-3p in cancer biology. Microrna 2018, 7, 156–166. [Google Scholar] [CrossRef]
- Iwamoto, N.; Vettori, S.; Maurer, B.; Brock, M.; Jüngel, A.; Calcagni, M.; Gay, R.E.; Distler, J.H.; Gay, S.; Kawakami, A. A3. 19 mIR-193B induces UPA in SSC and contributes to the proliferative vasculopathy via uPAR independent pathways. Ann. Rheum. Dis. 2014, 73, A49. [Google Scholar] [CrossRef]
- Li, X.; Yan, P.; Shao, Z. Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene 2009, 28, 3937–3948. [Google Scholar] [CrossRef]
- Iwamoto, N.; Vettori, S.; Maurer, B.; Brock, M.; Pachera, E.; Jüngel, A.; Calcagni, M.; Gay, R.E.; Whitfield, M.L.; Distler, J.H. Downregulation of miR-193b in systemic sclerosis regulates the proliferative vasculopathy by urokinase-type plasminogen activator expression. Ann. Rheum. Dis. 2016, 75, 303–310. [Google Scholar] [CrossRef]
- Ferreira, G.M.; Cuello, H.A.; Nogueira, A.C.; Castillo, J.O.; Rojo, S.; Gulino, C.A.; Segatori, V.I.; Gabri, M.R. The Essential Role of N-Glycosylation in Integrin αV and uPAR Interaction in Glioblastoma; Springer Science and Business Media LLC: New York, NY, USA, 2024. [Google Scholar]
- Raghu, H.; Gondi, C.S.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch-1 receptor. Mol. Cancer 2011, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Marra, P.; Marshall, C.J. uPAR promotes formation of the p130Cas–Crk complex to activate Rac through DOCK180. J. Cell Biol. 2008, 182, 777–790. [Google Scholar] [CrossRef] [PubMed]
- SARRA FERRARIS, G. Non-Integrin Cell Adhesion Triggers Ligand-Independent Integrin Signaling. Ph.D. Thesis, Università degli Studi di Milano, Milan, Italy, 2011. [Google Scholar]
- Baart, V.M.; van Manen, L.; Bhairosingh, S.S.; Vuijk, F.A.; Iamele, L.; de Jonge, H.; Scotti, C.; Resnati, M.; Cordfunke, R.A.; Kuppen, P.J. Side-by-side comparison of uPAR-targeting optical imaging antibodies and antibody fragments for fluorescence-guided surgery of solid tumors. Mol. Imaging Biol. 2023, 25, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Aghamiri, S.S.; Amin, R. Cancer Stem Cell Metastatic Checkpoints and Glycosylation Patterns: Implications for Therapeutic Strategies. Kinases Phosphatases 2024, 2, 151–165. [Google Scholar] [CrossRef]
- Xie, S.; Yang, G.; Wu, J.; Jiang, L.; Yuan, C.; Xu, P.; Huang, M.; Liu, Y.; Li, J. In silico screening of natural products as uPAR inhibitors via multiple structure-based docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2023, 43, 3064–3075. [Google Scholar] [CrossRef]
- Koller, L.; Stojkovic, S.; Richter, B.; Sulzgruber, P.; Potolidis, C.; Liebhart, F.; Mörtl, D.; Berger, R.; Goliasch, G.; Wojta, J. Soluble urokinase-type plasminogen activator receptor improves risk prediction in patients with chronic heart failure. JACC Heart Fail. 2017, 5, 268–277. [Google Scholar] [CrossRef]
- Hayek, S.S.; Tahhan, A.S.; Ko, Y.-A.; Alkhoder, A.; Zheng, S.; Bhimani, R.; Hartsfield, J.; Kim, J.; Wilson, P.; Shaw, L. Soluble urokinase plasminogen activator receptor levels and outcomes in patients with heart failure. J. Card. Fail. 2023, 29, 158–167. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Døssing, K.B.; Rossing, M.; Bagger, F.O.; Kjaer, A. uPAR (PLAUR) Marks Two Intra-Tumoral Subtypes of Glioblastoma: Insights from Single-Cell RNA Sequencing. Int. J. Mol. Sci. 2024, 25, 1998. [Google Scholar] [CrossRef]
- Magnussen, S.; Hadler-Olsen, E.; Latysheva, N.; Pirila, E.; Steigen, S.E.; Hanes, R.; Salo, T.; Winberg, J.-O.; Uhlin-Hansen, L.; Svineng, G. Tumour microenvironments induce expression of urokinase plasminogen activator receptor (uPAR) and concomitant activation of gelatinolytic enzymes. PLoS ONE 2014, 9, e105929. [Google Scholar] [CrossRef]
- Mason, S.D.; Joyce, J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011, 21, 228–237. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, L.; Zhou, Y. HDAC1 is indirectly involved in the epigenetic regulation of p38 MAPK that drive the lung cancer progression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5980–5986. [Google Scholar] [PubMed]
- Wang, M.; Chen, Y.; Bi, X.; Luo, X.; Hu, Z.; Liu, Y.; Shi, X.; Weng, W.; Mo, B.; Lu, Y. LncRNA NEAT1_1 suppresses tumor-like biologic behaviors of fibroblast-like synoviocytes by targeting the miR-221-3p/uPAR axis in rheumatoid arthritis. J. Leukoc. Biol. 2022, 111, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Leth, J.M.; Ploug, M. Targeting the urokinase-type plasminogen activator receptor (uPAR) in human diseases with a view to non-invasive imaging and therapeutic intervention. Front. Cell Dev. Biol. 2021, 9, 732015. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.R.A.; Bayraktutan, U. Urokinase plasminogen activator: A potential thrombolytic agent for ischaemic stroke. Cell. Mol. Neurobiol. 2020, 40, 347–355. [Google Scholar] [CrossRef]
- Luo, S.; Vasbinder, A.; Du-Fay-de-Lavallaz, J.M.; Gomez, J.M.D.; Suboc, T.; Anderson, E.; Tekumulla, A.; Shadid, H.; Berlin, H.; Pan, M. Soluble Urokinase Plasminogen Activator Receptor and Venous Thromboembolism in COVID-19. J. Am. Heart Assoc. 2022, 11, e025198. [Google Scholar] [CrossRef]
- Yepes, M. Urokinase-type plasminogen activator is a modulator of synaptic plasticity in the central nervous system: Implications for neurorepair in the ischemic brain. Neural Regen. Res. 2020, 15, 620–624. [Google Scholar] [CrossRef]
- Bychkov, M.L.; Isaev, A.B.; Andreev-Andrievskiy, A.A.; Petrov, K.; Paramonov, A.S.; Kirpichnikov, M.P.; Lyukmanova, E.N. Aβ1-42 Accumulation Accompanies Changed Expression of Ly6/uPAR Proteins, Dysregulation of the Cholinergic System, and Degeneration of Astrocytes in the Cerebellum of Mouse Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2023, 24, 14852. [Google Scholar] [CrossRef]
- Mateusiak, Ł.; Floru, S.; De Groof, T.W.; Wouters, J.; Declerck, N.B.; Debie, P.; Janssen, S.; Zeven, K.; Puttemans, J.; Vincke, C. Generation and Characterization of Novel Pan-Cancer Anti-uPAR Fluorescent Nanobodies as Tools for Image-Guided Surgery. Adv. Sci. 2024, 11, 2400700. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Skovgaard, D.; Brandt-Larsen, M.; Christensen, C.; Madsen, J.; Nielsen, C.H.; Thurison, T.; Klausen, T.L.; Holm, S.; Loft, A. First-in-human uPAR PET: Imaging of cancer aggressiveness. Theranostics 2015, 5, 1303. [Google Scholar] [CrossRef]
- Lawaetz, M.; Binderup, T.; Christensen, A.; Juhl, K.; Lelkaitis, G.; Lykke, E.; Knudsen, L.; von Buchwald, C.; Kjaer, A. Urokinase-Type Plasminogen Activator Receptor (uPAR) Expression and [64Cu] Cu-DOTA-AE105 uPAR-PET/CT in Patient-Derived Xenograft Models of Oral Squamous Cell Carcinoma. Mol. Imaging Biol. 2023, 25, 1034–1044. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Zhang, Y.; Li, L.; Zhang, S.; Wang, R.; Zhang, M.-R.; Hu, K. Imaging uPAR with cyclic peptide-based PET tracers. J. Nucl. Med. 2024, 65 (Suppl. S2), 241781. [Google Scholar]
- Zou, Z.; Sun, W.; Xu, Y.; Liu, W.; Zhong, J.; Lin, X.; Chen, Y. Application of multi-omics approach in sarcomas: A tool for studying mechanism, biomarkers, and therapeutic targets. Front. Oncol. 2022, 12, 946022. [Google Scholar] [CrossRef] [PubMed]
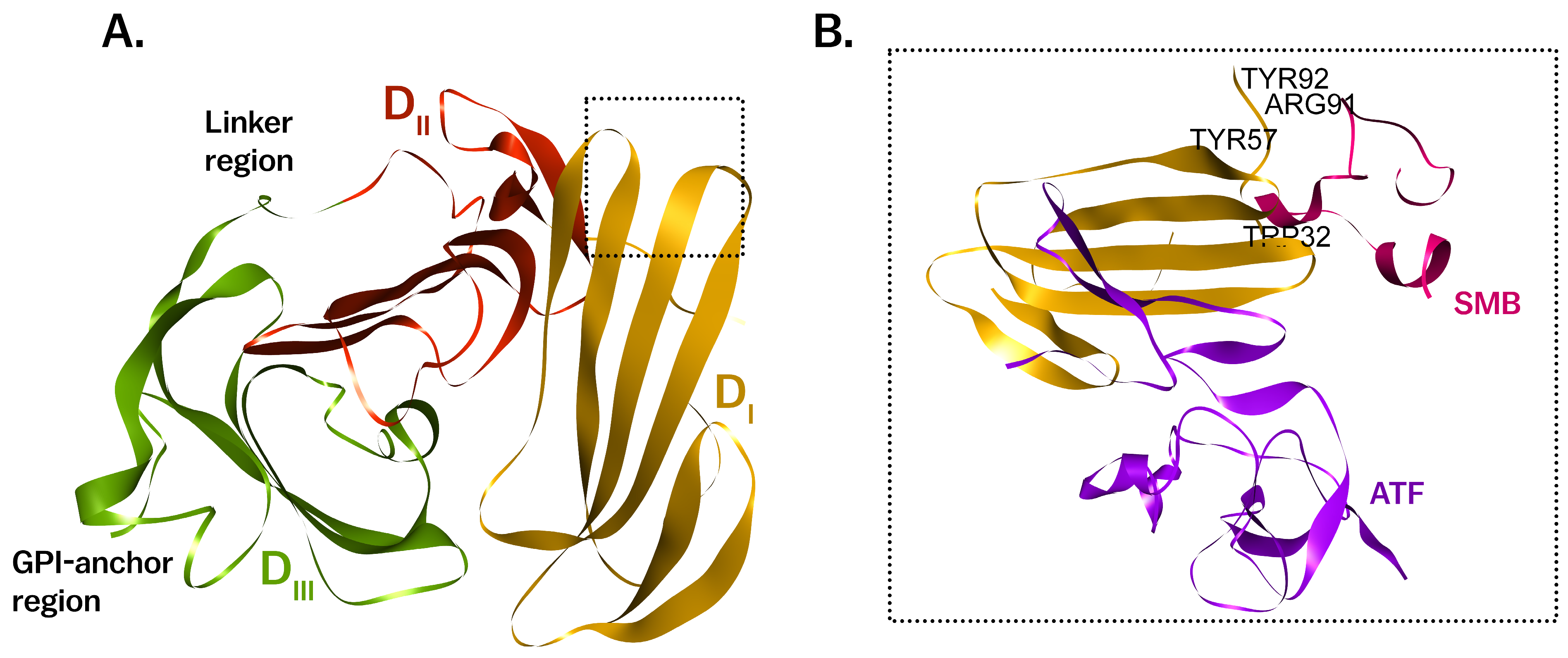
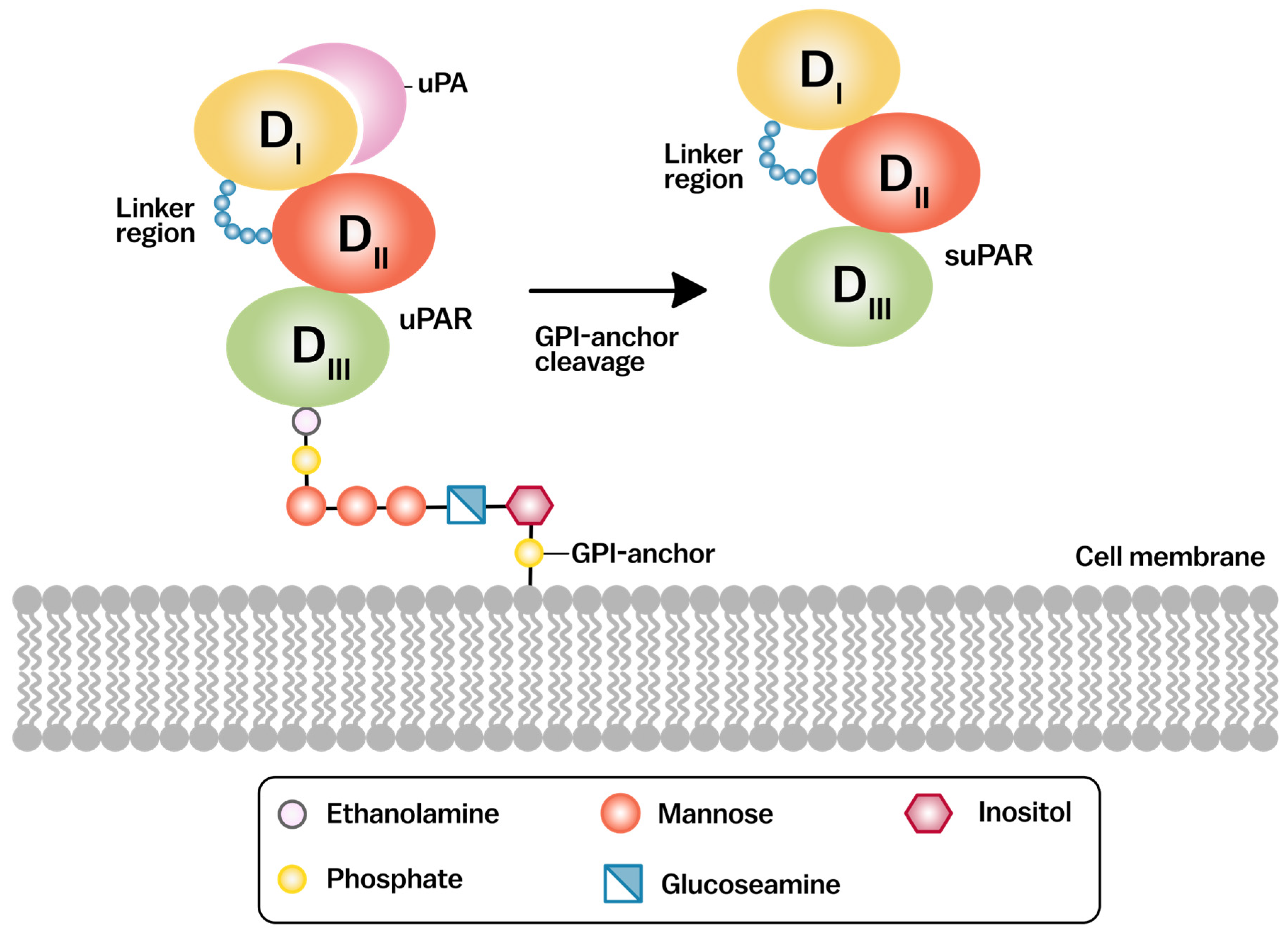
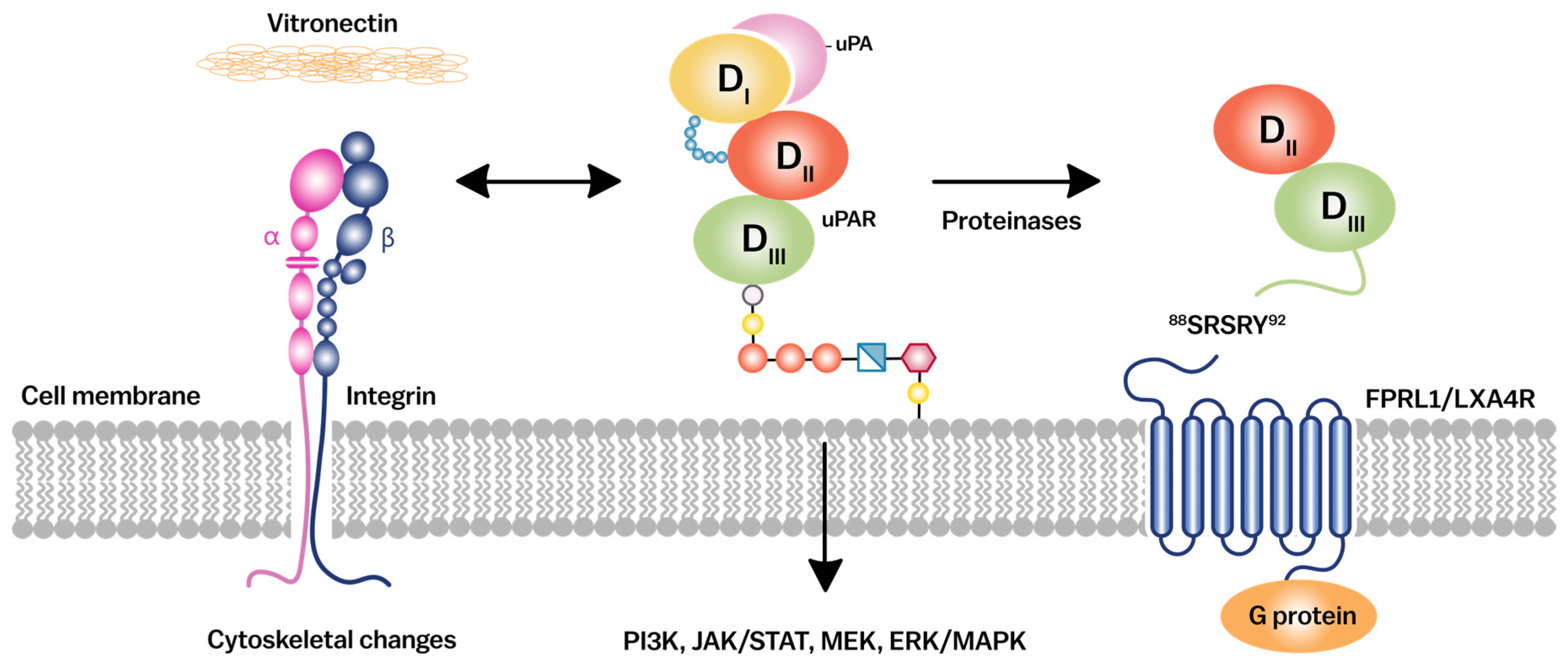
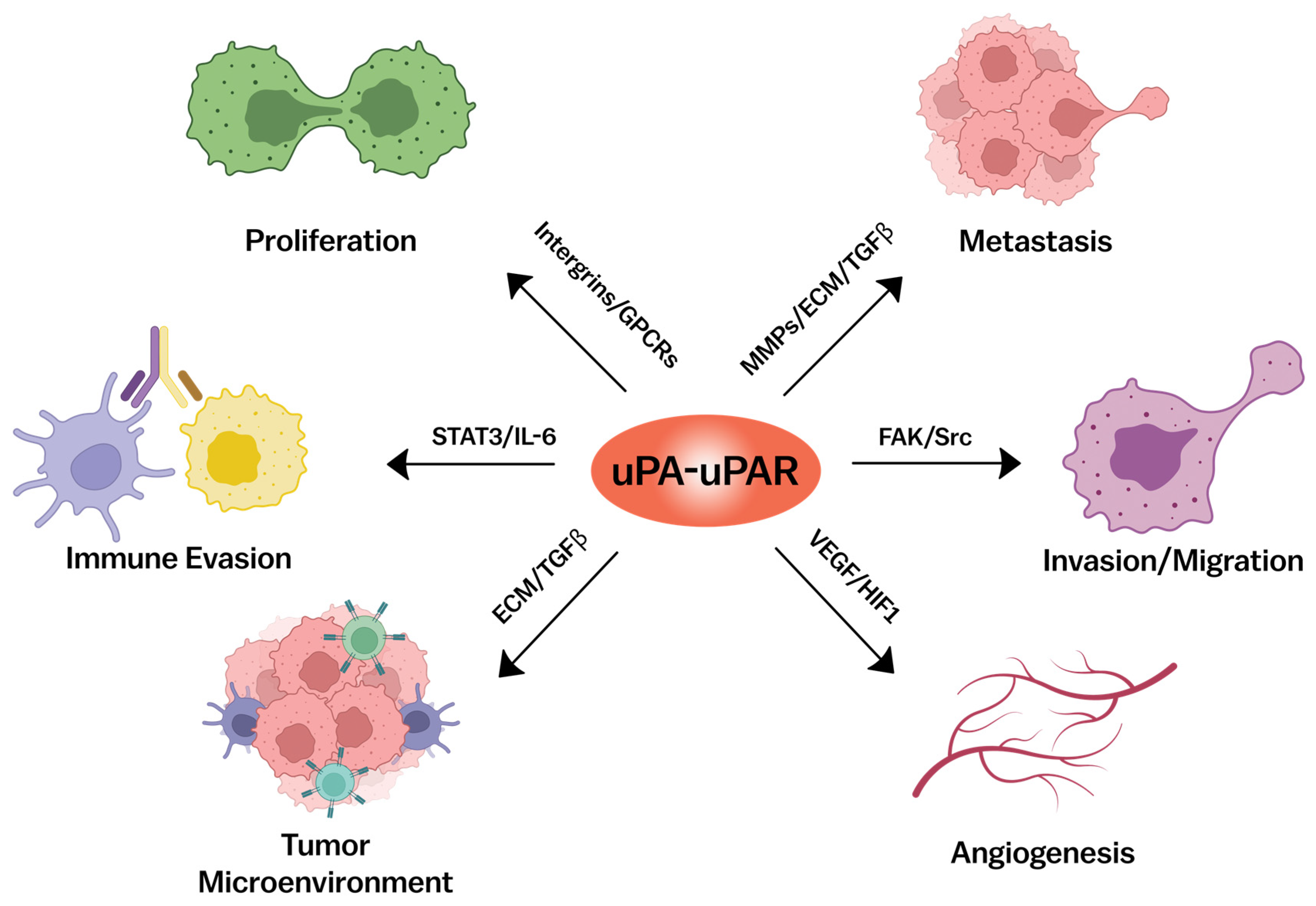
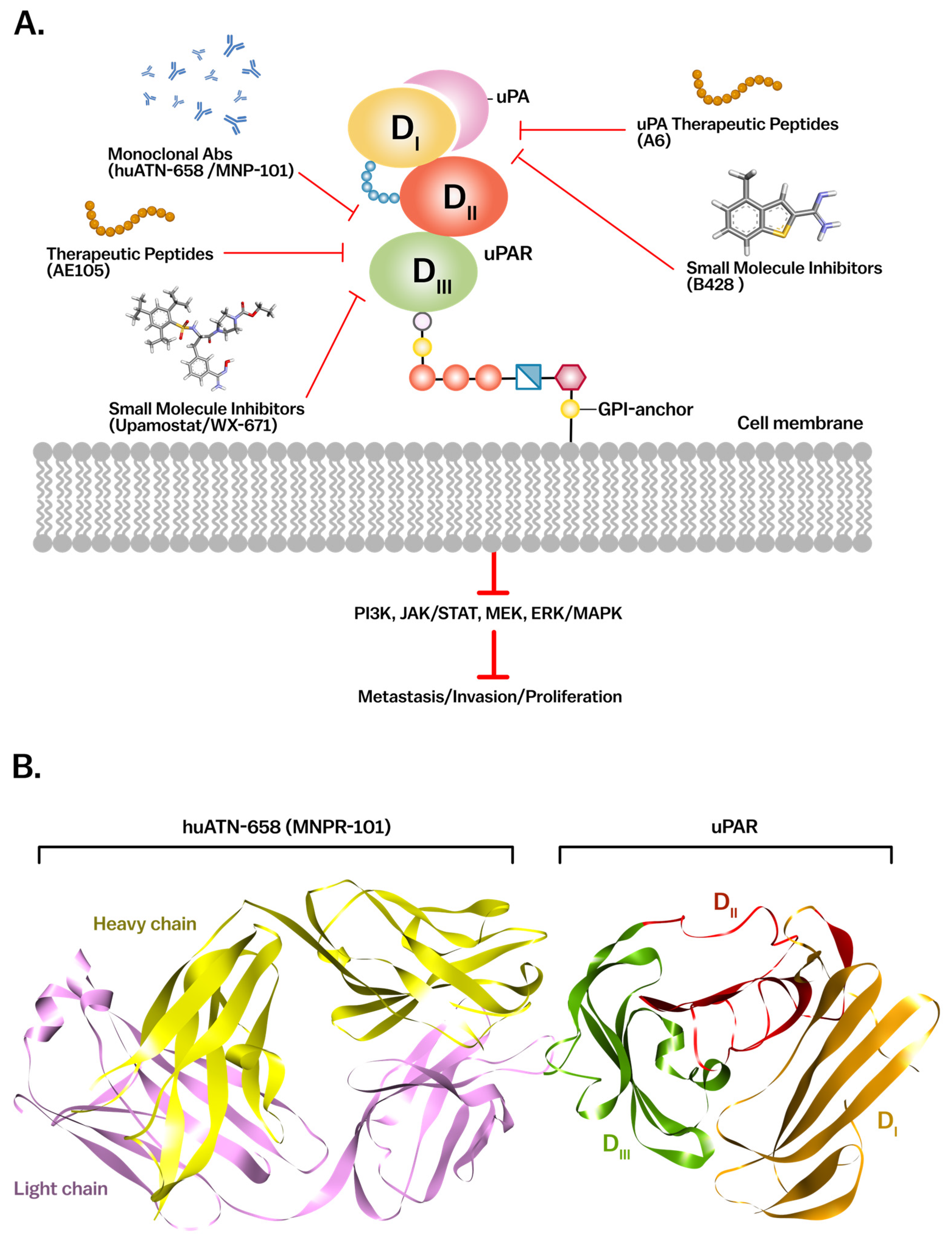
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftikhar, A.; Mahmood, N.; Rabbani, S.A. The Urokinase-Type Plasminogen Activator Receptor (uPAR) as a Mediator of Physiological and Pathological Processes: Potential Therapeutic Strategies. Cancers 2025, 17, 3309. https://doi.org/10.3390/cancers17203309
Iftikhar A, Mahmood N, Rabbani SA. The Urokinase-Type Plasminogen Activator Receptor (uPAR) as a Mediator of Physiological and Pathological Processes: Potential Therapeutic Strategies. Cancers. 2025; 17(20):3309. https://doi.org/10.3390/cancers17203309
Chicago/Turabian StyleIftikhar, Ali, Niaz Mahmood, and Shafaat A. Rabbani. 2025. "The Urokinase-Type Plasminogen Activator Receptor (uPAR) as a Mediator of Physiological and Pathological Processes: Potential Therapeutic Strategies" Cancers 17, no. 20: 3309. https://doi.org/10.3390/cancers17203309
APA StyleIftikhar, A., Mahmood, N., & Rabbani, S. A. (2025). The Urokinase-Type Plasminogen Activator Receptor (uPAR) as a Mediator of Physiological and Pathological Processes: Potential Therapeutic Strategies. Cancers, 17(20), 3309. https://doi.org/10.3390/cancers17203309







