Optimizing Post-Neoadjuvant Treatment in Early Triple-Negative Breast Cancer
Simple Summary
Abstract
1. Introduction
1.1. Breast Conservation
1.2. Survival
1.3. Response Assessment
1.4. Current Standard of Care
2. Post-Neoadjuvant Surgery
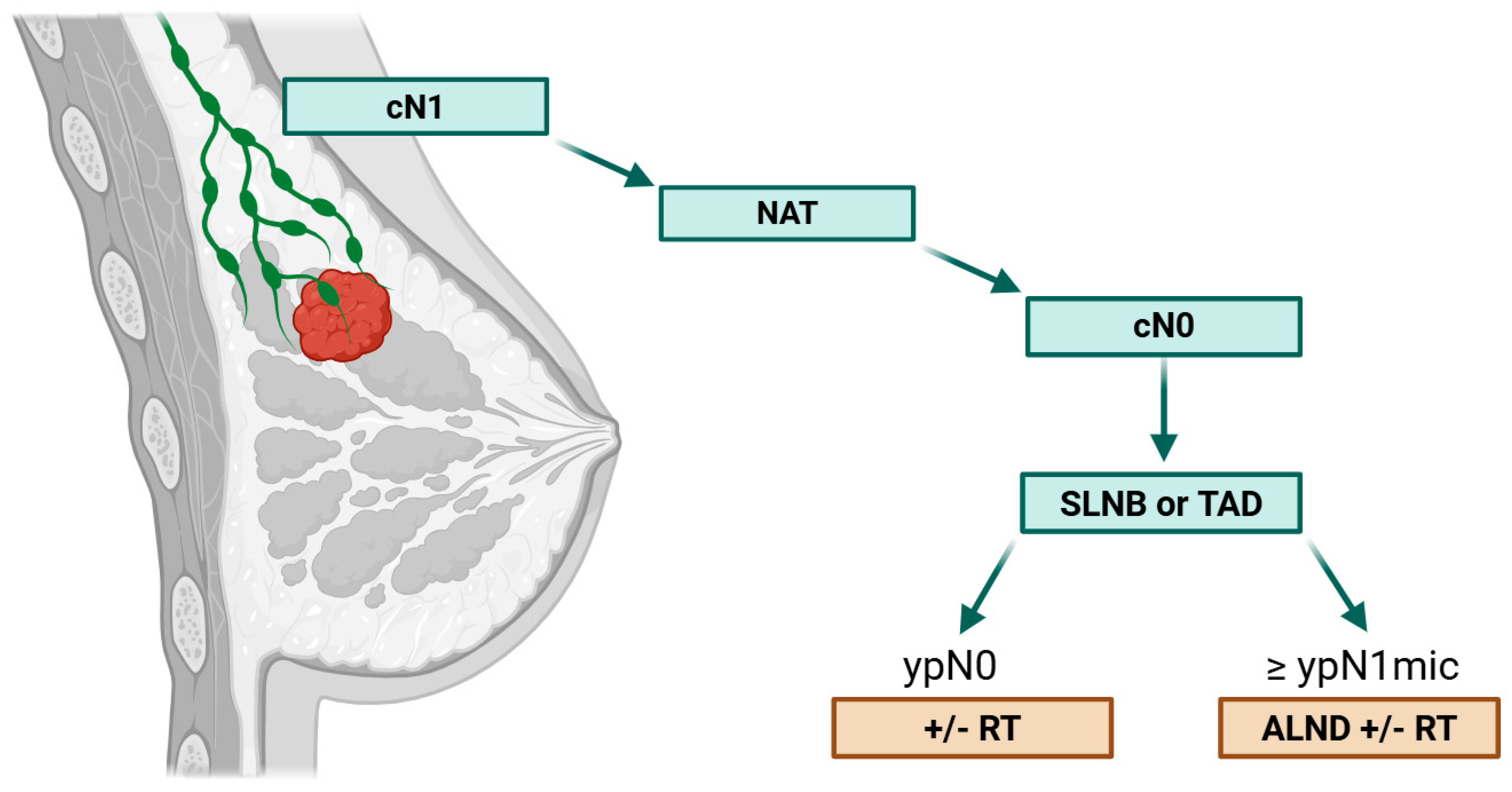
2.1. Axillary Management After NAC
2.2. Avoiding Surgery
3. Optimizing Post-Neoadjuvant Medical Treatment
3.1. Post-Neoadjuvant De-Escalation Approaches
3.2. Biomarkers-Driven De-Escalation
3.3. Post-Neoadjuvant Escalation Approaches
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| aPD-(L)1 | anti–PD-1 or PD-L1 agent |
| ADC | Antibody–Drug Conjugate |
| ALND | Axillary Lymph Node Dissection |
| AUS | Axillary Ultrasound |
| BCS | Breast-Conserving Surgery |
| cCR | Clinical Complete Response |
| ctDNA | Circulating Tumor DNA |
| DFS | Disease-Free Survival |
| Dato-DXd | Datopotamab Deruxtecan |
| EFS | Event-Free Survival |
| ER | Estrogen Receptor |
| FCRI | Fear of Cancer Recurrence Inventory |
| HR | Hazard Ratio |
| ICI | Immune Checkpoint Inhibitor |
| iDFS | Invasive Disease-Free Survival |
| ILC | Invasive Lobular Carcinoma |
| irAE | Immune-Related Adverse Event |
| ITT | Intention-To-Treat |
| MRI | Magnetic Resonance Imaging |
| NAC | Neoadjuvant Chemotherapy |
| NAT | Neoadjuvant Therapy |
| OS | Overall Survival |
| pCR | Pathological Complete Response |
| RD | Residual Disease |
| RCB | Residual Cancer Burden |
| RFS | Relapse-Free Survival |
| SLNB | Sentinel Lymph Node Biopsy |
| TAD | Targeted Axillary Dissection |
| TILs | Tumor-Infiltrating Lymphocytes |
| TNBC | Triple-Negative Breast Cancer |
| TPC | Treatment of Physician’s Choice |
| VAF | Variant Allele Frequency |
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-Term Outcomes for Neoadjuvant versus Adjuvant Chemotherapy in Early Breast Cancer: Meta-Analysis of Individual Patient Data from Ten Randomised Trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef]
- Miglietta, F.; Dieci, M.V.; Griguolo, G.; Guarneri, V. Neoadjuvant Approach as a Platform for Treatment Personalization: Focus on HER2-Positive and Triple-Negative Breast Cancer. Cancer Treat. Rev. 2021, 98, 102222. [Google Scholar] [CrossRef]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of Preoperative Chemotherapy on the Outcome of Women with Operable Breast Cancer. J. Clin. Oncol. 2023, 41, 1795–1808. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. Evaluating the Evidence behind the Surrogate Measures Included in the FDA’s Table of Surrogate Endpoints as Supporting Approval of Cancer Drugs. EClinicalMedicine 2020, 21, 100332. [Google Scholar] [CrossRef]
- Cortazar, P.; Kluetz, P.G. Neoadjuvant Breast Cancer Therapy and Drug Development. Clin. Adv. Hematol. Oncol. 2015, 13, 755–761. [Google Scholar] [PubMed]
- Jones, S.; Holmes, F.A.; O’Shaughnessy, J.; Blum, J.L.; Vukelja, S.J.; McIntyre, K.J.; Pippen, J.E.; Bordelon, J.H.; Kirby, R.L.; Sandbach, J.; et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J. Clin. Oncol. 2009, 27, 1177–1183. [Google Scholar] [CrossRef]
- Blum, J.L.; Flynn, P.J.; Yothers, G.; Asmar, L.; Geyer, C.E.; Jacobs, S.A.; Robert, N.J.; Hopkins, J.O.; O’Shaughnessy, J.A.; Dang, C.T.; et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 2017, 35, 2647–2655. [Google Scholar] [CrossRef]
- An, X.; Lei, X.; Huang, R.; Luo, R.; Li, H.; Xu, F.; Yuan, Z.; Wang, S.; de Nonneville, A.; Gonçalves, A.; et al. Adjuvant Chemotherapy for Small, Lymph Node-Negative, Triple-Negative Breast Cancer: A Single-Center Study and a Meta-Analysis of the Published Literature. Cancer 2020, 126 (Suppl. S16), 3837–3846. [Google Scholar] [CrossRef]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early Breast Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Schumacher-Wulf, E.; Matos, L.; Gelmon, K.; Aapro, M.S.; Bajpai, J.; Barrios, C.H.; Bergh, J.; Bergsten-Nordström, E.; et al. 6th and 7th International Consensus Guidelines for the Management of Advanced Breast Cancer (ABC Guidelines 6 and 7). Breast 2024, 76, 103756. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Cancer Netw. 2023, 21, 594–608. [Google Scholar] [CrossRef]
- Citron, M.L.; Berry, D.A.; Cirrincione, C.; Hudis, C.; Winer, E.P.; Gradishar, W.J.; Davidson, N.E.; Martino, S.; Livingston, R.; Ingle, J.N.; et al. Randomized Trial of Dose-Dense versus Conventionally Scheduled and Sequential versus Concurrent Combination Chemotherapy as Postoperative Adjuvant Treatment of Node-Positive Primary Breast Cancer: First Report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 2003, 21, 1431–1439. [Google Scholar] [CrossRef]
- Del Mastro, L.; Poggio, F.; Blondeaux, E.; De Placido, S.; Giuliano, M.; Forestieri, V.; De Laurentiis, M.; Gravina, A.; Bisagni, G.; Rimanti, A.; et al. Fluorouracil and Dose-Dense Adjuvant Chemotherapy in Patients with Early-Stage Breast Cancer (GIM2): End-of-Study Results from a Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 1571–1582. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the Dose Intensity of Chemotherapy by More Frequent Administration or Sequential Scheduling: A Patient-Level Meta-Analysis of 37,298 Women with Early Breast Cancer in 26 Randomised Trials. Lancet 2019, 393, 1440–1452. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP Inhibitor Veliparib plus Carboplatin or Carboplatin Alone to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (BrighTNess): A Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-Term Efficacy and Safety of Addition of Carboplatin with or without Veliparib to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: 4-Year Follow-up Data from BrighTNess, a Randomized Phase III Trial. Ann. Oncol. 2022, 33, 384–394. [Google Scholar] [CrossRef]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-Based Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Tagliamento, M.; Ceppi, M.; Bruzzone, M.; Conte, B.; Fregatti, P.; Punie, K.; de Azambuja, E.; Del Mastro, L.; Lambertini, M. Adding a Platinum Agent to Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer: The End of the Debate. Ann. Oncol. 2022, 33, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Bailey, A.; McArthur, H.; El-Abed, S.; de Azambuja, E.; Metzger, O.; Chui, S.Y.; Dieterich, M.; Perretti, T.; Shearer-Kang, E.; et al. Adjuvant Atezolizumab for Early Triple-Negative Breast Cancer: The ALEXANDRA/IMpassion030 Randomized Clinical Trial. JAMA 2025, 333, 1150–1160. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kümmel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Abstract LB1-07: Exploratory Biomarker Analysis of the Phase 3 KEYNOTE-522 Study of Neoadjuvant Pembrolizumab or Placebo Plus Chemotherapy Followed by Adjuvant Pembrolizumab or Placebo for Early-Stage TNBC. Clin. Cancer Res. 2025, 31, LB1-07. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant Atezolizumab in Combination with Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy versus Placebo and Chemotherapy in Patients with Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.-U.; Furlanetto, J.; Zahm, D.-M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant Durvalumab Improves Survival in Early Triple-Negative Breast Cancer Independent of Pathological Complete Response. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic Complete Response (pCR) to Neoadjuvant Treatment with or without Atezolizumab in Triple-Negative, Early High-Risk and Locally Advanced Breast Cancer: NeoTRIP Michelangelo Randomized Study. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Jonas, S.F.; Salgado, R.; Loi, S.; de Jong, V.; Carter, J.M.; Nielsen, T.O.; Leung, S.; Riaz, N.; Chia, S.; et al. Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. JAMA 2024, 331, 1135–1144. [Google Scholar] [CrossRef]
- Geurts, V.C.M.; Balduzzi, S.; Steenbruggen, T.G.; Linn, S.C.; Siesling, S.; Badve, S.S.; DeMichele, A.; Ignatiadis, M.; Leon-Ferre, R.A.; Goetz, M.P.; et al. Tumor-Infiltrating Lymphocytes in Patients With Stage I Triple-Negative Breast Cancer Untreated With Chemotherapy. JAMA Oncol. 2024, 10, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Salgado, R.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor Infiltrating Lymphocyte Stratification of Prognostic Staging of Early-Stage Triple Negative Breast Cancer. NPJ Breast Cancer 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Assaf, Z.J.; Harbeck, N.; Zhang, H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; et al. Peri-Operative Atezolizumab in Early-Stage Triple-Negative Breast Cancer: Final Results and ctDNA Analyses from the Randomized Phase 3 IMpassion031 Trial. Nat. Med. 2025, 31, 2397–2404. [Google Scholar] [CrossRef]
- Geyer, C.; Tang, G.; Nekljudova, V.; Rastogi, P.; Reinisch, M.; Acosta, J.; Schneeweiss, A.; Hilton, C.; Seiler, S.; Denkert, C.; et al. Abstract GS3-05: NSABP B-59/GBG-96-GeparDouze: A Randomized Double-Blind Phase III Clinical Trial of Neoadjuvant Chemotherapy with Atezolizumab or Placebo Followed by Adjuvant Atezolizumab or Placebo in Patients with Stage II and III Triple-Negative Breast Cancer. Clin. Cancer Res. 2025, 31, GS3-05. [Google Scholar] [CrossRef]
- Samiei, S.; Simons, J.M.; Engelen, S.M.E.; Beets-Tan, R.G.H.; Classe, J.-M.; Smidt, M.L.; EUBREAST Group. Axillary Pathologic Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-Analysis. JAMA Surg. 2021, 156, e210891. [Google Scholar] [CrossRef]
- Boughey, J.C.; Ballman, K.V.; Hunt, K.K.; McCall, L.M.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Le-Petross, H.T. Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J. Clin. Oncol. 2015, 33, 3386–3393. [Google Scholar] [CrossRef]
- Kahler-Ribeiro-Fontana, S.; Pagan, E.; Magnoni, F.; Vicini, E.; Morigi, C.; Corso, G.; Intra, M.; Canegallo, F.; Ratini, S.; Leonardi, M.C.; et al. Long-Term Standard Sentinel Node Biopsy after Neoadjuvant Treatment in Breast Cancer: A Single Institution Ten-Year Follow-Up. Eur. J. Surg. Oncol. 2021, 47, 804–812. [Google Scholar] [CrossRef]
- Montagna, G.; Mrdutt, M.M.; Sun, S.X.; Hlavin, C.; Diego, E.J.; Wong, S.M.; Barrio, A.V.; van den Bruele, A.B.; Cabioglu, N.; Sevilimedu, V.; et al. Omission of Axillary Dissection Following Nodal Downstaging With Neoadjuvant Chemotherapy. JAMA Oncol. 2024, 10, 793–798. [Google Scholar] [CrossRef]
- Muslumanoglu, M.; Cabioglu, N.; Igci, A.; Karanlık, H.; Kocer, H.B.; Senol, K.; Mantoglu, B.; Tukenmez, M.; Çakmak, G.K.; Ozkurt, E.; et al. Combined Analysis of the MF18-02/MF18-03 NEOSENTITURK Studies: ypN-Positive Disease Does Not Necessitate Axillary Lymph Node Dissection in Patients with Breast Cancer with a Good Response to Neoadjuvant Chemotherapy as Long as Radiotherapy Is Provided. Cancer 2025, 131, e35610. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, H.; Kim, J.; Chung, I.Y.; Kim, H.J.; Ko, B.S.; Lee, J.W.; Ahn, S.H.; Son, B.H. Prognosis According to Clinical and Pathologic Lymph Node Status in Breast Cancer Patients Who Underwent Sentinel Lymph Node Biopsy Alone after Neoadjuvant Therapy. PLoS ONE 2021, 16, e0251597. [Google Scholar] [CrossRef]
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody, H.S.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal Recurrence in Patients With Node-Positive Breast Cancer Treated With Sentinel Node Biopsy Alone After Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol. 2021, 7, 1851–1855. [Google Scholar] [CrossRef]
- Piltin, M.A.; Hoskin, T.L.; Day, C.N.; Davis, J.; Boughey, J.C. Oncologic Outcomes of Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy for Node-Positive Breast Cancer. Ann. Surg. Oncol. 2020, 27, 4795–4801. [Google Scholar] [CrossRef]
- Kuemmel, S.; Heil, J.; Bruzas, S.; Breit, E.; Schindowski, D.; Harrach, H.; Chiari, O.; Hellerhoff, K.; Bensmann, E.; Hanf, V.; et al. Safety of Targeted Axillary Dissection After Neoadjuvant Therapy in Patients With Node-Positive Breast Cancer. JAMA Surg. 2023, 158, 807–815. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.; Kim, S.-Y.; Lee, E.S.; Kang, H.-S.; Lee, S.; Jung, S.-Y.; Lee, E. Sentinel Lymph Node Biopsy in Breast Cancer Patients With Pathological Complete Response in the Axillary Lymph Node After Neoadjuvant Chemotherapy. J. Breast Cancer 2021, 24, 531–541. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, I.; Alsharif, E.; Park, S.; Kim, J.-M.; Ryu, J.M.; Nam, S.J.; Kim, S.W.; Yu, J.; Lee, S.K.; et al. Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis. J. Breast Cancer 2018, 21, 433–441. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Li, J.-W.; Wang, Y.-J.; Jin, K.-R.; Yang, B.-L.; Li, J.-J.; Yu, X.-L.; Mo, M.; Hu, N.; Shao, Z.-M.; et al. Clinical Feasibility and Oncological Safety of Non-Radioactive Targeted Axillary Dissection after Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: A Prospective Diagnostic and Prognostic Study. Int. J. Surg. 2023, 109, 1863–1870. [Google Scholar] [CrossRef]
- Martelli, G.; Barretta, F.; Miceli, R.; Folli, S.; Maugeri, I.; Listorti, C.; Scaperrotta, G.; Baili, P.; Pruneri, G.; Capri, G.; et al. Sentinel Node Biopsy Alone or With Axillary Dissection in Breast Cancer Patients After Primary Chemotherapy: Long-Term Results of a Prospective Interventional Study. Ann. Surg. 2022, 276, e544–e552. [Google Scholar] [CrossRef] [PubMed]
- Damin, A.P.; Zancan, M.; Melo, M.P.; Biazus, J.V. Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: Guiding a More Selective Axillary Approach. Breast Cancer Res. Treat. 2021, 186, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Basik, M.; Florianova, L.; Margolese, R.; Dumitra, S.; Muanza, T.; Carbonneau, A.; Ferrario, C.; Boileau, J.F. Oncologic Safety of Sentinel Lymph Node Biopsy Alone After Neoadjuvant Chemotherapy for Breast Cancer. Ann. Surg. Oncol. 2021, 28, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.K.; Amersi, F.; Chung, A.; Tseng, J.; Giuliano, A.E. Comparison of Targeted Axillary Dissection with Sentinel Node Biopsy Alone on Nodal Recurrence for Patients Who Have Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2025, 32, 4847–4854. [Google Scholar] [CrossRef]
- Lim, S.Z.; Yoo, T.-K.; Lee, S.B.; Kim, J.; Chung, I.Y.; Ko, B.S.; Lee, J.W.; Son, B.H.; Ahn, S.-H.; Kim, S.; et al. Long-Term Outcome in Patients with Nodal-Positive Breast Cancer Treated with Sentinel Lymph Node Biopsy Alone after Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2024, 203, 95–102. [Google Scholar] [CrossRef]
- Ring, A.; Webb, A.; Ashley, S.; Allum, W.H.; Ebbs, S.; Gui, G.; Sacks, N.P.; Walsh, G.; Smith, I.E. Is Surgery Necessary after Complete Clinical Remission Following Neoadjuvant Chemotherapy for Early Breast Cancer? J. Clin. Oncol. 2003, 21, 4540–4545. [Google Scholar] [CrossRef]
- Daveau, C.; Savignoni, A.; Abrous-Anane, S.; Pierga, J.-Y.; Reyal, F.; Gautier, C.; Kirova, Y.M.; Dendale, R.; Campana, F.; Fourquet, A.; et al. Is Radiotherapy an Option for Early Breast Cancers with Complete Clinical Response after Neoadjuvant Chemotherapy? Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1452–1459. [Google Scholar] [CrossRef]
- Clouth, B.; Chandrasekharan, S.; Inwang, R.; Smith, S.; Davidson, N.; Sauven, P. The Surgical Management of Patients Who Achieve a Complete Pathological Response after Primary Chemotherapy for Locally Advanced Breast Cancer. Eur. J. Surg. Oncol. 2007, 33, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Kuerer, H.M.; Valero, V.; Smith, B.D.; Krishnamurthy, S.; Diego, E.J.; Johnson, H.M.; Lin, H.; Shen, Y.; Lucci, A.; Shaitelman, S.F.; et al. Selective Elimination of Breast Surgery for Invasive Breast Cancer: A Nonrandomized Clinical Trial. JAMA Oncol. 2025, 11, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.; Espié, M.; Petit, T. Neoadjuvant Therapy: Current Landscape and Future Horizons for ER-Positive/HER2-Negative and Triple-Negative Early Breast Cancer. Curr. Treat. Options Oncol. 2024, 25, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis after Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.-S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual Cancer Burden after Neoadjuvant Chemotherapy and Long-Term Survival Outcomes in Breast Cancer: A Multicentre Pooled Analysis of 5161 Patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef]
- Winchester, D.J.; Singh, L.; Edge, S.B.; Allison, K.H.; Barlow, W.E.; Bossuyt, V.; Chavez-MacGregor, M.; Conant, E.F.; Connolly, J.L.; De Los Santos, J.F.; et al. Novel Postneoadjuvant Prognostic Breast Cancer Staging System. J. Clin. Oncol. 2025, 43, 1948–1960. [Google Scholar] [CrossRef]
- Rocque, G.B.; Williams, C.P.; Andrews, C.; Childers, T.C.; Wiseman, K.D.; Gallagher, K.; Tung, N.; Balch, A.; Lawhon, V.M.; Ingram, S.A.; et al. Patient Perspectives on Chemotherapy De-Escalation in Breast Cancer. Cancer Med. 2021, 10, 3288–3298. [Google Scholar] [CrossRef]
- Trapani, D.; Franzoi, M.A.; Burstein, H.J.; Carey, L.A.; Delaloge, S.; Harbeck, N.; Hayes, D.F.; Kalinsky, K.; Pusztai, L.; Regan, M.M.; et al. Risk-Adapted Modulation through de-Intensification of Cancer Treatments: An ESMO Classification. Ann. Oncol. 2022, 33, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.J.; Hilbers, F.S.; Bliss, J.M.; Caballero, C.; Frank, E.S.; Renault, P.; Naït Kaoudjt, R.; Schumacher, E.; Spears, P.A.; Regan, M.M.; et al. Road Map to Safe and Well-Designed De-Escalation Trials of Systemic Adjuvant Therapy for Solid Tumors. J. Clin. Oncol. 2020, 38, 4120–4129. [Google Scholar] [CrossRef]
- Hofherr, M.; Clifton, K.; January, S.; Owen, E.; Raheem, F.; Mina, L.A.; Karikalan, S.A.; Lyons, L.; Rose, M.W.; Madden, K.; et al. Abstract PS14-06: Real-World Analysis of Adverse Events in Patients with Triple Negative Breast Cancer Receiving Therapy per KEYNOTE-522. Cancer Res. 2024, 84, PS14-06. [Google Scholar] [CrossRef]
- Andrade, M.d.O.; Gutierres, I.G.; Tavares, M.C.; de Sousa, I.M.; Balint, F.C.; Marin Comini, A.C.; Gouveia, M.C.; Bines, J.; Madasi, F.; Ferreira, R.D.P.; et al. Immune-Related Adverse Events among Patients with Early-Stage Triple-Negative Breast Cancer Treated with Pembrolizumab plus Chemotherapy: Real-World Data from the Neo-Real/GBECAM 0123 Study. Breast 2025, 83, 104473. [Google Scholar] [CrossRef]
- Rached, L.; Peyre-Pradat, F.; Spotti, M.; Baldini, C.; Laparra, A.; Lambotte, O.; Sakkal, M.; Perret, A.; Viansone, A.; Michiels, S.; et al. Real-World Safety and Effectiveness of Neoadjuvant Chemotherapy Combination with Pembrolizumab in Triple-Negative Breast Cancer. ESMO Real World Data Digit. Oncol. 2024, 5, 100061. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Zaid, M.A.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bermas, B.; Braaten, T.; Budde, L.E.; et al. NCCN Guidelines® Insights: Management of Immunotherapy-Related Toxicities, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 582–592. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Sharma, P.; Stecklein, S.R.; Yoder, R.; Staley, J.M.; Schwensen, K.; O’Dea, A.; Nye, L.; Satelli, D.; Crane, G.; Madan, R.; et al. Clinical and Biomarker Findings of Neoadjuvant Pembrolizumab and Carboplatin Plus Docetaxel in Triple-Negative Breast Cancer: NeoPACT Phase 2 Clinical Trial. JAMA Oncol. 2024, 10, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.; Savard, J. Fear of Cancer Recurrence Inventory: Development and Initial Validation of a Multidimensional Measure of Fear of Cancer Recurrence. Support. Care Cancer 2009, 17, 241–251. [Google Scholar] [CrossRef]
- Valenza, C.; Trapani, D.; Loibl, S.; Chia, S.K.L.; Burstein, H.J.; Curigliano, G. Optimizing Postneoadjuvant Treatment in Patients With Early Breast Cancer Achieving Pathologic Complete Response. J. Clin. Oncol. 2024, 42, 2372–2376. [Google Scholar] [CrossRef]
- Elwyn, G.; Durand, M.A.; Song, J.; Aarts, J.; Barr, P.J.; Berger, Z.; Cochran, N.; Frosch, D.; Galasiński, D.; Gulbrandsen, P.; et al. A Three-Talk Model for Shared Decision Making: Multistage Consultation Process. BMJ 2017, 359, j4891. [Google Scholar] [CrossRef]
- Huober, J.; van Mackelenbergh, M.; Schneeweiss, A.; Seither, F.; Blohmer, J.-U.; Denkert, C.; Tesch, H.; Hanusch, C.; Salat, C.; Rhiem, K.; et al. Identifying Breast Cancer Patients at Risk of Relapse despite Pathological Complete Response after Neoadjuvant Therapy. NPJ Breast Cancer 2023, 9, 23. [Google Scholar] [CrossRef]
- Dawson, S.-J.; Tsui, D.W.Y.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.-F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Panet, F.; Papakonstantinou, A.; Borrell, M.; Vivancos, J.; Vivancos, A.; Oliveira, M. Use of ctDNA in Early Breast Cancer: Analytical Validity and Clinical Potential. NPJ Breast Cancer 2024, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Murillas, I.; Chopra, N.; Comino-Méndez, I.; Beaney, M.; Tovey, H.; Cutts, R.J.; Swift, C.; Kriplani, D.; Afentakis, M.; Hrebien, S.; et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol. 2019, 5, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Magbanua, M.J.M.; Brown Swigart, L.; Ahmed, Z.; Sayaman, R.W.; Renner, D.; Kalashnikova, E.; Hirst, G.L.; Yau, C.; Wolf, D.M.; Li, W.; et al. Clinical Significance and Biology of Circulating Tumor DNA in High-Risk Early-Stage HER2-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy. Cancer Cell 2023, 41, 1091–1102.e4. [Google Scholar] [CrossRef]
- Hunter, N.; Parsons, H.A.; Cope, L.; Canzoniero, J.V.; Denbow, R.; Navarro, F.; El-Refai, S.M.; Boyle, S.M.; Anampa, J.D.S.; Rimawi, M.; et al. Circulating Tumor DNA, Pathologic Response after Neoadjuvant Therapy, and Survival: First Results from TBCRC 040 (the PREDICT-DNA Trial). J. Clin. Oncol. 2025, 43, 1009. [Google Scholar] [CrossRef]
- Loi, S.; Niman, S.; Zdenkowski, N.; Francis, P.; Hay, S.B.; Fox, W.; Punie, K.; Menzies, A.M.; Angus, R.; Mavin, C.; et al. Abstract LBO1-03: Randomized Phase II Study of Neoadjuvant Nivolumab (N) 2 Week Lead-in Followed by 12 Weeks of Concurrent N+carboplatin plus Paclitaxel (CbP) vs Concurrent N+CbP in Triple Negative Breast Cancer (TNBC): (BCT1902/IBCSG 61-20 Neo-N). Cancer Res. 2024, 84, LBO1-03. [Google Scholar] [CrossRef]
- Nederlof, I.; Isaeva, O.I.; De Graaf, M.; Gielen, R.C.A.M.; Bakker, N.A.M.; Rolfes, A.L.; Garner, H.; Boeckx, B.; Traets, J.J.H.; Mandjes, I.A.M.; et al. Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Early-Stage Triple-Negative Breast Cancer: A Phase 2 Adaptive Trial. Nat. Med. 2024, 30, 3223–3235. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kümmel, S.; Bergh, J.; Park, Y.H.; Hui, R.; et al. Event-Free Survival by Residual Cancer Burden with Pembrolizumab in Early-Stage TNBC: Exploratory Analysis from KEYNOTE-522. Ann. Oncol. 2024, 35, 429–436. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Awada, G.; Cascone, T.; van der Heijden, M.S.; Blank, C.U.; Kok, M.; Chalabi, M. The Rapidly Evolving Paradigm of Neoadjuvant Immunotherapy across Cancer Types. Nat. Cancer 2025, 6, 967–987. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Mayer, I.A.; Zhao, F.; Arteaga, C.L.; Symmans, W.F.; Park, B.H.; Burnette, B.L.; Tevaarwerk, A.J.; Garcia, S.F.; Smith, K.L.; Makower, D.F.; et al. Randomized Phase III Postoperative Trial of Platinum-Based Chemotherapy Versus Capecitabine in Patients With Residual Triple-Negative Breast Cancer Following Neoadjuvant Chemotherapy: ECOG-ACRIN EA1131. J. Clin. Oncol. 2021, 39, 2539–2551. [Google Scholar] [CrossRef]
- Schneider, B.P.; Jiang, G.; Ballinger, T.J.; Shen, F.; Chitambar, C.; Nanda, R.; Falkson, C.; Lynce, F.C.; Gallagher, C.; Isaacs, C.; et al. BRE12-158: A Postneoadjuvant, Randomized Phase II Trial of Personalized Therapy Versus Treatment of Physician’s Choice for Patients With Residual Triple-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, 345–355. [Google Scholar] [CrossRef]
- Page, D.; Pucilowska, J.; Bennetts, L.; Kim, I.; Sanchez, K.; Martel, M.; Conlin, A.; Moxon, N.; Mellinger, S.; Acheson, A.; et al. Abstract P2-09-03: Updated Efficacy of First or Second-Line Pembrolizumab (Pembro) plus Capecitabine (Cape) in Metastatic Triple Negative Breast Cancer (mTNBC) and Correlations with Baseline Lymphocyte and Naïve CD4+ T-Cell Count. Cancer Res. 2019, 79, P2-09-03. [Google Scholar] [CrossRef]
- Shah, A.N.; Flaum, L.; Helenowski, I.; Santa-Maria, C.A.; Jain, S.; Rademaker, A.; Nelson, V.; Tsarwhas, D.; Cristofanilli, M.; Gradishar, W. Phase II Study of Pembrolizumab and Capecitabine for Triple Negative and Hormone Receptor-Positive, HER2−negative Endocrine-Refractory Metastatic Breast Cancer. J. Immunother. Cancer 2020, 8, e000173. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall Survival in the OlympiA Phase III Trial of Adjuvant Olaparib in Patients with Germline Pathogenic Variants in BRCA1/2 and High-Risk, Early Breast Cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.-A.; Park, Y.H.; Delord, J.-P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and Durvalumab in Patients with Germline BRCA-Mutated Metastatic Breast Cancer (MEDIOLA): An Open-Label, Multicentre, Phase 1/2, Basket Study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-Label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Carmagnani Pestana, R.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.; Curigliano, G. Antibody–Drug Conjugates: Smart Chemotherapy Delivery across Tumor Histologies. CA Cancer J. Clin. 2022, 72, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Giordano, A.; Giordano, S.; Schlam, I.; Tolaney, S.M.; Tarantino, P. Antibody-Drug Conjugates for Treating Early-Stage Breast Cancer: Current Use, Anticipated Evolutions. NPJ Breast Cancer 2025, 11, 81. [Google Scholar] [CrossRef]
| Trial Name (NCT) | GeparNuevo (NCT02685059) [27,32] | IMpassion031 (NCT03197935) [26,33] | NeoTRIP (NCT02620280) [28] | GeparDouze (NCT03281954) [34] | Keynote 522 (NCT03036488) [22,23] |
|---|---|---|---|---|---|
| ICI | Durvalumab | Atezolizumab | Atezolizumab | Atezolizumab | Pembrolizumab |
| Primary endpoint | pCR | pCR | EFS | EFS | pCR/EFS |
| Adjuvant ICI | No | Yes | No | Yes | Yes |
| Optional adjuvant therapy | TPC | TPC | Not allowed | TPC | Not allowed |
| n | 174 | 333 | 280 | 1550 | 1174 |
| pCR in ICI arm (improvement in pCR) | 53.4% (+9.2%) | 57.6% (+16.5%) | 48.6% (+4.6%) | 63.3% (+6.3%) | 63.1% (+13.6%) |
| 4-year EFS in ICI arm | If pCR: 95.5% If RD: 76.3% | If pCR: 94% If RD: 61.1% | ITT: 70.8% | If pCR: 93.2% If RD: 70.6% | If pCR: 93.4% If RD: 63.7% |
| Study | n | Population | Procedure | Follow-Up | % TNBC | % Nodal Recurrence |
|---|---|---|---|---|---|---|
| Montagna et al. [38] | 1144 | cN1/N2/N3 | SLNB/TAD | 42 months | 23% | 1% |
| Muslumanoglu et al. [39] | 501 | cN1/N2/N3 | SLNB/TAD | 40 months | 8.8% | 0.4% |
| Lee et al. [40] | 242 | cN1/N2/N3 | SLNB | 60 months | NA | 2.9% |
| Barrio et al. [41] | 234 | cN1 | SLNB | 40 months | 18% | 0.4% |
| Piltin et al. [42] | 159 | cN1/N2/N3 | SLNB | 34 months | 20.8% | 0.9% |
| Kahler–Ribeiro–Fontana et al. [37] | 123 | cN1/N2 | SLNB | 110 months | 18.5% | 1.6% |
| Kuemmel et al. [43] | 119 | cN1 | TAD | 43 months | 24.4% | 1.8% |
| Kim et al. [44] | 94 | cN1/N2/N3 | SLNB | 57 months | 41.5% | 4.3% |
| Choi et al. [45] | 85 | cN1/N2/N3 | SLNB | 51 months | 29.2% | 2.4% |
| Wu et al. [46] | 85 | cN1/N2/N3 | TAD | 36.6 months | 23.5% | 0% |
| Martelli et al. [47] | 81 | cN1 | SLNB | 87 months | 9.4% | 0% |
| Damin et al. [48] | 59 | cN1/N2 | SLNB | 55.8 months | NA | 2.6% |
| Wong et al. [49] | 58 | cN1/N2 | SLNB | 36 months | 23.5% | 0% |
| Boyle et al. [50] | 44 | cN1/N2/N3 | SLNB/TAD | 28 months | 25% | 0% |
| Lim et al. [51] | 477 | cN1 | SLNB | 65 months | 26.4% | 3.2% |
| Clinical Scenario | Prognosis | Current Standard of Care | Emerging Strategies/Biomarkers |
|---|---|---|---|
| pCR (RCB 0) | Excellent—5-year EFS ≈ 90–95% | Adjuvant pembrolizumab (per Keynote-522) | De-escalation trials (Optimice-pCR, OPT-PEMBRO); role of TILs and ctDNA clearance |
| Minimal RD (RCB I) | Good—5-year EFS ≈ 80% | Continue pembrolizumab; consider adding capecitabine if BRCA wild-type; add olaparib if germline BRCA-mutated | Biomarker-driven tailoring (ctDNA, TILs) |
| Moderate RD (RCB II) | Intermediate—5-year EFS ≈ 66% | Same as above | ADCs (sacituzumab govitecan, datopotamab deruxtecan); ctDNA-guided intensification |
| Extensive RD (RCB III) | Poor—5-year EFS < 30% | Same as above | High-priority population for ADC trials and novel escalation strategies |
| Trial | NCT | Population | Estimated n | Study Drugs | Primary Endpoint |
|---|---|---|---|---|---|
| SASCIA | NCT04595565 | RD after ≥16 week of neoadjuvant taxane-based therapy with or without anthracyclines * | 1332 | Sacituzumab govitecan (vs. capecitabine) | iDFS |
| ASCENT05/OptimICE-RD | NCT05633654 | RD after ≥6 cycles of neoadjuvant anthracycline and/or taxane-based chemotherapy with or without an aPD-(L)1 agent or platinum agent | 1514 | Sacituzumab govitecan + pembrolizumab for 8 cycles (vs. pembrolizumab or pembrolizumab + capecitabine) | iDFS |
| TROPION-Breast03 | NCT05629585 | RD after ≥6 cycles of neoadjuvant anthracycline and/or a taxane with or without platinum chemotherapy, with or without pembrolizumab | 1174 | Datopotamab deruxtecan with or without durvalumab for 8 cycles (vs. capecitabine or pembrolizumab + capecitabine) | iDFS for Dato-Dxd vs. TPC |
| SWOG1418/BR006 | NCT02954874 | RD after neoadjuvant chemotherapy of TPC, without aPD-(L)1 agent; 6 cycles of adjuvant capecitabine allowed | 1155 | Pembrolizumab for 1 year (vs. observation) | iDFS |
| MK-2870-012 | NCT06393374 | RD after neoadjuvant KEYNOTE-522 regimen (pembrolizumab with carboplatin/taxanes and with anthracycline-based chemotherapy) | 1530 | Sacituzumab tirumotecan + pembrolizumab for 24 weeks (vs. pembrolizumab or pembrolizumab + capecitabine) | iDFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischoff, H.; Somme, L.; Petit, T. Optimizing Post-Neoadjuvant Treatment in Early Triple-Negative Breast Cancer. Cancers 2025, 17, 3288. https://doi.org/10.3390/cancers17203288
Bischoff H, Somme L, Petit T. Optimizing Post-Neoadjuvant Treatment in Early Triple-Negative Breast Cancer. Cancers. 2025; 17(20):3288. https://doi.org/10.3390/cancers17203288
Chicago/Turabian StyleBischoff, Hervé, Laura Somme, and Thierry Petit. 2025. "Optimizing Post-Neoadjuvant Treatment in Early Triple-Negative Breast Cancer" Cancers 17, no. 20: 3288. https://doi.org/10.3390/cancers17203288
APA StyleBischoff, H., Somme, L., & Petit, T. (2025). Optimizing Post-Neoadjuvant Treatment in Early Triple-Negative Breast Cancer. Cancers, 17(20), 3288. https://doi.org/10.3390/cancers17203288






