Simple Summary
Infections are common after definitive treatment in patients with non-small cell lung cancer (NSCLC), but their prognostic significance has not been clearly established. In this retrospective study, 214 patients with NSCLC who underwent definitive treatment were evaluated. Infections were found to be frequent within the first year after treatment, mainly caused by Gram-negative bacteria such as Pseudomonas aeruginosa and Acinetobacter baumannii. A substantial proportion of the isolated pathogens were healthcare-associated. Patients with positive cultures had significantly shorter overall survival. These findings demonstrate that infections are a strong prognostic factor in NSCLC after definitive treatment. Early microbiological evaluation, appropriate therapy, and strict infection prevention strategies are critical to improving overall survival in this vulnerable group.
Abstract
Background: Infections are common complications in patients with non-small cell lung cancer (NSCLC) and may adversely influence clinical outcomes. Their prognostic impact after definitive treatment is not well established. This study aimed to investigate the incidence, microbiological profile, and prognostic significance of infections occurring within one year after definitive treatment in patients with NSCLC. Methods: We retrospectively analyzed patients with NSCLC who completed definitive treatment between 1 January 2016, and 31 December 2023. Microbiological culture results obtained within one-year post-treatment and inflammatory markers measured one month after treatment were evaluated. Pathogens were classified as healthcare-associated infection (HAI) or non-HAI agents. Overall survival (OS) was estimated using the Kaplan–Meier method, and prognostic factors were assessed using Cox regression analysis. Results: Among 214 eligible patients, 45 had positive microbiological cultures. Gram-negative bacteria predominated (n = 24), with Pseudomonas aeruginosa (n = 8) and Acinetobacter baumannii (n = 6) being the most frequently isolated species. Among all isolates, 20 Gram-negative and 6 Gram-positive microorganisms were identified as HAI pathogens. In multivariate analysis, culture positivity (HR: 2.75, p < 0.001) remained an independent prognostic factor for worse OS. Conclusion: Infections within the first year after definitive treatment, particularly those caused by HAI-related Gram-negative pathogens, are associated with reduced OS in NSCLC. Early microbiological diagnosis, targeted antimicrobial therapy, and strict infection prevention strategies may help improve outcomes in this high-risk population.
1. Introduction
Lung cancer remains the leading cause of cancer-related death in both men and women worldwide. Survival has improved in recent years due to advances in staging, earlier detection, and more effective treatments including radiotherapy, chemotherapy, immunotherapy and targeted therapies [1,2,3]. Patients with non-small cell lung cancer (NSCLC) are highly susceptible to infections. This is related to tumor-induced structural lung damage, treatment-related immunosuppression, and frequent invasive procedures [4,5]. Such infections complicate cancer therapy and increase both morbidity and mortality [6,7]. Infection-related deaths are a significant non-cancer cause of mortality, particularly among younger patients and those with advanced disease [8,9]. Despite these risks, there is limited evidence on the spectrum of infectious pathogens in NSCLC patients undergoing definitive treatment and on the prognostic impact of these infections. Slow and insensitive microbiological methods may delay effective treatment. In the absence of clear guidelines, empirical antibiotic use is common, which may promote antimicrobial resistance and worsen outcomes [10].
Advances in radiotherapy, such as intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT), have reduced toxicities like pneumonitis and esophagitis. However, treatment-related infections remain a persistent problem [11,12]. Chemotherapy-induced neutropenia and radiotherapy-induced lymphopenia further increase vulnerability to severe infections. Furthermore, targeted therapies such as new-generation EGFR tyrosine kinase inhibitors have also been reported to influence host immunity and contribute to infection risk [13].
Given the lack of definitive evidence, further research is needed to clarify the microbiological profile of post-treatment infections and their prognostic relevance in unresectable NSCLC. The present study aimed to identify infectious agents occurring within one year after definitive treatment and to evaluate their association with overall survival (OS) in patients with NSCLC.
2. Materials and Methods
2.1. Patient Selection
Between 1 January 2016, and 31 December 2023, 597 patients with NSCLC who received anti-cancer treatment were retrospectively evaluated. 383 of them were excluded from the study because they did not meet the inclusion criteria. A total of 214 patients who underwent definitive treatment were included in the final cohort. The study design flowchart is shown in Figure 1.
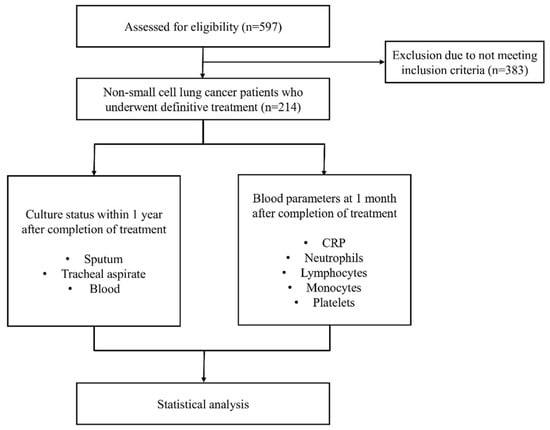
Figure 1.
Study design flowchart.
The inclusion criteria for this study were as follows: (1) histopathologically confirmed NSCLC deemed inoperable before treatment, with staging verified by positron emission tomography and computed tomography; (2) completion of definitive treatment, including concurrent chemoradiotherapy, induction chemotherapy followed by concurrent chemoradiotherapy, or definitive radiotherapy alone; and (3) no evidence of active infection at the beginning of follow-up, based on laboratory findings and microbiological records. Patients were excluded if they had undergone surgical resection, received palliative radiotherapy, or had a known diagnosis of interstitial lung disease prior to cancer diagnosis. Patients who received immune checkpoint inhibitors, maintenance immunotherapy, or molecularly targeted therapies during the study period were also excluded.
2.2. Treatment and Follow-Up
A total of 151 patients received concurrent chemoradiotherapy, 59 patients were treated with induction chemotherapy followed by chemoradiotherapy, and 4 patients received radiotherapy alone. All patients underwent external beam radiotherapy delivered via three-dimensional conformal radiotherapy, IMRT, or helical IMRT techniques. Radiotherapy was administered with a daily fraction dose of 2 Gy to a total dose ranging from 60 to 66 Gy. Following treatment completion, patients were monitored through routine physical examinations and radiological imaging as part of standard follow-up protocols.
2.3. Data Collection
Clinical data were collected retrospectively from the institutional electronic medical records. Sociodemographic and physiological variables were also recorded, including education level, place of residence, income level, body mass index (BMI), and the presence of comorbid conditions. Comorbidity burden was assessed using the Charlson comorbidity index (CCI) [14]. Laboratory parameters, including C-reactive protein (CRP), platelet count, lymphocyte count, neutrophil count, and monocyte count, measured at the first month following completion of definitive treatment were recorded. In addition, microbiological culture results from sputum, tracheal aspirate, and blood samples obtained within one year after completion of therapy were reviewed. In patients with positive culture results, the isolated microorganisms were identified and classified according to whether they met the definition of healthcare-associated infection (HAI). HAI was defined according to the Centers for Disease Control and Prevention (CDC) / National Healthcare Safety Network (NHSN) surveillance criteria, which classify cases as infections that were not present or were incubating at the time of hospital admission [15]. Bacterial identification was performed using matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Bremen, Germany). Antibiotic susceptibility tests were performed using the fully automated BD Phoenix System (Becton Dickinson, Franklin Lakes, NJ, USA) and evaluated according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria. Antibiotics that were not included in the EUCAST-based institutional testing panel for the isolated infectious agents were marked as untested.
2.4. Statistical Analysis
All statistical analyses were performed using IBM SPSS, version 24.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant. Descriptive statistics were used to summarize patient and microbiological characteristics. OS was defined as the time from the completion of definitive treatment to either the date of death or the date of last follow-up. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive performance of variables for OS, with optimal cut-off values selected at the point where sensitivity and specificity were closest [16]. To identify potential prognostic factors for OS, univariate Cox regression analyses were performed. Variables that demonstrated statistical significance in univariate analysis were subsequently included in a multivariate Cox regression model to determine independent prognostic factors for OS. OS curves were estimated using the Kaplan–Meier method and differences between groups were compared using the log-rank test.
3. Results
Patient characteristics are summarized in Table 1. The median age was 65 years, and most patients were male (87.4%). The predominant histological subtype was squamous cell carcinoma (57.9%). Most patients had advanced-stage disease, with 85.6% presenting with stage III at diagnosis.

Table 1.
Patient characteristics (n = 214).
Among the study population, microbiological culture testing was performed in 111 patients (51.9%) during the one-year follow-up period following treatment. Of these, 45 patients had at least one positive culture result, while 66 patients had negative cultures. No culture samples were available for 103 patients. The most common sampling site was sputum (n = 29), followed by blood (n = 10) and tracheal aspirate (n = 6). Monomicrobial growth was observed in 38 cases, while 7 patients exhibited dual-organism growth. The distribution of sampling sites (sputum, tracheal aspirate, blood) and their HAI associations are shown in Table 2.

Table 2.
Culture characteristics.
A total of 52 microorganisms were isolated. The distribution of pathogens by culture site is shown in Figure 2. Gram-negative bacteria were the most frequently detected pathogens, with Pseudomonas aeruginosa (P. aeruginosa) (n = 8) and Acinetobacter baumannii (A. baumannii) (n = 6) being the most common. Other isolated Gram-negative species included Escherichia coli (E. coli) (n = 3), Stenotrophomonas maltophilia (n = 2), Klebsiella pneumoniae (K. pneumoniae) (n = 1), Acinetobacter pittii (n = 1), Haemophilus influenzae (n = 1), Moraxella catarrhalis (n = 1), and Salmonella spp. (n = 1). Of the Gram-negative isolates (n = 24), 20 were classified as HAI pathogens. Gram-positive bacteria (n = 15) included Staphylococcus aureus (S. aureus) (n = 5), Corynebacterium spp. (n = 4), Micrococcus luteus (n = 2), Enterococcus faecium (E. faecium) (n = 1), and Streptococcus spp. (n = 3), and 6 of these were identified as HAI agents. Fungal isolates consisted primarily of Candida albicans (n = 11), as well as Candida glabrata (n = 1) and Magnusiomyces capitatus (n = 1).
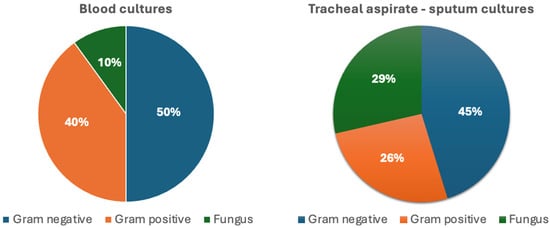
Figure 2.
Distribution of pathogen groups isolated from blood and tracheal aspirate–sputum cultures.
Antibiotic resistance rates are shown in Table 3 and Table 4. Antibiotics marked with an asterisk (*) in Table 3 were not tested, as they were not included in the EUCAST-based institutional testing panel for the isolated infectious agents. The antibiotic resistance status of agents not listed in the table was also evaluated. Two Stenotrophomonas maltophilia strains were found to be susceptible to levofloxacin and intermediate to imipenem. Salmonella spp. was resistant to ampicillin and ciprofloxacin but susceptible to SXT and ceftriaxone. Haemophilus influenzae was resistant to AMC and susceptible to other antibiotics, including meropenem, ampicillin, cefotaxime, SXT, and cefuroxime axetil. Moraxella catarrhalis was resistant to erythromycin but susceptible to other antibiotics, such as AMC, cefotaxime, and levofloxacin. Streptococcus pneumoniae strains were resistant to oxacillin, SXT, levofloxacin, cefotaxime, and penicillin G but susceptible to vancomycin, erythromycin, meropenem, and daptomycin. No resistance was detected in Streptococcus agalactiae and Streptococcus constellatus strains, which were susceptible to penicillin G and other antibiotics. Corynebacterium striatum and Corynebacterium amycolatum strains were resistant to gentamicin, ciprofloxacin, tetracycline, rifampin, and clindamycin but susceptible to vancomycin and linezolid.

Table 3.
Gram-negative bacteria antibiotic resistance rates, n (%).

Table 4.
S. aureus and E. faecium antibiotic resistance rates, n (%).
Among Gram-negative bacteria, the highest resistance was to ampicillin and AMC, while the most effective agents were imipenem, meropenem, and amikacin. Among Gram-positive bacteria, resistance was most common to ampicillin and penicillin G, while linezolid proved to be the most effective antibiotic. Candida glabrata strain was found to be susceptible to anidulafungin.
Optimal cut-off values were established through ROC curve analysis. The cut-off points for lymphocyte count, neutrophil count, and monocyte count were 1.015 × 103/μL (sensitivity: 58.6%, specificity: 57.1%), 3.935 × 103/μL (sensitivity: 44.8%, specificity: 44.9%), and 0.645 × 103/μL (sensitivity: 52.6%, specificity: 52.0%), respectively. The optimal cut-off values for platelet count and CRP were 239 × 103/μL (sensitivity: 39.7%, specificity: 39.8%) and 19.64 mg/L (sensitivity: 60.3%, specificity: 60.2%), respectively. Additionally, the optimal cut-off point for BMI was 24.75 kg/m2 (sensitivity: 49.1%, specificity: 49%). These values were subsequently used to categorize patients in survival analyses.
In univariate Cox regression analysis, diabetes mellitus (HR: 0.53, 95% CI: 0.34–0.82, p = 0.005), CCI (HR: 2.25, 95% CI: 1.50–3.36, p < 0.001), platelet count (HR: 1.59, 95% CI: 1.09–2.32, p = 0.01), lymphocyte count (HR: 0.63, 95% CI: 0.43–0.91, p = 0.01), CRP level (HR: 1.67, 95% CI: 1.15–2.42, p = 0.007), and culture positivity (HR: 3.29, 95% CI: 2.20–4.92, p < 0.001) were significantly associated with OS. In the multivariate Cox regression analysis, culture positivity (HR: 2.75, 95% CI: 1.78–4.27, p < 0.001) remained an independent prognostic factor for OS (Table 5).

Table 5.
Univariate and multivariate Cox regression analysis for the prediction of overall survival.
Kaplan–Meier survival analysis revealed significantly reduced OS in patients with positive culture results compared to those with negative or no cultures (p < 0.001) (Figure 3). Similarly, patients with HAI-related pathogens demonstrated a trend toward worse OS compared to those without, with the difference approaching statistical significance (p = 0.08) (Figure 4). When stratified by the most common pathogens, P. aeruginosa and A. baumannii were each associated with worse OS (Figure 5).
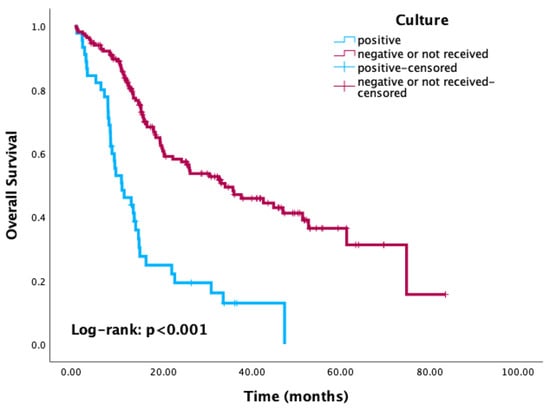
Figure 3.
Kaplan–Meier curves of overall survival according to culture status.
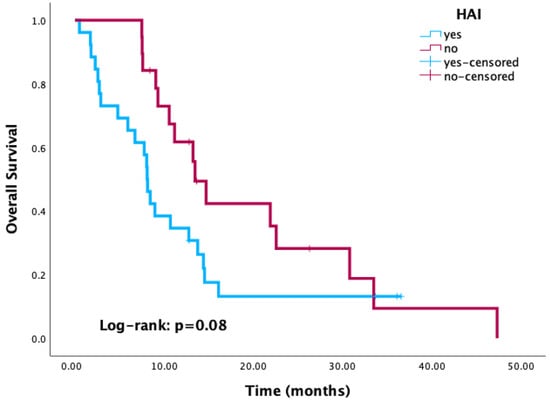
Figure 4.
Kaplan–Meier curves of overall survival according to healthcare-associated infection status.
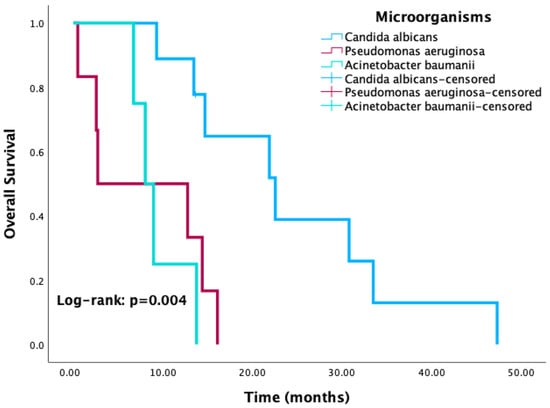
Figure 5.
Kaplan–Meier curves for overall survival according to the three most common isolated microorganisms.
4. Discussion
This study demonstrates that infections occurring within the first year after definitive treatment are associated with significantly reduced OS in patients with NSCLC. Culture positivity and the presence of HAI pathogens emerged as strong adverse prognostic indicators. These findings align with recent evidence underscoring that infections remain a major contributor to non-cancer mortality in NSCLC, even in the era of improved multimodal therapy [17,18].
The microbiological profile in this cohort was dominated by Gram-negative bacteria, most notably P. aeruginosa and A. baumannii, the majority of which were classified as HAI-related. Gram-positive isolates included methicillin-resistant S. aureus (MRSA), and fungal pathogens were predominantly Candida albicans with occasional Candida glabrata. This distribution is consistent with previous reports showing Gram-negative predominance in NSCLC patients treated with chemoradiotherapy or radiotherapy [19,20,21], as well as studies in elderly populations reporting frequent polymicrobial infections and opportunistic fungal involvement [22]. The predominance of HAI-related Gram-negative pathogens is particularly concerning, given their established association with prolonged hospitalizations, treatment delays, and worse oncologic outcomes.
Antimicrobial resistance patterns observed in this study have direct therapeutic implications. Gram-negative isolates exhibited high resistance to ampicillin and amoxicillin/clavulanic acid, while carbapenems and amikacin retained substantial efficacy. Among Gram-positive pathogens, resistance to penicillin and ampicillin was common, with linezolid demonstrating consistent activity. The isolation of multidrug-resistant A. baumannii and MRSA is in line with prior intensive care unit-based oncology series, which have emphasized the necessity of empiric regimens with broad coverage in high-risk patients [23]. The observed susceptibility of Stenotrophomonas maltophilia to levofloxacin, along with the preserved activity of glycopeptides and oxazolidinones against Corynebacterium species, highlights the importance of early microbiological identification to enable timely de-escalation of therapy, thereby minimizing toxicity and limiting further resistance development. Additionally, it should be noted that some antibiotics were not tested, as EUCAST-based institutional laboratory protocols recommend testing to agents considered clinically relevant for the isolated microorganisms.
Both treatment-related and disease-related mechanisms drive the adverse impact of infection on OS after definitive treatment in NSCLC. Treatment causes profound and sustained immunosuppression through neutropenia, mucositis, and impaired mucociliary clearance [5]. Radiotherapy has been shown to cause lymphopenia, which compromises the host’s immune defense [24]. Even at low doses, it can alter blood counts and other immune parameters, thereby increasing susceptibility to infections [25]. Qin et al. identified multiple chemotherapy cycles, radiotherapy, and elevated baseline neutrophil counts as independent predictors of pulmonary infection [19]. Tumor-related airway obstruction also predisposes patients to post-obstructive pneumonia, which is often difficult to eradicate and prone to recurrence [26]. Furthermore, tumor progression has been associated with reduced numbers and impaired function of natural killer (NK) cells, which weakens innate immune defense and may increase susceptibility to infections [27]. These risk factors were also present in our cohort, where HAIs and a predominance of Gram-negative pathogens were frequently observed.
Survival analyses in this study revealed a marked reduction in OS among patients with positive cultures, with a clear adverse trend among those infected with HAI-related pathogens. In the multivariate model, culture positivity remained an independent prognostic factor for worse OS. Kaplan–Meier survival curves and the clinical interpretation uniformly indicate that infection was detrimental to OS. These findings are consistent with the observations of Patel et al., who reported that pneumonia in NSCLC patients significantly increased both in-hospital mortality and overall comorbidity burden [18]. Similarly, Sarihan et al. found that nearly half of NSCLC patients developed infections during thoracic radiotherapy, most caused by Gram-negative bacteria, and that the presence of infection was associated with significantly shorter OS [4].
Although immune checkpoint inhibitor (ICI)-treated patients were excluded from this analysis, modern treatment paradigms frequently incorporate ICIs into first-line therapy. Evidence indicates that the combination of ICIs with chemotherapy increases infection risk through overlapping toxicities, such as ICI-related pneumonitis and chemotherapy-induced neutropenia [28,29,30]. By focusing exclusively on patients treated with radiotherapy and/or chemotherapy, our study provides a clearer assessment of the infection burden attributable to standard multimodality treatment, but it also suggests that infection rates could be even higher in current practice with ICI-based combinations.
The present findings underscore the need for vigilant post-treatment monitoring, rapid microbiological work-up, and early targeted antimicrobial therapy in patients with suspected infection. Strict adherence to infection prevention measures, including minimizing the use of invasive devices and reinforcing hospital infection control protocols, is crucial for reducing the incidence of HAI-related pathogens. Local antimicrobial resistance data should inform empiric treatment choices, with prompt de-escalation once susceptibility results are available. Attention should also be given to optimizing supportive care in patients with tumor-related airway obstruction, which may involve bronchial hygiene interventions or palliative procedures to restore airflow and reduce the risk of infection. Pneumococcal vaccination should be encouraged where appropriate, although routine antibiotic prophylaxis is not recommended due to the potential for resistance development and adverse effects [18,22].
This study has several limitations. Its retrospective design introduces the potential for selection bias, and the absence of culture results in nearly half of the cohort limits the ability to fully characterize the epidemiology of infection. Microbiological sampling was more likely to have been performed in clinically unstable patients, which may have inflated the association between culture positivity and mortality. Furthermore, treating infection as a fixed covariate in OS analysis does not account for its time-dependent nature and may underestimate its dynamic impact on survival. Future prospective, multicenter studies incorporating time-varying models and cause-specific mortality endpoints are warranted to validate these findings. The exclusion of ICI-treated patients may limit the applicability of this study to modern regimens; however, it also provides an opportunity to evaluate the independent effect of chemoradiotherapy-related infections on long-term outcomes.
5. Conclusions
Infections within the first year after definitive treatment, particularly those caused by HAI-related Gram-negative pathogens, are associated with a significant reduction in OS in NSCLC. Strengthening infection prevention strategies and implementing rapid, targeted antimicrobial treatment may mitigate this impact. These findings underscore the crucial importance of proactive infection surveillance and management as essential components of post-treatment care in this vulnerable population.
Author Contributions
Conceptualization, Ö.K.; methodology, Ö.K. and M.B.Ş.; software, U.K.K. and R.A.A.; validation, U.K.K. and A.F.K.; formal analysis, M.B.Ş. and R.A.A.; investigation, T.K., M.B.Ş., and R.A.A.; resources, T.K.; data curation, U.K.K. and M.B.Ş.; writing—original draft, Ö.K., U.K.K., and R.A.A.; writing—review and editing, Ö.K. and A.F.K.; supervision, T.K. and A.F.K.; project administration, Ö.K., T.K., and A.F.K.; funding acquisition, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Akdeniz University Medical Scientific Research Ethics Committee (Approval No: 630, Date: 31 July 2025).
Informed Consent Statement
Informed consent was waived due to the retrospective design of the study and the de-identification of patient data.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Obradović, J.; Niševic-Lazović, J.; Sekeruš, V.; Milašin, J.; Perin, B.; Jurisic, V. Investigating the Frequencies of EGFR Mutations and EGFR Single Nucleotide Polymorphisms Genotypes and Their Predictive Role in NSCLC Patients in Republic of Serbia. Mol. Biol. Rep. 2025, 52, 350. [Google Scholar] [CrossRef]
- Jurišić, V.; Obradovic, J.; Pavlović, S.; Djordjevic, N. Epidermal Growth Factor Receptor Gene in Non-Small-Cell Lung Cancer: The Importance of Promoter Polymorphism Investigation. Anal. Cell. Pathol. 2018, 2018, 6192187. [Google Scholar] [CrossRef]
- Sarihan, S.; Ercan, I.; Saran, A.; Çetintas, S.K.; Akalin, H.; Engin, K. Evaluation of Infections in Non-Small Cell Lung Cancer Patients Treated with Radiotherapy. Cancer Detect. Prev. 2005, 29, 181–188. [Google Scholar] [CrossRef]
- Vento, S.; Cainelli, F.; Temesgen, Z. Lung Infections after Cancer Chemotherapy. Lancet Oncol. 2008, 9, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Perlin, E.; Bang, K.M.; Shah, A.; Hursey, P.D.; Whittingham, W.L.; Hashmi, K.; Campbell, L.; Kassim, O.O. The Impact of Pulmonary Infections on the Survival of Lung Cancer Patients. Cancer 1990, 66, 593–596. [Google Scholar] [CrossRef]
- Remiszewski, P.; Słodkowska, J.; Wiatr, E.; Zych, J.; Radomski, P.; Rowińska-Zakrzewska, E. Fatal Infection in Patients Treated for Small Cell Lung Cancer in the Institute of Tuberculosis and Chest Diseases in the Years 1980–1994. Lung Cancer 2001, 31, 101–110. [Google Scholar] [CrossRef]
- Liu, C.; Cui, H.; Gu, D.; Zhang, M.; Fang, Y.; Chen, S.; Tang, M.; Zhang, B.; Chen, H. Genetic Polymorphisms and Lung Cancer Risk: Evidence from Meta-Analyses and Genome-Wide Association Studies. Lung Cancer 2017, 113, 18–29. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of Death among Cancer Patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Radouani, F.; El Yazouli, L.; Elyazghi, Z.; Hejaji, H.; Alami, A.A.; Elmdaghri, N. Chlamydia Pneumoniae Sero-Prevalence in Moroccan Patients with Cardiovascular Diseases. Infect. Dis. Health 2019, 24, 67–74. [Google Scholar] [CrossRef]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Dillman, R.O.; Herndon, J.; Seagren, S.L.; Eaton, W.L.; Green, M.R. Improved Survival in Stage III Non-Small-Cell Lung Cancer: Seven-Year Follow-up of Cancer and Leukemia Group B (CALGB) 8433 Trial. JNCI J. Natl. Cancer Inst. 1996, 88, 1210–1215. [Google Scholar] [CrossRef]
- Obradovic, J.; Todosijevic, J.; Jurisic, V. Side Effects of Tyrosine Kinase Inhibitors Therapy in Patients with Non-Small Cell Lung Cancer and Associations with EGFR Polymorphisms: A Systematic Review and Meta-Analysis. Oncol. Lett. 2023, 25, 62. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care—Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qin, X.; Lu, Y.; Yao, L.; Liu, A.; Yu, Q.; Jiang, W.; Liang, J.; Li, Y.; Zhou, S.; et al. Pathogen Spectrum and Clinical Characteristics of Lung Cancer Patients: A 10-year Retrospective Study. Int. J. Cancer 2025, 156, 1470–1479. [Google Scholar] [CrossRef]
- Patel, A.J.; Nightingale, P.; Naidu, B.; Drayson, M.T.; Middleton, G.W.; Richter, A. Characterising the Impact of Pneumonia on Outcome in Non-Small Cell Lung Cancer: Identifying Preventative Strategies. J. Thorac. Dis. 2020, 12, 2236–2246. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; You, T.; Guo, T.; Tian, R.; Cui, X.; Wang, P. The Inter-Relationships Among the Risk Factors for Pulmonary Infection and the Diagnostic Utility of Inflammatory Markers in Patients with Non-Small Cell Lung Cancer. Infect. Drug Resist. 2025, 18, 1111–1123. [Google Scholar] [CrossRef]
- Shan, H.; Wang, J.; Zhang, Q.; Ming, Z.; Zhang, Y.; He, P.; Fang, P.; Zhang, M.; Li, W.; Shi, H.; et al. Pathogen Surveillance and Risk Factors for Pulmonary Infection in Patients with Lung Cancer: A Retrospective Single-Center Study. Open Med. 2025, 20, 20251180. [Google Scholar] [CrossRef]
- Petrovic, S.; Beovic, B.; Tomic, V.; Bitenc, M.; Malovrh, M.M.; Dimitric, V.; Luznik, D.; Miklavcic, M.; Bozic, T.; Gabrovec, T.; et al. Bronchial Bacterial Colonization and the Susceptibility of Isolated Bacteria in Patients with Lung Malignancy. Radiol. Oncol. 2025, 59, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Wang, Z.; Lu, Y.; Wen, X.; Li, H.; Zhao, H. A Retrospective Study of Risk and Prognostic Factors in Relation to Lower Respiratory Tract Infection in Elderly Lung Cancer Patients. Am. J. Cancer Res. 2015, 5, 423–432. [Google Scholar]
- Abd-Elmonsef, M.M.E.; Elsharawy, D.; Abd-Elsalam, A.S. Mechanical Ventilator as a Major Cause of Infection and Drug Resistance in Intensive Care Unit. Environ. Sci. Pollut. Res. 2018, 25, 30787–30792. [Google Scholar] [CrossRef]
- de Kermenguy, F.; Morel, D.; El-Aichi, M.; Barbolosi, D.; Deutsch, E.; Robert, C. Radiation-Induced Lymphopenia: From Mathematical Modeling Towards Mechanistic Learning. Int. J. Radiat. Oncol. Biol. Phys. 2025; in press. [Google Scholar] [CrossRef]
- Pajic, J.; Milic, M.; Jurisic, V.; Vinnikov, V. Editorial: Research on Low Dose Ionizing Radiation Health Effects. Front. Public. Health 2025, 13, 1566179. [Google Scholar] [CrossRef] [PubMed]
- Valvani, A.; Martin, A.; Devarajan, A.; Chandy, D. Postobstructive Pneumonia in Lung Cancer. Ann. Transl. Med. 2019, 7, 357. [Google Scholar] [CrossRef]
- Jurisic, V.; Colovic, N.; Konjevic, G.; Minic, I.; Colovic, M. An Aggressive Extramedullary Cutaneous Plasmacytoma Associated with Extreme Alterations in the Innate Immune System. Onkologie 2010, 33, 113–115. [Google Scholar] [CrossRef]
- Bertaglia, V.; Morelli, A.M.; Solinas, C.; Aiello, M.M.; Manunta, S.; Denaro, N.; Tampellini, M.; Scartozzi, M.; Novello, S. Infections in Lung Cancer Patients Undergoing Immunotherapy and Targeted Therapy: An Overview on the Current Scenario. Crit. Rev. Oncol. Hematol. 2023, 184, 103954. [Google Scholar] [CrossRef]
- Belluomini, L.; Caldart, A.; Avancini, A.; Dodi, A.; Trestini, I.; Kadrija, D.; Sposito, M.; Tregnago, D.; Casali, M.; Riva, S.T.; et al. Infections and Immunotherapy in Lung Cancer: A Bad Relationship? Int. J. Mol. Sci. 2020, 22, 42. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Hu, W.; Zhang, J. Incidence and Risk Factors of Serious Infections Occurred in Patients with Lung Cancer Following Immune Checkpoint Blockade Therapy. BMC Cancer 2025, 25, 307. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).