Evaluating the Impact of Index Metastasis Resection in Patients with Multiple Brain Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Surgery
2.3. Tumor Volumetric Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Functional Outcome After Surgery
3.3. Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brastianos, P.K.; Curry, W.T.; Oh, K.S. Clinical discussion and review of the management of brain metastases. J. Natl. Compr. Cancer Netw. 2013, 11, 1153–1164. [Google Scholar] [CrossRef]
- Fox, B.D.; Cheung, V.J.; Patel, A.J.; Suki, D.; Rao, G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 2011, 22, 1–6. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Patel, K.R.; Burri, S.H.; Boselli, D.; Symanowski, J.T.; Asher, A.L.; Sumrall, A.; Fraser, R.W.; Press, R.H.; Zhong, J.; Cassidy, R.J.; et al. Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: A multi-institutional analysis. J. Neurooncol. 2017, 131, 611–618. [Google Scholar] [CrossRef]
- Weltman, E.; Salvajoli, J.V.; Brandt, R.A.; de Morais Hanriot, R.; Prisco, F.E.; Cruz, J.C.; de Oliveira Borges, S.R.; Wajsbrot, D.B. Radiosurgery for brain metastases: A score index for predicting prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1155–1161. [Google Scholar] [CrossRef]
- Kalkanis, S.N.; Kondziolka, D.; Gaspar, L.E.; Burri, S.H.; Asher, A.L.; Cobbs, C.S.; Ammirati, M.; Robinson, P.D.; Andrews, D.W.; Loeffler, J.S.; et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 33–43. [Google Scholar] [CrossRef]
- Al-Shamy, G.; Sawaya, R. Management of brain metastases: The indispensable role of surgery. J. Neurooncol. 2009, 92, 275–282. [Google Scholar] [CrossRef]
- Sivasanker, M.; Madhugiri, V.S.; Moiyadi, A.V.; Shetty, P.; Subi, T.S. Surgery for brain metastases: An analysis of outcomes and factors affecting survival. Clin. Neurol. Neurosurg. 2018, 168, 153–162. [Google Scholar] [CrossRef]
- Winther, R.R.; Hjermstad, M.J.; Skovlund, E.; Aass, N.; Helseth, E.; Kaasa, S.; Yri, O.E.; Vik-Mo, E.O. Surgery for brain metastases—Impact of the extent of resection. Acta Neurochir. 2022, 164, 2773–2780. [Google Scholar] [CrossRef]
- Wagner, A.; Ille, S.; Liesenhoff, C.; Aftahy, K.; Meyer, B.; Krieg, S.M. Improved potential quality of intraoperative transcranial motor-evoked potentials by navigated electrode placement compared to the conventional ten-twenty system. Neurosurg. Rev. 2022, 45, 585–593. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Wildrick, D.M.; Sawaya, R. The role of surgical resection in patients with brain metastases. Ecancermedicalscience 2013, 7, 308. [Google Scholar] [PubMed]
- Paek, S.H.; Audu, P.B.; Sperling, M.R.; Cho, J.; Andrews, D.W. Reevaluation of surgery for the treatment of brain metastases: Review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery 2005, 56, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Kim, D.G.; Kim, J.W.; Han, J.H.; Kim, Y.H.; Park, C.-K.; Kim, C.-Y.; Paek, S.H.; Jung, H.-W. The role of surgical resection in the management of brain metastasis: A 17-year longitudinal study. Acta Neurochir. 2013, 155, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Salvati, M.; Tropeano, M.P.; Maiola, V.; Lavalle, L.; Brogna, C.; Colonnese, C.; Frati, A.; D’eLia, A. Multiple brain metastases: A surgical series and neurosurgical perspective. Neurol. Sci. 2018, 39, 671–677. [Google Scholar] [CrossRef]
- Ersoy, T.F.; Brainman, D.; Coras, R.; Berger, B.; Weissinger, F.; Grote, A.; Simon, M. Defining the role of surgery for patients with multiple brain metastases. J. Neurooncol. 2024, 169, 317–328. [Google Scholar] [CrossRef]
- Mahajan, U.V.; Desai, A.; Shost, M.D.; Cai, Y.; Anthony, A.; Labak, C.M.; Herring, E.Z.; Wijesekera, O.; Mukherjee, D.; Sloan, A.E.; et al. Stereotactic radiosurgery and resection for treatment of multiple brain metastases: A systematic review and analysis. Neurosurg. Focus 2022, 53, E9. [Google Scholar] [CrossRef]
- Schackert, G.; Lindner, C.; Petschke, S.; Leimert, M.; Kirsch, M. Retrospective study of 127 surgically treated patients with multiple brain metastases: Indication, prognostic factors, and outcome. Acta Neurochir. 2013, 155, 379–387. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar]
- Diehl, C.D.; Pigorsch, S.U.; Gempt, J.; Krieg, S.M.; Reitz, S.; Waltenberger, M.; Barz, M.; Meyer, H.S.; Wagner, A.; Wilkens, J.; et al. Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience. Cancers 2022, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- O’sUllivan, C.C.; Davarpanah, N.N.; Abraham, J.; Bates, S.E. Current challenges in the management of breast cancer brain metastases. Semin. Oncol. 2017, 44, 85–100. [Google Scholar] [CrossRef]
- Ene, C.I.; Ferguson, S.D. Surgical Management of Brain Metastasis: Challenges and Nuances. Front. Oncol. 2022, 12, 847110. [Google Scholar] [CrossRef]
- Ranasinghe, M.G.; Sheehan, J.M. Surgical management of brain metastases. Neurosurg. Focus 2007, 22, 1–7. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; E Gaspar, L.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. Neuro Oncol. 2022, 24, 331–357. [Google Scholar] [CrossRef]
- Soliman, H.; Das, S.; Larson, D.A.; Sahgal, A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 2016, 7, 12318. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.K.; Perlow, H.K.; Upadhyay, R.; McCalla, A.; Raval, R.R.; Thomas, E.M.; Blakaj, D.M.; Beyer, S.J.; Palmer, J.D. Advances in Radiotherapy for Brain Metastases. Surg. Oncol. Clin. 2023, 32, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.R.; Bentzen, S.M.; Hanna, A.; Choi, E.; Boggs, D.H.; Kwok, Y.; Hyder, J.; Feigenberg, S.J.; Regine, W.F.; Woodworth, G.F.; et al. Prognostic models for patients with brain metastases after stereotactic radiosurgery with or without whole brain radiotherapy: A validation study. J. Neurooncol. 2018, 140, 341–349. [Google Scholar] [CrossRef]
- Steindl, A.; Berghoff, A.S. Brain metastases in metastatic cancer: A review of recent advances in systemic therapies. Expert Rev. Anticancer. Ther. 2021, 21, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Tonse, R.; Tom, M.C.; Mehta, M.P.; Ahluwalia, M.S.; Kotecha, R. Integration of Systemic Therapy and Stereotactic Radiosurgery for Brain Metastases. Cancers 2021, 13, 3682. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, S.; Schmutzer-Sondergeld, M.; Weller, J.; Katzendobler, S.; Kirchleitner, S.; Forbrig, R.; Harter, P.N.; Baumgarten, L.V.; Schichor, C.; Stoecklein, V.; et al. Neurosurgical resection of multiple brain metastases: Outcomes, complications, and survival rates in a retrospective analysis. J. Neurooncol. 2024, 169, 349–358. [Google Scholar] [CrossRef]
- Stumpo, V.; Carretta, A.; Bellomo, J.; Padevit, L.; Staartjes, V.; Maldaner, N.; Coker, P.; Fierstra, J.; Weller, M.; Le Rhun, E.; et al. Surgical tumor volume reduction in patients with brain metastases: A systematic review and meta-analysis. Cancer Treat. Rev. 2025, 139, 102981. [Google Scholar] [CrossRef]
- Lin, J.; Kaiser, Y.; Wiestler, B.; Bernhardt, D.; Combs, S.E.; Delbridge, C.; Meyer, B.; Gempt, J.; Aftahy, A.K. Cytoreduction of Residual Tumor Burden Is Decisive for Prolonged Survival in Patients with Recurrent Brain Metastases—Retrospective Analysis of 219 Patients. Cancers 2023, 15, 5067. [Google Scholar] [CrossRef]
- Aftahy, A.K.; Barz, M.; Lange, N.; Baumgart, L.; Thunstedt, C.; Eller, M.A.; Wiestler, B.; Bernhardt, D.; Combs, S.E.; Jost, P.J.; et al. The Impact of Postoperative Tumor Burden on Patients With Brain Metastases. Front. Oncol. 2022, 12, 869764. [Google Scholar] [CrossRef]
- Freeman, M.; Ennis, M.; Jerzak, K.J. Karnofsky Performance Status (KPS) ≤60 Is Strongly Associated With Shorter Brain-Specific Progression-Free Survival Among Patients With Metastatic Breast Cancer With Brain Metastases. Front. Oncol. 2022, 12, 867462. [Google Scholar] [CrossRef]
- Schneider, M.; Heimann, M.; Schaub, C.; Eichhorn, L.; Potthoff, A.-L.; Giordano, F.A.; Güresir, E.; Ko, Y.-D.; Landsberg, J.; Lehmann, F.; et al. Comorbidity Burden and Presence of Multiple Intracranial Lesions Are Associated with Adverse Events after Surgical Treatment of Patients with Brain Metastases. Cancers 2020, 12, 3209. [Google Scholar] [CrossRef]
- Wagner, A.; Wostrack, M.; Hartz, F.; Heim, J.; Hameister, E.; Hildebrandt, M.; Meyer, B.; Winter, C. The role of extended coagulation screening in adult cranial neurosurgery. Brain Spine 2023, 3, 101756. [Google Scholar] [CrossRef] [PubMed]
- Bahna, M.; Heimann, M.; Bode, C.; Borger, V.; Eichhorn, L.; Güresir, E.; Hamed, M.; Herrlinger, U.; Ko, Y.-D.; Lehmann, F.; et al. Tumor-associated epilepsy in patients with brain metastases: Necrosis-to-tumor ratio forecasts postoperative seizure freedom. Neurosurg. Rev. 2022, 45, 545–551. [Google Scholar] [CrossRef]
- Sauvageot, S.; Mollevi, C.; Thomas, Q.D.; Charissoux, M.; Darlix, A.; Rigau, V.; Bauchet, L.; Quantin, X.; Pujol, J.L.; Roch, B.; et al. Prognostic impact of the number and total tumor burden of secondary cerebral lesions in patients with resected brain metastases of non–small cell lung cancers. J. Neurosurg. 2024, 141, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.M. The Management of Brain Metastases—Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef] [PubMed]


| Variable | Category | N (%) |
|---|---|---|
| Sex | Male | 87 (54.4) |
| Female | 73 (45.6) | |
| Age, years | Median (IQR, range) | 67 (56–74, 20–90) |
| History of epileptic seizures before surgery | Yes | 27 (16.9) |
| No | 133 (83.1) | |
| History of epileptic seizures after surgery | Yes | 41 (25.6) |
| No | 119 (74.4) | |
| Extracranial disease | Metachronous | 64 (40.0) |
| Synchronous | 96 (60.0) | |
| Primary tumor histology | Lung | 53 (33.1) |
| Breast | 14 (8.8) | |
| Melanoma | 24 (15.0) | |
| CUP | 5 (3.1) | |
| Gastrointestinal (GI) | 15 (9.4) | |
| Kidney | 12 (7.5) | |
| Urothelial | 5 (3.1) | |
| Other | 32 (20.0) | |
| No. of brain metastases | Single metastasis | 77 (48.1) |
| Oligometastases (2–3) | 35 (21.9) | |
| Multiple (>3) | 48 (30.0) | |
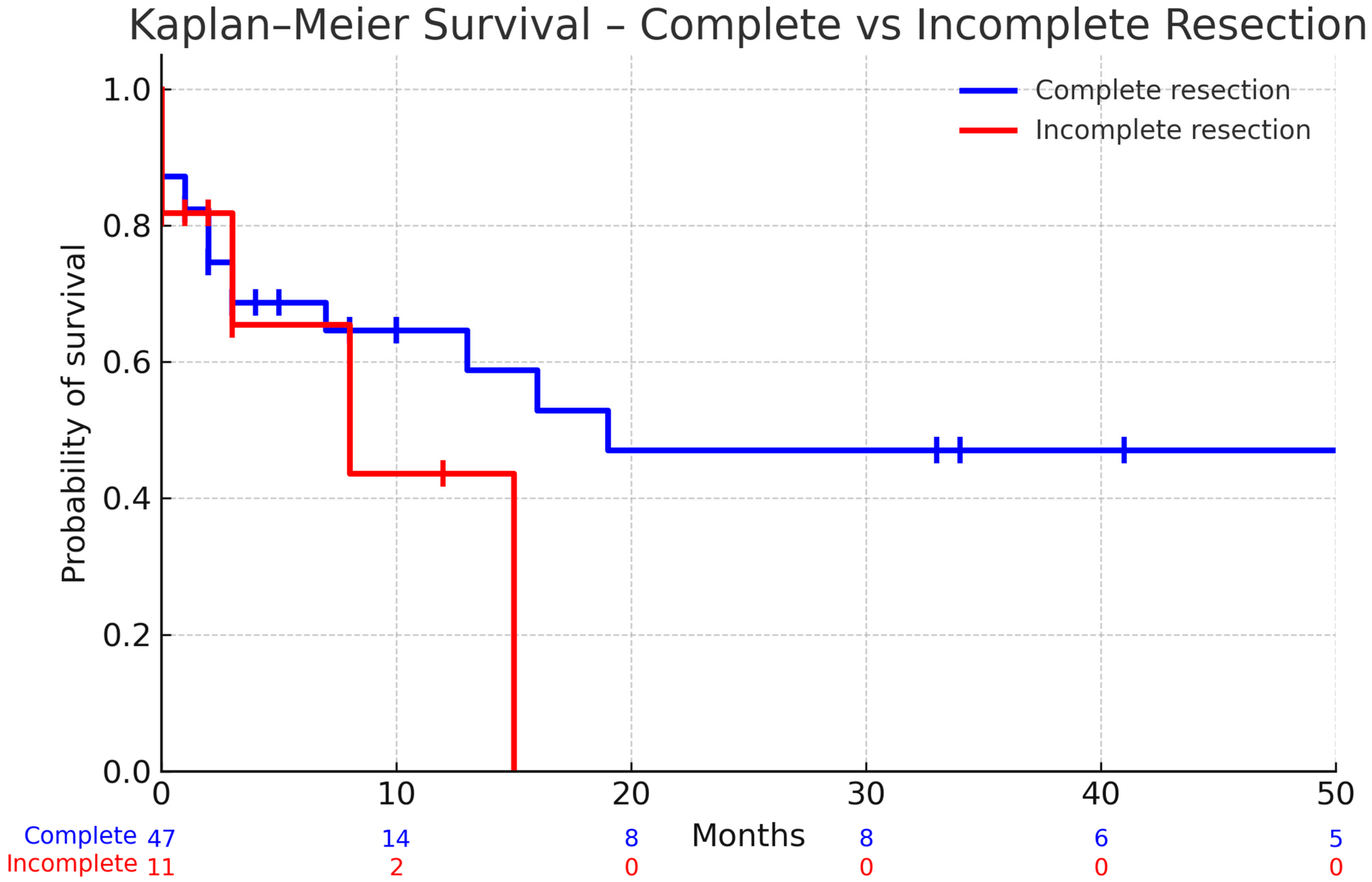
| Complete resection of index metastasis | Yes | 134 (83.8) |
| No | 26 (16.3) | |
| Total number of metastases | Median (IQR, range) | 2 (1–4, 1–16) |
| Total tumor volume (cm3) | Median (IQR) | 14 (7–31) |
| Volume of resected lesion (cm3) | Median (IQR) | 12 (6–26) |
| Preoperative KPS | Median (IQR) | 80 (60–90) |
| Postoperative KPS | Median (IQR) | 80 (60–90) |
| Hospital stay, days | Median (IQR, range) | 10 (6–17, 3–74) |
| Adverse effects (Clavien–Dindo grade) | Any adverse effect | 37 (23.1) |
| Grade I | 13 (35.1) | |
| Grade II | 2 (5.4) | |
| Grade IIIa | 1 (2.7) | |
| Grade IIIb | 5 (13.5) | |
| Grade IVb | 7 (18.9) | |
| Grade V | 9 (24.3) | |
| Radiation therapy (post- or intraoperatively) | Yes | 135 (84.4) |
| No | 25 (15.6) | |
| Systemic therapy postoperatively | Yes | 108 (67.5) |
| No | 52 (32.5) |
| Patient Group | Preoperative KPS Median (IQR), Mean Rank | Postoperative KPS Median (IQR), Mean Rank | p-Value |
|---|---|---|---|
| Single metastasis | 80 (70–90), 15.91 | 80 (70–90), 17.17 | 0.900 |
| Oligometastases | 80 (60–90), 14.50 | 70 (50–90), 7.15 | 0.123 |
| Multiple metastases | 70 (60–80), 12.93 | 70 (50–80), 9.00 | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldberg, M.; Kraus, L.M.; Vatcheva, C.; Bernhardt, D.; Combs, S.E.; Negwer, C.; Meyer, B.; Wagner, A. Evaluating the Impact of Index Metastasis Resection in Patients with Multiple Brain Metastases. Cancers 2025, 17, 3281. https://doi.org/10.3390/cancers17203281
Goldberg M, Kraus LM, Vatcheva C, Bernhardt D, Combs SE, Negwer C, Meyer B, Wagner A. Evaluating the Impact of Index Metastasis Resection in Patients with Multiple Brain Metastases. Cancers. 2025; 17(20):3281. https://doi.org/10.3390/cancers17203281
Chicago/Turabian StyleGoldberg, Maria, Luisa Mona Kraus, Cvetina Vatcheva, Denise Bernhardt, Stephanie E. Combs, Chiara Negwer, Bernhard Meyer, and Arthur Wagner. 2025. "Evaluating the Impact of Index Metastasis Resection in Patients with Multiple Brain Metastases" Cancers 17, no. 20: 3281. https://doi.org/10.3390/cancers17203281
APA StyleGoldberg, M., Kraus, L. M., Vatcheva, C., Bernhardt, D., Combs, S. E., Negwer, C., Meyer, B., & Wagner, A. (2025). Evaluating the Impact of Index Metastasis Resection in Patients with Multiple Brain Metastases. Cancers, 17(20), 3281. https://doi.org/10.3390/cancers17203281





