Development of a Simplified Geriatric Score-4 (SGS-4) to Predict Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged over 50
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
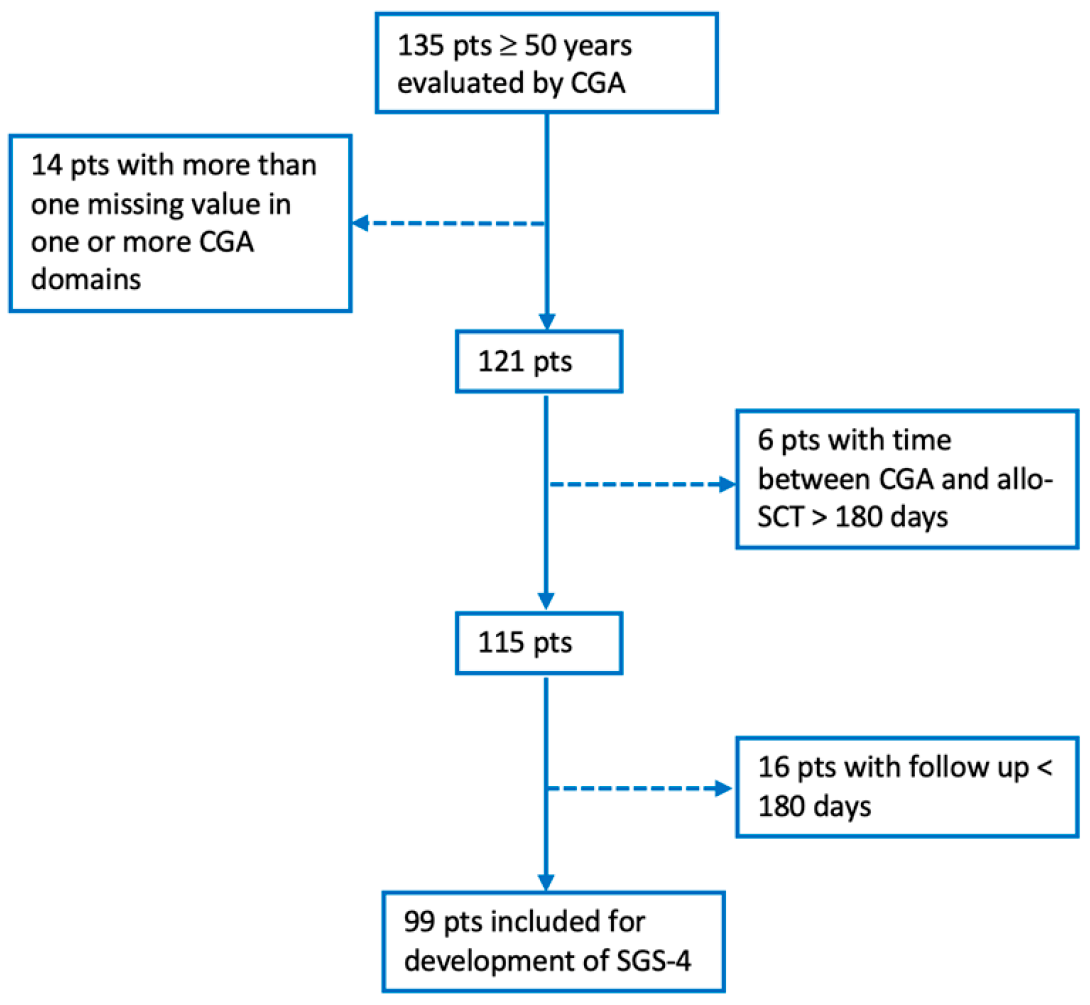
2.1. Patient Population and Geriatric Assessment
2.2. Statistical Analysis and Mathematical Model
3. Results
3.1. Patients and Transplant Characteristics
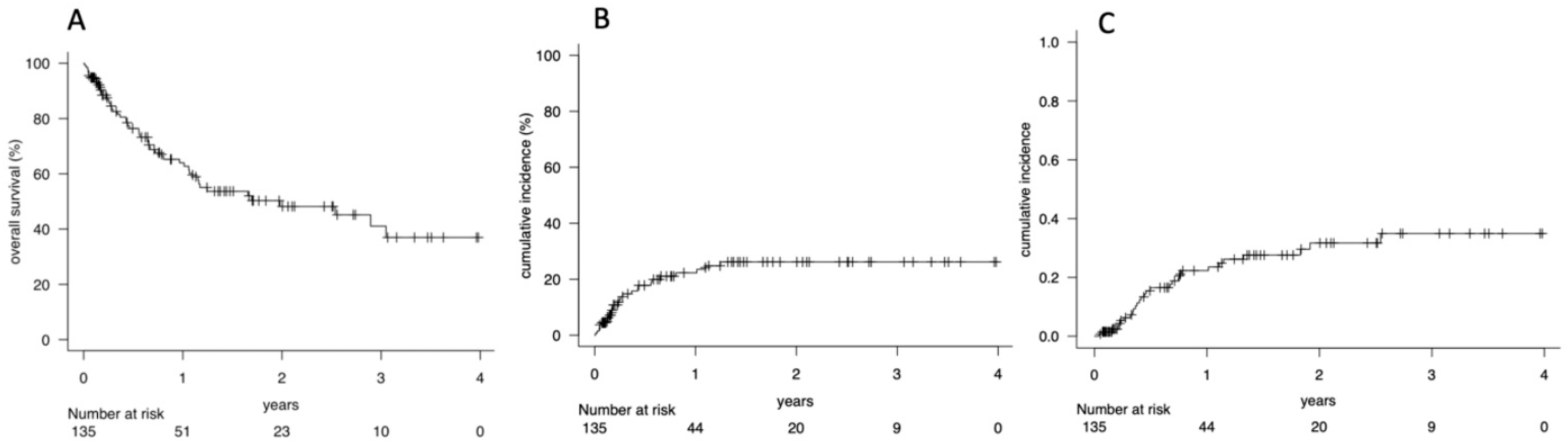
3.2. Overall Survival (OS), Non-Relapse Mortality (NRM) and Cumulative Incidence of Relapse (CIR)
3.3. Comprehensive Geriatric Assessment and Outcomes
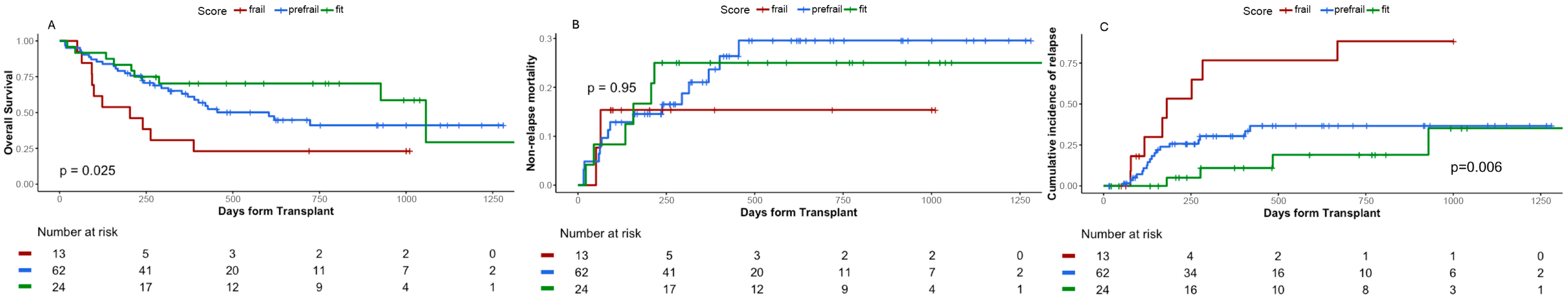
3.4. Simplified Geriatric Score-4 (SGS-4) Design
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | Activities of daily Living |
| aGVHD | acute Graft-versus-Host Disease |
| cGVHD | chronic Graft-versus-Host Disease |
| Allo-HCT | Allogeneic Hematopoietic Cell Transplantation |
| AML | Acute Myeloid Leukemia |
| ATG | Anti-T Lymphocyte Globulin |
| CGA | Comprehensive Geriatric Assessment |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| CSF | Clinical Frailty Scale |
| CIR | Cumulative Incidence of Relapse |
| CyA | Cyclosporine A |
| DRI | Disease Risk Index |
| FA | Factor Analysis |
| FIL | Fondazione Italiana Linfomi |
| G8 | Geriatric 8 Screening Tool |
| GITMO | Gruppo Italiano Trapianto di Midollo Osseo |
| GVHD | Graft-versus-Host Disease |
| GVL | Graft-versus-leukemia |
| HCT-CI | Hematopoietic Cell Transplantation–Comorbidity Index |
| IADL | Instrumental Activities of daily Living |
| KPS | Karnofsky Performance Status |
| MAC | Myeloablative Conditioning |
| MMF | Mycophenolate Mofetil |
| MMSE | Mini Mental State examination |
| MTX | Methotrexate |
| MUD | Matched Unrelated Donor |
| NRM | Non-Relapse Mortality |
| OS | Overall Survival |
| PBSC | Peripheral Blood Stem Cells |
| PTCy | Post-Transplant Cyclophosphamide |
| RIC | Reduced-Intensity Conditioning |
| SGS-4 | Simplified Geriatric Score (4 variables) |
| VES-13 | Vulnerable Elders Survey-13 |
References
- Passweg, J.R.; Baldomero, H.; Atlija, M.; Kleovoulou, I.; Witaszek, A.; Alexander, T.; Angelucci, E.; Averbuch, D.; Bazarbachi, A.; Ciceri, F.; et al. The 2023 EBMT report on hematopoietic cell transplantation and cellular therapies. Increased use of allogeneic HCT for myeloid malignancies and of CAR-T at the expense of autologous HCT. Bone Marrow Transplant. 2025, 60, 519–528. [Google Scholar] [CrossRef]
- Loke, J.; Malladi, R.; Moss, P.; Craddock, C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: A triumph of hope and experience. Br. J. Haematol. 2019, 188, 129–146. [Google Scholar] [CrossRef]
- Lin, R.J.; Artz, A.S. Allogeneic Hema to Poi Etic Cell Trans Plan Ta Tion for Older Patients. Hematology 2021, 2021, 254–263. [Google Scholar] [CrossRef]
- Loke, J.; Vyas, H.; Craddock, C. Optimizing Transplant Approaches and Post-Transplant Strategies for Patients With Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 666091. [Google Scholar] [CrossRef]
- Malagola, M.; Polverelli, N.; Rubini, V.; Martino, M.; Patriarca, F.; Bruno, B.; Giaccone, L.; Grillo, G.; Bramanti, S.; Bernasconi, P.; et al. GITMO Registry Study on Allogeneic Transplantation in Patients Aged ≥60 Years from 2000 to 2017: Improvements and Criticisms. Biol. Blood Marrow Transplant. 2022, 28, 96.e1–96.e11. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Logan, B.R.; Zhu, X.; Rizzo, J.D.; Cooke, K.R.; McCarthy, P.L.; Ho, V.T.; Horowitz, M.M.; Pasquini, M.C. Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol. Blood Marrow Transplant. 2015, 21, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Elsawy, M.; Sorror, M.L. Up-to-Date Tools for Risk Assessment before Allogeneic Hemato-poietic Cell Transplantation. Bone Marrow Transpl. 2016, 51, 1283–1300. [Google Scholar] [CrossRef]
- Hurria, A.; Li, D.; Hansen, K.; Patil, S.; Gupta, R.; Nelson, C.; Lichtman, S.M.; Tew, W.P.; Hamlin, P.; Zuckerman, E.; et al. Distress in Older Patients With Cancer. J. Clin. Oncol. 2009, 27, 4346–4351. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The index of Adl: A standardized measure of biological and phychological funcation. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Yokom, D.W.; Alibhai, S.M.; Sattar, S.; Krzyzanowska, M.K.; Puts, M.T. Geriatric oncology screening tools for CGA-based interventions: Results from a phase II study of geriatric assessment and management for older adults with cancer. J. Geriatr. Oncol. 2018, 9, 683–686. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- McLean, R.R.; Kiel, D.P. Developing Consensus Criteria for Sarcopenia: An Update. J. Bone Miner. Res. 2015, 30, 588–592. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The Mini-Cog: A Cognitive “vital Signs” Measure for Dementia Screening in Multi-Lingual Elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Aiki, S.; Okuyama, T.; Sugano, K.; Kubota, Y.; Imai, F.; Nishioka, M.; Ito, Y.; Iida, S.; Komatsu, H.; Ishida, T.; et al. Cognitive dysfunction among newly diagnosed older patients with hematological malignancy: Frequency, clinical indicators and predictors. Ultrasound Med. Biol. 2017, 48, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tucci, A.; Martelli, M.; Rigacci, L.; Riccomagno, P.; Cabras, M.G.; Salvi, F.; Stelitano, C.; Fabbri, A.; Storti, S.; Fogazzi, S.; et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: A prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk. Lymphoma 2014, 56, 921–926. [Google Scholar] [CrossRef]
- Salas, M.Q.; Atenafu, E.G.; Bascom, O.; Wilson, L.; Lam, W.; Law, A.D.; Pasic, I.; Kim, D.H.; Michelis, F.V.; Al-Shaibani, Z.; et al. Pilot prospective study of Frailty and Functionality in routine clinical assessment in allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2020, 56, 60–69. [Google Scholar] [CrossRef]
- Muffly, L.S.; Boulukos, M.; Swanson, K.; Kocherginsky, M.; del Cerro, P.; Schroeder, L.; Pape, L.; Extermann, M.; Van Besien, K.; Artz, A.S. Pilot Study of Comprehensive Geriatric Assessment (CGA) in Allogeneic Transplant: CGA Captures a High Prevalence of Vulnerabilities in Older Transplant Recipients. Biol. Blood Marrow Transplant. 2013, 19, 429–434. [Google Scholar] [CrossRef]
- Muffly, L.S.; Kocherginsky, M.; Stock, W.; Chu, Q.; Bishop, M.R.; Godley, L.A.; Kline, J.; Liu, H.; Odenike, O.M.; Larson, R.A.; et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014, 99, 1373–1379. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Souza, P.M.R.; de Oliveira Muniz Koch, L.; Hamerschlak, N. The use of comprehensive geriatric assessment in older patients before allologeneic hematopoietic stem cell transplantation: A cross-sectional study. J. Geriatr. Oncol. 2020, 11, 100–106. [Google Scholar] [CrossRef]
- Lew, M.V.; Ren, Y.; Lowder, Y.P.; Siamakpour-Reihani, S.; Ramalingam, S.; Romero, K.M.; Thompson, J.C.; Bohannon, L.M.; McIntyre, J.; Tang, H.; et al. Geriatric Assessment Reveals Actionable Impairments in Hematopoietic Stem Cell Transplantation Candidates Age 18 to 80 Years. Biol. Blood Marrow Transplant. 2022, 28, 498.e1–498.e9. [Google Scholar] [CrossRef]
- Polverelli, N.; Tura, P.; Battipaglia, G.; Malagola, M.; Bernardi, S.; Gandolfi, L.; Zollner, T.; Zanaglio, C.; Farina, M.; Morello, E.; et al. Multidimensional geriatric assessment for elderly hematological patients (≥60 years) submitted to allogeneic stem cell transplantation. A French–Italian 10-year experience on 228 patients. Bone Marrow Transplant. 2020, 55, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; Ihorst, G.; Schnitzler, S.; Bertz, H.; Finke, J. Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: A prospective risk factor and serial assessment analysis. Bone Marrow Transplant. 2018, 53, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Derman, B.A.; Kordas, K.; Ridgeway, J.; Chow, S.; Dale, W.; Lee, S.M.; Aguada, E.; Jakubowiak, A.J.; Jasielec, J.; Kline, J.; et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv. 2019, 3, 3488–3498. [Google Scholar] [CrossRef]
- Malagola, M.; Castagna, L.; Farina, M.; Pellizzeri, S.; Degrandi, E.; Oldani, E.; Bassi, S.; Battipaglia, G.; Borghero, C.; Brunello, L.; et al. Assessment of frailty and comorbidities in cellular therapies patients in Italy: Results of the GITMO Elderly-Survey (GITMO ELD-Survey). Leuk. Lymphoma 2025, 66, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Dunne, R.F.; Giri, S.; Shachar, S.S.; Caan, B.J. Sarcopenia in the Older Adult With Cancer. J. Clin. Oncol. 2021, 39, 2068–2078. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef]
- Conti, C.; Paillaud, E.; Laurent, M.; Poisson, J.; Boudou-Rouquette, P.; Frelaut, M.; Gay, P.; Canovas, J.; Caillet, P.; Mebarki, S.; et al. Prognostic impact of neurocognitive disorders in older patients with cancer: The ELCAPA prospective cohort study. J. Nutr. Health Aging 2024, 28, 100215. [Google Scholar] [CrossRef]
- Augschoell, J.; Kemmler, G.; Hamaker, M.E.; Stauder, R. PPT and VES-13 in elderly patients with cancer: Evaluation in multidimensional geriatric assessment and prediction of survival. J. Geriatr. Oncol. 2014, 5, 415–421. [Google Scholar] [CrossRef]
- Merli, F.; Luminari, S.; Tucci, A.; Arcari, A.; Rigacci, L.; Hawkes, E.; Chiattone, C.S.; Cavallo, F.; Cabras, G.; Alvarez, I.; et al. Simplified Geriatric Assessment in Older Patients With Diffuse Large B-Cell Lymphoma: The Prospective Elderly Project of the Fondazione Italiana Linfomi. J. Clin. Oncol. 2021, 39, 1214–1222. [Google Scholar] [CrossRef]
- Sung, A.D.; Koll, T.; Gier, S.H.; Racioppi, A.; White, G.; Lew, M.; Free, M.; Agarwal, P.; Bohannon, L.M.; Johnson, E.J.; et al. Preconditioning Frailty Phenotype Influences Survival and Relapse for Older Allogeneic Transplantation Recipients. Biol. Blood Marrow Transplant. 2024, 30, 415.e1–415.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cueto, J.; Ko, N.Y.; Hoskins, K.F.; Nabulsi, N.A.; Asfaw, A.A.; Hubbard, C.C.; Mitra, D.; Calip, G.S.; Law, E.H. Population-based recurrence rates among older women with HR-positive, HER2-negative early breast cancer: Clinical risk factors, frailty status, and differences by race. Breast 2021, 59, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Guarinoni, M.G.; Arena, F.; Andreoli, M.; Bernardi, S.; Malagola, M.; Turra, A.; Polverelli, N.; Russo, D. A Systematic Review of the Literature and Perspectives on the Role of Biomarkers in the Management of Malnutrition After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2021, 11, 535890. [Google Scholar] [CrossRef]
- Malard, F.; Jenq, R.R. The Microbiome and Its Impact on Allogeneic Hematopoietic Cell Transplantation. Cancer J. 2023, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Artz, A.S.; Logan, B.; Zhu, X.; Akpek, G.; Bufarull, R.M.; Gupta, V.; Lazarus, H.M.; Litzow, M.; Loren, A.; Majhail, N.S.; et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016, 101, 1426–1433. [Google Scholar] [CrossRef]
- Yamamoto, W.; Fujii, E.; Matsumoto, K.; Yamamoto, E.; Aoki, J.; Tanaka, M.; Ishigatsubo, Y.; Kanamori, H. Prognostic value of pretransplant serum C-reactive protein in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2016, 103, 444–452. [Google Scholar] [CrossRef]
- Salas, M.Q.; Solano, M.T.; González, M.B.; Gómez, M.A.; Fox, L.; Artigas, M.d.M.P.; Santamaría, A.; González, M.d.C.Q.; Salinas, A.S.; Camacho, J.M.S.; et al. Frailty assessment in adults undergoing allogeneic hematopoietic cell transplantation: Insights from a multicenter GETH-TC study to optimize outcomes and care. Front. Immunol. 2025, 15, 1512154. [Google Scholar] [CrossRef] [PubMed]
| Values | Gait Speed (m/s) | Hand Grip (kg) | Sex |
|---|---|---|---|
| 1 | ≤1.04 | ≤24.5 | M |
| 2 | 1.04 < X ≤ 1.17 | 24.5 < X ≤ 32.7 | F |
| 3 | 1.17 < X ≤ 1.29 | 32.7 < X ≤ 41.3 | |
| 4 | 1.29 < X ≤ 1.70 | 41.3 < X ≤ 57.6 |
| Characteristics | N (%) |
|---|---|
| Number | 135 (100) |
| Age at allo-SCT, years, median (range) | 63 (50–75) |
| Male, n (%) | 86 (63.7) |
| Diagnosis, n (%) | |
| AML | 53 (39.3) |
| MDS | 17 (12.6) |
| ALL | 11 (8.1) |
| Lymphoma | 13 (9.6) |
| MF | 25 (18.5) |
| Other | 16 (11.9) |
| DRI, n (%) | |
| Low | 3 (2.2) |
| Intermediate | 95 (70.4) |
| High | 35 (25.9) |
| Very high | 2 (1.5) |
| KPS, n (%) | |
| 90–100 | 126 (93.4) |
| 70–80 | 9 (6.6) |
| HCT-CI, n (%) | |
| 0 | 27 (20) |
| 1–2 | 37 (27.4) |
| ≥3 | 71 (52.6) |
| FIL score, n (%) | |
| Fit | 132 (97.8) |
| Unfit | 3 (2.2) |
| Disease status, n (%) | |
| 1st CR | 53 (39.3) |
| 2nd or subsequent CR | 82 (60.7) |
| Donor type, n (%) | |
| Related | 33 (24.4) |
| Unrelated | 63 (46.7) |
| Haploidentical | 39 (28.9) |
| Stem cell source, n(%) | |
| PBSC | 124 (91.9) |
| BM | 11 (8.1) |
| Conditioning regimen, n (%) | |
| MAC | 85 (63) |
| RIC | 50 (37) |
| GVHD prophylaxis, n (%) | |
| CyA + MFF | 3 (2.2) |
| CyA + MTX | 4 (3) |
| CyA + MTX + ATG | 78 (57.8) |
| PTCy + Other | 27 (20) |
| Other | 23 (17) |
| Follow-up, months, median (range) | 7.5 (0.01–47.8) |
| Pts Evaluated | OS 1 y | OS 2 y | p | NRM 1y | NRM 2 y | p | CIR 1 y | CIR 2 y | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| ADL | ||||||||||
| Any limitations | 4 | 50 | - | 25 | - | 25 | - | |||
| No limitations | 130 | 63 | 49 | 0.5 | 24 | 26 | 0.95 | 23 | 30 | 0.73 |
| IADL | ||||||||||
| Any limitations | 118 | 30 | 50 | 46 | 24 | 35 | 35 | |||
| No limitations | 15 | 66 | 30 | 0.11 | 21 | 46 | 0.11 | 22 | 21 | 0.72 |
| G8, n | ||||||||||
| ≤13 | 51 | 52 | 46 | 24 | 24 | 31 | 37 | |||
| >13 | 83 | 67 | 48 | 0.7 | 24 | 29 | 0.9 | 17 | 26 | 0.23 |
| VES 13, n | ||||||||||
| ≤3 | 14 | 11 | 11 | 30 | 30 | 58 | 58 | |||
| >3 | 120 | 67 | 52 | 0.04 | 23 | 26 | 0.93 | 18 | 28 | 0.01 |
| FIL score | ||||||||||
| FIT | 132 | 65 | 50 | 24 | 27 | 21 | 30 | |||
| UNFIT | 3 | 0 | - | <0.01 | 0 | 23 | 0.41 | 100 | - | <0.01 |
| CFS | ||||||||||
| FIT | 75 | 63 | 55 | 23 | 23 | 22 | 35 | |||
| UNFIT | 12 | 45 | - | 0.6 | 28 | - | 0.9 | 27 | - | 0.8 |
| Gait Speed, m/s | ||||||||||
| ≤1.1 | 80 | 73 | 60 | 20 | 22 | 25 | 31 | |||
| >1.1 | 49 | 41 | 27 | 0.06 | 31 | 34 | 0.31 | 40 | 40 | 0.2 |
| Chair Stand, times | ||||||||||
| <14 | 90 | 65 | 50 | 21 | 22 | 25 | 32 | |||
| ≥14 | 41 | 54 | 40 | 0.5 | 27 | 34 | 0.37 | 24 | 31 | 0.83 |
| Hand Grip, Kg | ||||||||||
| <26 | 37 | 45 | 30 | 34 | 38 | 28 | 41 | |||
| ≥26 | 97 | 68 | 54 | <0.01 | 21 | 21 | 0.05 | 20 | 28 | 0.6 |
| Mini-Cog, n | ||||||||||
| 1–2 | 56 | 64 | 58 | 21 | 21 | 19 | 29 | |||
| 3 | 77 | 60 | 39 | 0.28 | 24 | 29 | 0.44 | 26 | 31 | 0.45 |
| MMSE, n | ||||||||||
| ≤24 | 13 | 19 | 19 | 52 | 52 | 29 | 48 | |||
| >24 | 121 | 66 | 50 | 0.03 | 22 | 24 | 0.2 | 22 | 29 | 0.56 |
| HCT-CI, n | ||||||||||
| 0 | 27 | 88 | 33 | 17 | 17 | 18 | 25 | |||
| 1–2 | 37 | 70 | 49 | 18 | 18 | 22 | 38 | |||
| ≥3 | 71 | 55 | 37 | 0.2 | 29 | 34 | 0.2 | 28 | 30 | 0.88 |
| Characteristics | Total N = 99 | Frail N = 13 | Prefrail N = 62 | Fit N = 24 | p-Value |
|---|---|---|---|---|---|
| Age, years (range) | 63.0 (56.0, 66.0) | 64.0 (56.0, 68.0) | 62.0 (57.0, 66.0) | 63.0 (56.0, 65.0) | >0.9 |
| Diagnosis, n (%) | |||||
| AL | 41 (41.4) | 10 (76.9) | 23 (37.1) | 8 (33.3) | 0.10 |
| MDS | 11 (11.1) | 2 (15.4) | 6 (9.7) | 3 (12.5) | |
| Lymphoma | 14 (14.1) | 1 (8) | 11 (17.7) | 3 (12.5) | |
| MPN | 1 (1.0) | 0 (0) | 0 (0) | 1 (4.2) | |
| MM | 5 (5.1) | 0 (0) | 5 (8.1) | 0 (0) | |
| Other | 27 (27.3) | 1 (7.7) | 17 (27.4) | 9 (37.5) | |
| DRI, n (%) | |||||
| 1 | 2 (2.0) | 0 (0) | 1 (1.6) | 1 (4.2) | 0.4 |
| 2 | 30 (30.3) | 7 (53.8) | 19 (30.6) | 4 (17.4) | |
| 3 | 24 (24.5) | 6 (46.2) | 19 (30.6) | 1 (4.3) | |
| 4 | 2 (2.0) | 0 (0) | 1 (1.6) | 1 (4.2) | |
| Missing | 0 | 0 | 0 | 0 | |
| KPS, n (%) | |||||
| 90–100 | 92 (92.9) | 11 (84.6) | 57 (91.9) | 24 (100) | 0.5 |
| 70–80 | 7 (7.1) | 2 (15.4) | 5 (8.1) | 0 (0) | |
| HCT-CI, n (%) | |||||
| 0 | 21 (21.2) | 1 (7.7) | 12 (19.4) | 8 (33.3) | 0.13 |
| 1–2 | 28 (28.3) | 3 (23.1) | 16 (25.8) | 9 (37.5) | |
| ≥3 | 50 (50.5) | 9 (69.2) | 34 (54.8) | 7 (29.2) | |
| Disease status, n (%) | |||||
| 1st CR | 33 (33.3) | 6 (46.2) | 20 (32.3) | 7 (29.2) | 0.6 |
| 2nd or subsequent CR | 66 (66.7) | 7 (53.8) | 42 (67.7) | 17 (70.8) | |
| Line of Therapy, n (%) | |||||
| 1 | 48 (49.5) | 8 (61.5) | 28 (45.2) | 12 (52.0) | 0.5 |
| ≥2 | 50 (50.5) | 5 (38.5) | 34 (54.8) | 11 (48.0) | |
| Missing | 1 | 0 | 0 | 1 | |
| Stem cell source, n (%) | |||||
| PBSC | 91 (92.0) | 12 (92.3) | 56 (90.3) | 23 (95.8) | 0.9 |
| BM | 8 (8.0) | 1 (7.7) | 6 (9.7) | 1 (4.2) | |
| Conditioning regimen, n (%) | |||||
| MAC | 40 (40.4) | 4 (30.7) | 25 (40.3) | 11 (45.8) | 0.7 |
| RIC | 59 (59.6) | 9 (69.3) | 37 (59.7) | 13 (54.2) | |
| TCI score, n | 2.5 | 2.8 | 2.7 | 3.0 | 0.4 |
| Donor age, years (range) | 37 (28–50) | 40 (28–45) | 36 (28–47)) | 38 (28–55) | 0.9 |
| Donor, n (%) | |||||
| Related | 23 (23.2) | 3 (23.1) | 13 (21.0) | 7 (29.2) | 0.9 |
| Unrelated | 44 (44.5) | 6 (46.1) | 27 (43.5) | 11 (45.8) | |
| Haploidentical | 32 (32.3) | 4 (30.8) | 22 (35.5) | 6 (25.0) | |
| Time to allo-SCT, days (range) | 11.3 (6.5–31.9) | 6.3 (5.7–10.2) | 12.4 (7.1–29.0) | 19.5 (6.7–54.9) | 0.03 |
| aGVHD | 48 (48.5) | 4 (30.8) | 29 (46.8) | 15 (62.5) | 0.17 |
| cGVHD | 16 (16.2) | 9 (0.0) | 10 (16.1) | 6 (25.0) | 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accorsi Buttini, E.; Zucchelli, A.; Tura, P.; Bianco, G.; Avenoso, D.; Campisi, G.; Farina, M.; Magliano, G.; Morello, E.; Radici, V.; et al. Development of a Simplified Geriatric Score-4 (SGS-4) to Predict Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged over 50. Cancers 2025, 17, 3278. https://doi.org/10.3390/cancers17203278
Accorsi Buttini E, Zucchelli A, Tura P, Bianco G, Avenoso D, Campisi G, Farina M, Magliano G, Morello E, Radici V, et al. Development of a Simplified Geriatric Score-4 (SGS-4) to Predict Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged over 50. Cancers. 2025; 17(20):3278. https://doi.org/10.3390/cancers17203278
Chicago/Turabian StyleAccorsi Buttini, Eugenia, Alberto Zucchelli, Paolo Tura, Gianluca Bianco, Daniele Avenoso, Giovanni Campisi, Mirko Farina, Gabriele Magliano, Enrico Morello, Vera Radici, and et al. 2025. "Development of a Simplified Geriatric Score-4 (SGS-4) to Predict Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged over 50" Cancers 17, no. 20: 3278. https://doi.org/10.3390/cancers17203278
APA StyleAccorsi Buttini, E., Zucchelli, A., Tura, P., Bianco, G., Avenoso, D., Campisi, G., Farina, M., Magliano, G., Morello, E., Radici, V., Polverelli, N., Russo, D., Marengoni, A., & Malagola, M. (2025). Development of a Simplified Geriatric Score-4 (SGS-4) to Predict Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged over 50. Cancers, 17(20), 3278. https://doi.org/10.3390/cancers17203278






