Genetic Variations in MDM2 Gene Contribute to Renal Cell Carcinoma Susceptibility: A Genotype–Phenotype Correlation Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Subjects
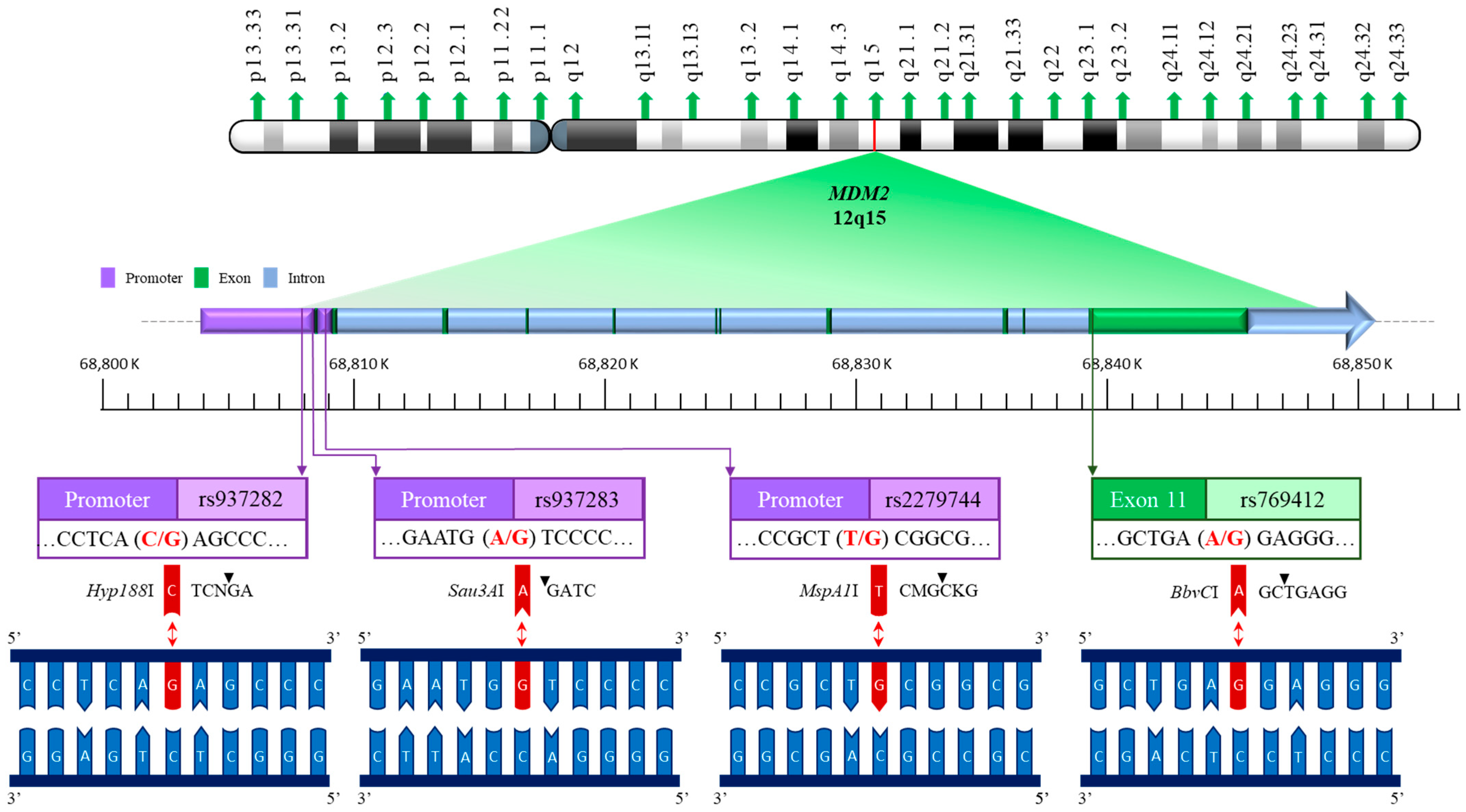
2.2. DNA Extraction and MDM2 Genotyping Methodology
2.3. MDM2 RNA Level Measurement by Quantitative Real-Time PCR
2.4. Statistical Analysis
3. Results
3.1. Comparison of Characteristics Between RCC Patients and Healthy Controls
3.2. Association Between MDM2 Genotypes and RCC Risk in Taiwan
3.3. Stratified Analysis of MDM2 rs2279744 Genotypes According to Epidemiological and Clinical Risk Factors
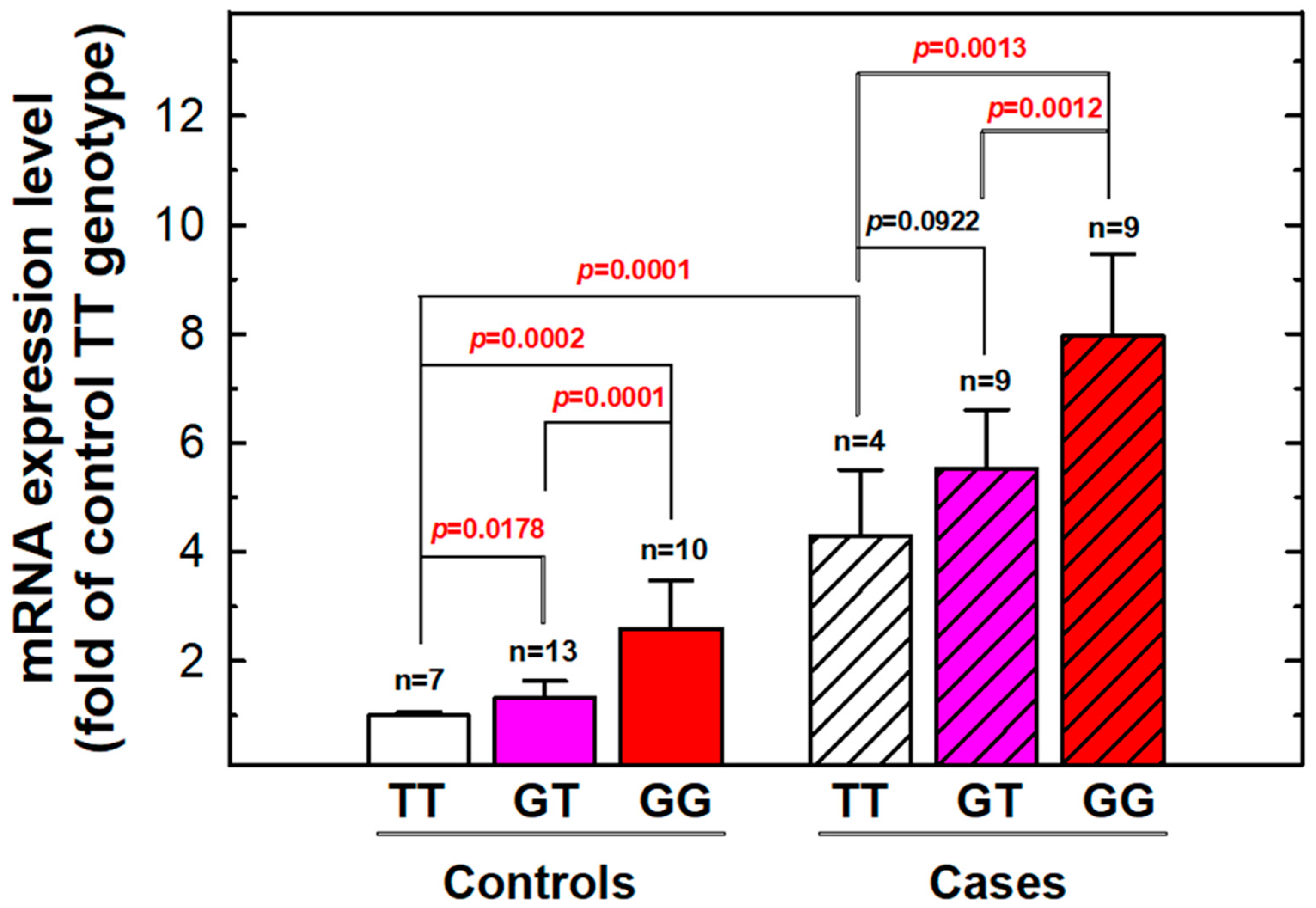
3.4. The Expression Levels of MDM2 mRNA According to Individual Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Rose, T.L.; Kim, W.Y. Renal Cell Carcinoma: A Review. JAMA 2024, 332, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Alaghehbandan, R.; Siadat, F.; Trpkov, K. What’s new in the WHO 2022 classification of kidney tumours? Pathologica 2022, 115, 8–22. [Google Scholar] [CrossRef]
- Kabaria, R.; Klaassen, Z.; Terris, M.K. Renal cell carcinoma: Links and risks. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 45–52. [Google Scholar] [CrossRef]
- Khaleghi Mehr, F.; Abian, N.; Abolhasani, M.; Moradi, Y. Asymptomatic ureteral metastasis of chromophobe renal cell carcinoma after radical nephrectomy: A case report and review of literature. Int. J. Surg. Case Rep. 2024, 120, 109907. [Google Scholar] [CrossRef] [PubMed]
- Beecroft, N.; Gauntner, T.D.; Upadhyay, R.; Wang, S.J.; Yang, Y.; Singer, E.A.; Dason, S. Active Surveillance in Metastatic Renal Cell Carcinoma. J. Kidney Cancer VHL 2024, 11, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Riveros, C.; Ranganathan, S.; Xu, J.; Patel, A.; Slawin, J.; Ordonez, A.; Aghazadeh, M.; Morgan, M.; Miles, B.J.; et al. Morbidity and mortality of multivisceral resection with radical nephrectomy for locally advanced renal cell carcinoma: An analysis of the National Surgical Quality Improvement Program (NSQIP) database. Urol. Oncol. 2023, 41, 209.e1–209.e9. [Google Scholar] [CrossRef]
- Maffezzoli, M.; Buti, S. Prognostication for surgically treated papillary renal cell carcinoma: Which model is the optimal choice? Future Oncol. 2024, 20, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Chang, W.S.; Xu, Y.; Huang, M.; Tamboli, P.; Wood, C.G.; Bau, D.T.; Gu, J. Prognostic significance of circulating insulin growth-like factor 1 and insulin growth-like factor binding protein 3 in renal cell carcinoma patients. Am. J. Cancer Res. 2022, 12, 852–860. [Google Scholar]
- Chen, M.; Tsai, C.W.; Chang, W.S.; Xiong, G.Y.; Xu, Y.; Bau, D.T.; Gu, J. High circulating insulin-like growth factor-1 reduces the risk of renal cell carcinoma: A Mendelian randomization study. Carcinogenesis 2021, 42, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Tsai, C.L.; Chang, S.Y.; Lin, Y.H.; Wang, Y.C.; Huang, W.C.; Mong, M.C.; Yang, Y.C.; Wu, W.T.; Chen, J.C.; et al. Impacts of Matrix Metalloproteinase 9 Genotypes on Renal Cell Carcinoma. Vivo 2023, 37, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Chien, W.C.; Chang, S.Y.; Lin, Y.H.; Wang, Y.C.; Huang, W.C.; Mong, M.C.; Yang, Y.C.; Wu, W.T.; Chen, J.C.; et al. Associations of Matrix Metalloproteinase-8 Genotypes to Renal Cell Carcinoma in Taiwan. Anticancer Res. 2024, 44, 1931–1938. [Google Scholar] [CrossRef]
- Chang, W.S.; Shen, T.C.; Yeh, W.L.; Yu, C.C.; Lin, H.Y.; Wu, H.C.; Tsai, C.W.; Bau, D.T. Contribution of Inflammatory Cytokine Interleukin-18 Genotypes to Renal Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 1563. [Google Scholar] [CrossRef]
- Alhopuro, P.; Ylisaukko-Oja, S.K.; Koskinen, W.J.; Bono, P.; Arola, J.; Jarvinen, H.J.; Mecklin, J.P.; Atula, T.; Kontio, R.; Makitie, A.A.; et al. The MDM2 promoter polymorphism SNP309T-->G and the risk of uterine leiomyosarcoma, colorectal cancer, and squamous cell carcinoma of the head and neck. J. Med. Genet. 2005, 42, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Knappskog, S.; Lonning, P.E. Effects of the MDM2 promoter SNP285 and SNP309 on Sp1 transcription factor binding and cancer risk. Transcription 2011, 2, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Kikuno, N.; Kawamoto, K.; Suehiro, Y.; Tanaka, Y.; Dahiya, R. MDM2 SNP309 polymorphism as risk factor for susceptibility and poor prognosis in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Su, C.T.; Chu, J.S.; Huang, S.P.; Pu, Y.S.; Yang, H.Y.; Chung, C.J.; Wu, C.C.; Hsueh, Y.M. The polymorphisms of P53 codon 72 and MDM2 SNP309 and renal cell carcinoma risk in a low arsenic exposure area. Toxicol. Appl. Pharmacol. 2011, 257, 349–355. [Google Scholar] [CrossRef] [PubMed]
- de Martino, M.; Taus, C.; Wessely, I.S.; Lucca, I.; Hofbauer, S.L.; Haitel, A.; Shariat, S.F.; Klatte, T. The T309G murine double minute 2 gene polymorphism is an independent prognostic factor for patients with renal cell carcinoma. DNA Cell Biol. 2015, 34, 107–112. [Google Scholar] [CrossRef]
- Haitel, A.; Wiener, H.G.; Baethge, U.; Marberger, M.; Susani, M. mdm2 expression as a prognostic indicator in clear cell renal cell carcinoma: Comparison with p53 overexpression and clinicopathological parameters. Clin. Cancer Res. 2000, 6, 1840–1844. [Google Scholar] [PubMed]
- Yang, M.D.; Lin, K.C.; Lu, M.C.; Jeng, L.B.; Hsiao, C.L.; Yueh, T.C.; Fu, C.K.; Li, H.T.; Yen, S.T.; Lin, C.W.; et al. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine 2017, 7, 10. [Google Scholar] [CrossRef]
- Deng, Y.; Ke, T.W.; Yueh, T.C.; Chin, Y.T.; Wang, Y.C.; Hung, Y.C.; Mong, M.C.; Yang, Y.C.; Wu, W.T.; Chang, W.S.; et al. The Contribution of DNA Ligase 4 Polymorphisms to Colorectal Cancer. Vivo 2024, 38, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.R.; Ma, H.H.; Chang, C.H.; Liao, C.H.; Chang, W.S.; Mong, M.C.; Yang, Y.C.; Gu, J.; Bau, D.T.; Tsai, C.W. Contribution of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 to Upper Tract Urothelial Cancer Risk in Taiwan. Life 2024, 14, 801. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Tsai, C.L.; Chin, Y.T.; Wang, Y.C.; Liu, C.H.; Lin, M.L.; Chen, S.S.; He, J.L.; Tsai, C.W.; Su, C.H.; et al. Impacts of Matrix Metalloproteinase-2 Promoter Genotypes on Breast Cancer Risk. Cancer Genom. Proteom. 2024, 21, 502–510. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, W.S.; Pei, J.S.; Kuo, C.C.; Wang, C.H.; Wang, Y.C.; Hsu, P.C.; He, J.L.; Gu, J.; Bau, D.T.; et al. Non-homologous End-joining Genotype, mRNA Expression, and DNA Repair Capacity in Childhood Acute Lymphocytic Leukemia. Cancer Genom. Proteom. 2024, 21, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Arreola, M.P.; Marquez-Rosales, M.G.; Sanchez-Corona, J.; Figuera, L.E.; Zuniga-Gonzalez, G.; Puebla-Perez, A.M.; Delgado-Saucedo, J.I.; Montoya-Fuentes, H. Association of the Del1518 Promoter (rs3730485) Polymorphism in the MDM2 Gene with Breast Cancer in a Mexican Population. Ann. Clin. Lab Sci. 2017, 47, 291–297. [Google Scholar]
- Okamoto, K.; Tsunematsu, R.; Tahira, T.; Sonoda, K.; Asanoma, K.; Yagi, H.; Yoneda, T.; Hayashi, K.; Wake, N.; Kato, K. SNP55, a new functional polymorphism of MDM2-P2 promoter, contributes to allele-specific expression of MDM2 in endometrial cancers. BMC Med. Genet. 2015, 16, 67. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Zhu, H.; Fu, G.; Wang, S.; Wu, D.; Zhou, J.; Wei, Q.; Zhang, Z. A novel functional polymorphism C1797G in the MDM2 promoter is associated with risk of bladder cancer in a Chinese population. Clin. Cancer Res. 2008, 14, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, S.; Ye, H.; Li, J.; Du, Y.; Chen, L.; Liu, X.; Ding, Y.; Li, Q.; Mao, Y.; et al. Association of p53 rs1042522, MDM2 rs2279744, and p21 rs1801270 polymorphisms with retinoblastoma risk and invasion in a Chinese population. Sci. Rep. 2015, 5, 13300. [Google Scholar] [CrossRef]
- Chen, X.; Sturgis, E.M.; Lei, D.; Dahlstrom, K.; Wei, Q.; Li, G. Human papillomavirus seropositivity synergizes with MDM2 variants to increase the risk of oral squamous cell carcinoma. Cancer Res. 2010, 70, 7199–7208. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, J.J.; van Marion, R.; Tilanus, H.W.; Dinjens, W.N. Functional polymorphisms associated with disease-free survival in resected carcinoma of the esophagus. J. Gastrointest. Surg. 2011, 15, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.K.; Guo, S.; Yang, F.; Zhao, L.S.; Wang, L.D. MDM2 and its functional polymorphism SNP309 contribute to the development of esophageal carcinoma. J. Gene Med. 2019, 21, e3086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, X.; Guo, Y.; Tan, W.; Zhou, Y.; Sun, T.; Wang, Y.; Lin, D. Genetic polymorphisms in cell cycle regulatory genes MDM2 and TP53 are associated with susceptibility to lung cancer. Hum. Mutat. 2006, 27, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wheatley-Price, P.; Zhou, W.; Park, S.; Heist, R.S.; Asomaning, K.; Wain, J.C.; Lynch, T.J.; Su, L.; Christiani, D.C. Genetic polymorphisms of MDM2, cumulative cigarette smoking and nonsmall cell lung cancer risk. Int. J. Cancer 2008, 122, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ren, B.; Hu, Z.; Chen, J.; Hu, L.; Dai, J.; Jin, G.; Xu, L.; Shen, H. MDM2 SNP309 contributes to non-small cell lung cancer survival in Chinese. Mol. Carcinog. 2011, 50, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, Y.; Gao, C.; Wei, Y.; Cao, G.; Lu, N.; Hou, Y.; Jiang, X.; Wang, J. Polymorphisms of p53 and MDM2 genes are associated with severe toxicities in patients with non-small cell lung cancer. Cancer Biol. Ther. 2014, 15, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, L.; Cui, J.; Ma, K.; Chen, X.; Li, W. Mouse double minute-2 homolog (MDM2)-rs2279744 polymorphism associated with lung cancer risk in a Northeastern Chinese population. Thorac. Cancer 2015, 6, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, W.S.; Shen, T.C.; Li, H.T.; Li, C.H.; Hsiau, Y.C.; Wang, Y.C.; Wu, C.N.; Gong, C.L.; Wang, Z.H.; et al. Association of Murine Double Minute 2 Genotypes and Lung Cancer Risk. Vivo 2020, 34, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Reza, H.A.; Anamika, W.J.; Chowdhury, M.M.K.; Mostafa, M.G.; Uddin, M.A. A cohort study on the association of MDM2 SNP309 with lung cancer risk in Bangladeshi population. Korean J. Intern. Med. 2020, 35, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Tas, A.; Donmez, G.; Kacan, T.; Silig, Y. Significant Association of the MDM2 T309G Polymorphism with Breast Cancer Risk in a Turkish Population. Asian Pac. J. Cancer Prev. 2018, 19, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Floris, M.; Pira, G.; Castiglia, P.; Idda, M.L.; Steri, M.; De Miglio, M.R.; Piana, A.; Cossu, A.; Azara, A.; Arru, C.; et al. Impact on breast cancer susceptibility and clinicopathological traits of common genetic polymorphisms in TP53, MDM2 and ATM genes in Sardinian women. Oncol. Lett. 2022, 24, 331. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Ho, B.; Yang, Y.; Huang, Z.; Zhang, Z.; Zhang, G. Interaction of Helicobacter pylori with genetic variants in the MDM2 promoter, is associated with gastric cancer susceptibility in Chinese patients. Helicobacter 2009, 14, 114–119. [Google Scholar] [CrossRef]
- Elingarami, S.; Liu, H.; Kalinjuma, A.V.; Hu, W.; Li, S.; He, N. Polymorphisms in NEIL-2, APE-1, CYP2E1 and MDM2 Genes are Independent Predictors of Gastric Cancer Risk in a Northern Jiangsu Population (China). J. Nanosci. Nanotechnol. 2015, 15, 4815–4828. [Google Scholar] [CrossRef]
- Tas, A.; Atabey, M.; Caglayan, G.; Bostanci, M.E.; Sahin Bolukbasi, S.; Topcu, O.; Silig, Y. Investigation of the association between the MDM2 T309G polymorphism and gastric cancer. Biomed. Rep. 2017, 7, 469–473. [Google Scholar] [CrossRef]
- Akkiz, H.; Sumbul, A.T.; Bayram, S.; Bekar, A.; Akgollu, E. MDM2 promoter polymorphism is associated with increased susceptibility to hepatocellular carcinoma in Turkish population. Cancer Epidemiol. 2010, 34, 448–452. [Google Scholar] [CrossRef]
- Di Vuolo, V.; Buonaguro, L.; Izzo, F.; Losito, S.; Botti, G.; Buonaguro, F.M.; Tornesello, M.L. TP53 and MDM2 gene polymorphisms and risk of hepatocellular carcinoma among Italian patients. Infect. Agent. Cancer 2011, 6, 13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ezzikouri, S.; Essaid El Feydi, A.; Afifi, R.; Benazzouz, M.; Hassar, M.; Pineau, P.; Benjelloun, S. Impact of TP53 codon 72 and MDM2 promoter 309 allelic dosage in a Moroccan population with hepatocellular carcinoma. Int. J. Biol. Markers 2011, 26, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, M.; Gu, D.; Zhu, L.; Chu, H.; Tong, N.; Zhang, Z.; Xu, Z.; Wang, M. MDM2 SNP309 polymorphism is associated with colorectal cancer risk. Sci. Rep. 2014, 4, 4851. [Google Scholar] [CrossRef] [PubMed]
- Yueh, T.C.; Hung, Y.W.; Shih, T.C.; Wu, C.N.; Wang, S.C.; Lai, Y.L.; Hsu, S.W.; Wu, M.H.; Fu, C.K.; Wang, Y.C.; et al. Contribution of Murine Double Minute 2 Genotypes to Colorectal Cancer Risk in Taiwan. Cancer Genom. Proteom. 2018, 15, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Atabey, M.; Tas, A.; Sari, I.; Bostanci, M.E.; Topcu, O.; Silig, Y. Significant association between MDM2 T309G polymorphism and colorectal cancer. J. BUON 2019, 24, 1137–1142. [Google Scholar] [PubMed]
- Ashton, K.A.; Proietto, A.; Otton, G.; Symonds, I.; McEvoy, M.; Attia, J.; Gilbert, M.; Hamann, U.; Scott, R.J. Polymorphisms in TP53 and MDM2 combined are associated with high grade endometrial cancer. Gynecol. Oncol. 2009, 113, 109–114. [Google Scholar] [CrossRef]
- Zajac, A.; Smolarz, B.; Stachowiak, G.; Wilczynski, J.R. TP53 and MDM2 polymorphisms and the risk of endometrial cancer in postmenopausal women. Med. Oncol. 2014, 31, 286. [Google Scholar] [CrossRef][Green Version]
- Gansmo, L.B.; Bjornslett, M.; Halle, M.K.; Salvesen, H.B.; Romundstad, P.; Hveem, K.; Vatten, L.; Dorum, A.; Lonning, P.E.; Knappskog, S. MDM2 promoter polymorphism del1518 (rs3730485) and its impact on endometrial and ovarian cancer risk. BMC Cancer 2017, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Luo, X. The association between murine double minute 2 (MDM2) rs2279744 and endometrial cancer risk in a Chinese Han population. Cell Mol. Biol. 2017, 63, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Hussain, S.; Thakur, N.; Batra, S.; Salhan, S.; Bhambani, S.; Bharadwaj, M. Association of MDM2 and p53 polymorphisms with the advancement of cervical carcinoma. DNA Cell Biol. 2013, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sivonova, M.K.; Jurecekova, J.; Kaplan, P.; Hives, M.; Grendar, M.; Tomascova, A.; Dusenka, R.; Drobkova, H.; Evin, D.; Kliment, J. Association of MDM2 T309G (rs2279744) Polymorphism and Expression Changes With Risk of Prostate Cancer in the Slovak Population. Anticancer Res. 2020, 40, 6257–6264. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.V.; Autuori, I.; Luo, L.; LaPilla, E.; Yoo, S.; Sharma, A.; Busam, K.J.; Olilla, D.W.; Dwyer, T.; Anton-Culver, H.; et al. Sex-Specific Associations of MDM2 and MDM4 Variants with Risk of Multiple Primary Melanomas and Melanoma Survival in Non-Hispanic Whites. Cancers 2023, 15, 2707. [Google Scholar] [CrossRef] [PubMed]
- Do, T.N.; Ucisik-Akkaya, E.; Davis, C.F.; Morrison, B.A.; Dorak, M.T. TP53 R72P and MDM2 SNP309 polymorphisms in modification of childhood acute lymphoblastic leukemia susceptibility. Cancer Genet. Cytogenet. 2009, 195, 31–36. [Google Scholar] [CrossRef]
- Bartnykaite, A.; Savukaityte, A.; Ugenskiene, R.; Dauksaite, M.; Korobeinikova, E.; Gudaitiene, J.; Juozaityte, E. Associations of MDM2 and MDM4 Polymorphisms with Early-Stage Breast Cancer. J. Clin. Med. 2021, 10, 866. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Jiang, Z.; Wu, Y.; Chen, X.; Xiao, X.; Yu, H. A Functional Polymorphism (rs937283) in the MDM2 Promoter Region is Associated with Poor Prognosis of Retinoblastoma in Chinese Han Population. Sci. Rep. 2016, 6, 31240. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, L.; Wei, Q.; Song, X.; Sturgis, E.M.; Li, G. Significance of MDM2 and P14 ARF polymorphisms in susceptibility to differentiated thyroid carcinoma. Surgery 2013, 153, 711–717. [Google Scholar] [CrossRef]
- Shebli, W.T.Y.; Alotibi, M.K.H.; Al-Raddadi, R.I.; Al-Amri, R.J.; Fallatah, E.I.Y.; Alhujaily, A.S.; Mohamed, H.S. Murine Double Minute 2 Gene (MDM2) rs937283A/G variant significantly increases the susceptibility to breast cancer in Saudi Women. Saudi J. Biol. Sci. 2021, 28, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, J.; Wang, J.; Zhang, J.; Gu, X.; Feng, X. The Study of MDM2 rs937283 Variant and Cancer Susceptibility in a Central Chinese Population. Technol. Cancer Res. Treat. 2018, 17, 1533033818801550. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, J.; Chen, Y.; Gu, X.; Feng, X. The MDM2 rs937283 A > G variant significantly increases the risk of lung and gastric cancer in Chinese population. Int. J. Clin. Oncol. 2018, 23, 867–876. [Google Scholar] [CrossRef]
- Ahmad, D.; Bakairy, A.K.; Katheri, A.M.; Tamimi, W. MDM2 (RS769412) G>A Polymorphism in Cigarette Smokers: A Clue for the Susceptibility to Smoking and Lung Cancer Risk. Asian Pac. J. Cancer Prev. 2015, 16, 4057–4060. [Google Scholar] [CrossRef]
- Sapkota, Y.; Mackey, J.R.; Lai, R.; Franco-Villalobos, C.; Lupichuk, S.; Robson, P.J.; Kopciuk, K.; Cass, C.E.; Yasui, Y.; Damaraju, S. Assessing SNP-SNP interactions among DNA repair, modification and metabolism related pathway genes in breast cancer susceptibility. PLoS ONE 2014, 8, e64896. [Google Scholar] [CrossRef] [PubMed]
- Gazdzicka, J.; Golabek, K.; Strzelczyk, J.K.; Kiczmer, P.; Miskiewicz-Orczyk, K.; Krakowczyk, L.; Ostrowska, Z.; Misiolek, M. Selected CDKN2A and MDM2 polymorphisms in oral cavity cancer. Acta Biochim. Pol. 2020, 67, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Bartpho, T.S.; Wattanawongdon, W.; Tongtawee, T. The mouse double minute 2 polymorphism is associated with both decreased p53 expression and poor clinicopathological outcomes of gastric cancer. J. Cancer Res. Ther. 2021, 17, 1438–1445. [Google Scholar] [CrossRef]
- Dang, J.; Kuo, M.L.; Eischen, C.M.; Stepanova, L.; Sherr, C.J.; Roussel, M.F. The RING domain of Mdm2 can inhibit cell proliferation. Cancer Res. 2002, 62, 1222–1230. [Google Scholar] [PubMed]
- Fenton, M.; Borcherds, W.; Chen, L.; Anbanandam, A.; Levy, R.; Chen, J.; Daughdrill, G. The MDMX Acidic Domain Uses Allovalency to Bind Both p53 and MDMX. J. Mol. Biol. 2022, 434, 167844. [Google Scholar] [CrossRef]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef]
- Li, F.; Aljahdali, I.A.M.; Zhang, R.; Nastiuk, K.L.; Krolewski, J.J.; Ling, X. Kidney cancer biomarkers and targets for therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 254. [Google Scholar] [CrossRef]
- Klein, A.M.; de Queiroz, R.M.; Venkatesh, D.; Prives, C. The roles and regulation of MDM2 and MDMX: It is not just about p53. Genes Dev. 2021, 35, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef]
- Basu, S.; Murphy, M.E. Genetic Modifiers of the p53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026302. [Google Scholar] [CrossRef]
- Carroll, V.A.; Ashcroft, M. Regulation of angiogenic factors by HDM2 in renal cell carcinoma. Cancer Res. 2008, 68, 545–552. [Google Scholar] [CrossRef]
- Twarda-Clapa, A. An update patent review of MDM2-p53 interaction inhibitors (2019–2023). Expert Opin. Ther. Pat. 2024, 34, 1177–1198. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Cases (n = 135) | Controls (n = 590) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age, mean ± SD | 59.0 ± 9.6 | 58.2 ± 9.9 | 0.4769 | ||
| ≤60 Years | 70 | 51.7% | 310 | 52.5% | 0.9606 |
| >60 Years | 65 | 48.3% | 280 | 47.5% | |
| Sex | |||||
| Male | 86 | 64.4% | 380 | 64.4% | 0.9567 |
| Female | 49 | 35.6% | 210 | 35.6% | |
| Smoking status | |||||
| Smoker | 55 | 40.7% | 229 | 38.8% | 0.7519 |
| Non-smoker | 80 | 59.3% | 361 | 61.2% | |
| Alcohol drinking status | |||||
| Drinker | 54 | 41.5% | 217 | 36.8% | 0.5491 |
| Non-drinker | 81 | 58.5% | 373 | 63.2% | |
| Hypertension | |||||
| Yes | 91 | 66.9% | 296 | 50.2% | 0.0004 * |
| No | 44 | 33.1% | 294 | 49.8% | |
| Diabetes | |||||
| Yes | 33 | 22.0% | 108 | 18.3% | 0.1323 |
| No | 102 | 78.0% | 482 | 81.7% | |
| Family history cancer | |||||
| Yes | 14 | 9.3% | 18 | 3.1% | 0.0005 * |
| No | 121 | 90.7% | 572 | 96.9% | |
| Histological type | |||||
| Clear-cell | 105 | 77.1% | |||
| Non-clear-cell | 30 | 22.9% | |||
| Histological grade | |||||
| Low | 72 | 53.4% | |||
| Middle and high | 63 | 46.6% | |||
| Polymorphisms | Primer Sequences | RE | Polymorphic Genotype | DNA Fragment Size (bp) |
|---|---|---|---|---|
| rs937282 | F: 5′-GGTAACAGCGACACGGAGAT-3′ R: 5′-CGCATCCGGGCATTTGTGC-3′ | Hyp188I | C G | 307 182 + 125 |
| rs937283 | F: 5′-CGGATTAGTGCGTACGAGCG-3′ R: 5′-TCAGAGCCCAGACCCAAAAG-3′ | Sau3AI | G A | 202 154 + 48 |
| rs2279744 | F: 5′-TTCGCAGCCTTTGTGCGGTT-3′ R: 5′-GAACGTGTCTGAACTTGACC-3′ | MspA1I | T G | 368 223 + 145 |
| rs769412 | F: 5′-GGTTACAGAAACTGACTGTG-3′ R: 5′-CACATCTTCTTGGCTGCTAT-3′ | BbvCI | A G | 371 196 + 175 |
| Polymorphism | Genotype | Cases | Controls | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| rs937282 | CC | 74 (54.8%) | 320 (54.2%) | 1.00 (Ref) | |
| CG | 47 (34.8%) | 221 (37.5%) | 0.7610 | 0.92 (0.61–1.38) | |
| GG | 14 (10.4%) | 49 (8.3%) | 0.6376 | 1.24 (0.65–2.36) | |
| Ptrend | 0.6861 | ||||
| PHWE | 0.2197 | ||||
| CG + GG | 61 (45.2%) | 270 (45.8%) | 0.9795 | 0.98 (0.67–1.42) | |
| rs937283 | AA | 73 (54.1%) | 331 (56.1%) | 1.00 (Ref) | |
| AG | 50 (37.0%) | 219 (37.1%) | 0.9454 | 1.04 (0.70–1.54) | |
| GG | 12 (8.9%) | 40 (6.8%) | 0.4942 | 1.36 (0.68–2.72) | |
| Ptrend | 0.6829 | ||||
| PHWE | 0.6448 | ||||
| AG + GG | 62 (45.9%) | 259 (43.9%) | 0.7400 | 1.09 (0.75–1.58) | |
| rs2279744 | TT | 21 (15.5%) | 136 (23.0%) | 1.00 (Ref) | |
| GT | 61 (45.2%) | 293 (49.7%) | 0.3346 | 1.35 (0.79–2.30) | |
| GG | 53 (39.3%) | 161 (27.3%) | 0.0098 * | 2.13 (1.22–3.71) | |
| Ptrend | 0.0133 * | ||||
| PHWE | 0.9035 | ||||
| GT + GG | 114 (84.5%) | 454 (77.0%) | 0.0732 | 1.63 (0.98–2.69) | |
| rs769412 | AA | 130 (96.3%) | 561 (95.1%) | 1.00 (Ref) | |
| AG | 5 (3.7%) | 28 (4.7%) | 0.7650 | 0.77 (0.29–2.03) | |
| GG | 0 (0.0%) | 1 (0.2%) | 1.0000 | -- | |
| Ptrend | 0.7758 | ||||
| PHWE | 0.3040 | ||||
| AG + GG | 5 (3.7%) | 29 (4.9%) | 0.7076 | 0.74 (0.28–1.96) |
| Allelic Type | Cases | Controls | p-Value | OR (95% CI) |
|---|---|---|---|---|
| rs937282 | ||||
| C | 195 (72.2%) | 861 (73.0%) | 1.00 (Ref) | |
| G | 75 (27.8%) | 319 (27.0%) | 0.8634 | 1.04 (0.77–1.40) |
| rs937283 | ||||
| A | 196 (72.6%) | 881 (74.7%) | 1.00 (Ref) | |
| G | 74 (27.4%) | 299 (25.3%) | 0.5325 | 1.11 (0.83–1.50) |
| rs2279744 | ||||
| T | 103 (38.1%) | 565 (47.9%) | 1.00 (Ref) | |
| G | 167 (61.9%) | 615 (52.1%) | 0.0047 * | 1.49 (1.14–1.95) |
| rs769412 | ||||
| A | 265 (98.1%) | 1150 (97.5%) | 1.00 (Ref) | |
| G | 5 (1.9%) | 30 (2.5%) | 0.6548 | 0.72 (0.28–1.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-Y.; Chang, W.-S.; Shih, H.-Y.; Chang, C.-H.; Wu, H.-C.; Tsai, C.-W.; Wang, Y.-C.; Gu, J.; Bau, D.-T. Genetic Variations in MDM2 Gene Contribute to Renal Cell Carcinoma Susceptibility: A Genotype–Phenotype Correlation Study. Cancers 2025, 17, 177. https://doi.org/10.3390/cancers17020177
Chang S-Y, Chang W-S, Shih H-Y, Chang C-H, Wu H-C, Tsai C-W, Wang Y-C, Gu J, Bau D-T. Genetic Variations in MDM2 Gene Contribute to Renal Cell Carcinoma Susceptibility: A Genotype–Phenotype Correlation Study. Cancers. 2025; 17(2):177. https://doi.org/10.3390/cancers17020177
Chicago/Turabian StyleChang, Shu-Yu, Wen-Shin Chang, Hou-Yu Shih, Chao-Hsiang Chang, Hsi-Chin Wu, Chia-Wen Tsai, Yun-Chi Wang, Jian Gu, and Da-Tian Bau. 2025. "Genetic Variations in MDM2 Gene Contribute to Renal Cell Carcinoma Susceptibility: A Genotype–Phenotype Correlation Study" Cancers 17, no. 2: 177. https://doi.org/10.3390/cancers17020177
APA StyleChang, S.-Y., Chang, W.-S., Shih, H.-Y., Chang, C.-H., Wu, H.-C., Tsai, C.-W., Wang, Y.-C., Gu, J., & Bau, D.-T. (2025). Genetic Variations in MDM2 Gene Contribute to Renal Cell Carcinoma Susceptibility: A Genotype–Phenotype Correlation Study. Cancers, 17(2), 177. https://doi.org/10.3390/cancers17020177






