A Scoping Review of Primary Breast Cancer Risk Reduction Strategies in East and Southeast Asia
Simple Summary
Abstract
1. Introduction
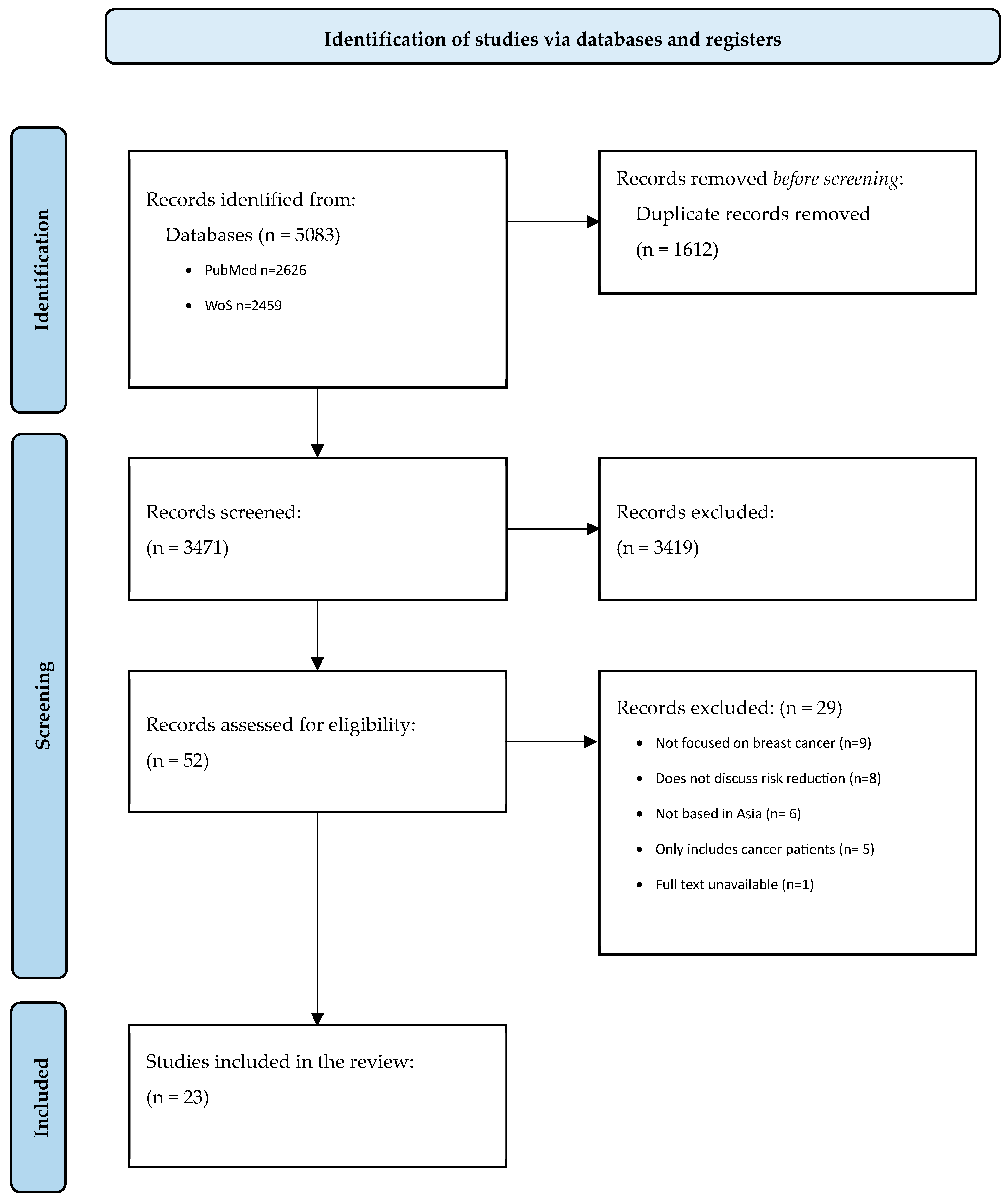
2. Methods
2.1. Search Strategy
2.2. Eligibility and Study Selection
2.3. Data Extraction and Analysis
3. Results
3.1. Scope of Studies
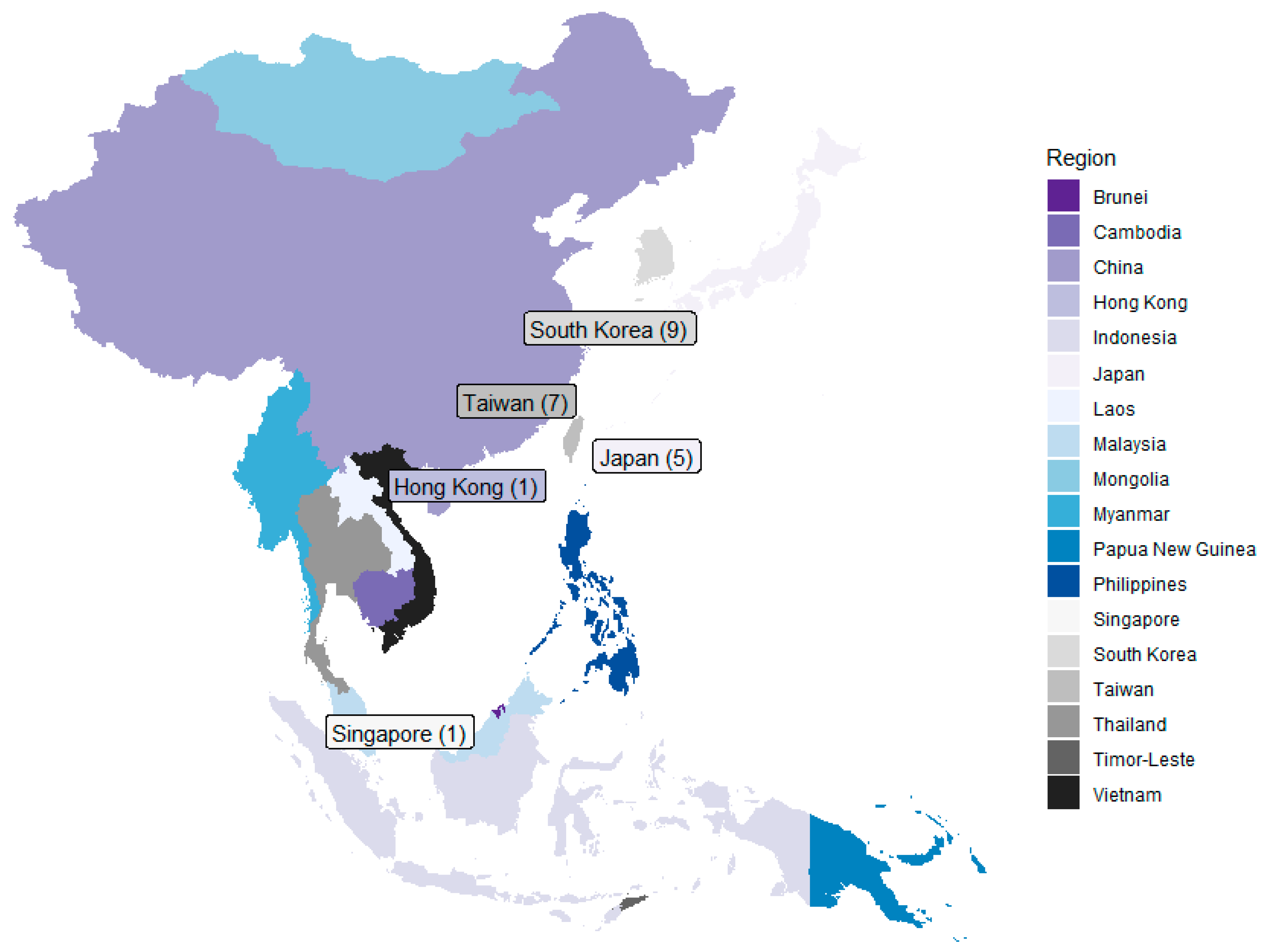
3.2. Geographical Distribution
3.3. Study Designs and Methodologies
3.4. High-Risk Groups Targeted
3.5. Prophylactic Surgery
3.6. Chemopreventive Agents
4. Discussion
4.1. Prevalence of Surgical Risk Reduction over Chemoprevention
4.2. Factors Influencing Uptake of Risk-Reducing Surgery Versus Chemoprevention
4.3. Differences in the Definition of High Risk
4.4. Gaps and Opportunities for Research
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A. Randomized Controlled Mammography Screening Trials Revisited. J. Breast Imaging 2022, 4, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Allweis, T.M.; Hermann, N.; Berenstein-Molho, R.; Guindy, M. Personalized Screening for Breast Cancer: Rationale, Present Practices, and Future Directions. Ann. Surg. Oncol. 2021, 28, 4306–4317. [Google Scholar] [CrossRef]
- Lim, Y.X.; Lim, Z.L.; Ho, P.J.; Li, J. Breast Cancer in Asia: Incidence, Mortality, Early Detection, Mammography Programs, and Risk-Based Screening Initiatives. Cancers 2022, 14, 4218. [Google Scholar] [CrossRef]
- Clift, A.K.; Dodwell, D.; Lord, S.; Petrou, S.; Brady, S.M.; Collins, G.S.; Hippisley-Cox, J. The current status of risk-stratified breast screening. Br. J. Cancer 2022, 126, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Grimm, L.J.; Avery, C.S.; Hendrick, E.; Baker, J.A. Benefits and Risks of Mammography Screening in Women Ages 40 to 49 Years. J. Prim. Care Community Health 2022, 13, 21501327211058322. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Lee, C.I.; Wang, P.C.; Hsu, W.; Brentnall, A.R.; Hoyt, A.C.; Naeim, A.; Elmore, J.G. Variability Among Breast Cancer Risk Classification Models When Applied at the Level of the Individual Woman. J. Gen. Intern. Med. 2023, 38, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.H.; Wang, Q.; Bolla, M.K.; et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet. 2019, 104, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, M.; Eriksson, M.; Hammarstrom, M.; Borgquist, S.; Leifland, K.; Czene, K.; Hall, P. Cohort Profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int. J. Epidemiol. 2017, 46, 1740–1741g. [Google Scholar] [CrossRef] [PubMed]
- Mars, N.; Kerminen, S.; Tamlander, M.; Pirinen, M.; Jakkula, E.; Aaltonen, K.; Meretoja, T.; Heinavaara, S.; Widen, E.; Ripatti, S.; et al. Comprehensive Inherited Risk Estimation for Risk-Based Breast Cancer Screening in Women. J. Clin. Oncol. 2024, 42, 1477–1487. [Google Scholar] [CrossRef]
- Liu, J.; Ho, P.J.; Tan, T.H.L.; Yeoh, Y.S.; Chew, Y.J.; Mohamed Riza, N.K.; Khng, A.J.; Goh, S.A.; Wang, Y.; Oh, H.B.; et al. BREAst screening Tailored for HEr (BREATHE)-A study protocol on personalised risk-based breast cancer screening programme. PLoS ONE 2022, 17, e0265965. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Bibbins-Domingo, K. Toward Risk-Based Breast Cancer Screening. Ann. Intern. Med. 2021, 174, 710–711. [Google Scholar] [CrossRef]
- Esserman, L.; Eklund, M.; Veer, L.V.; Shieh, Y.; Tice, J.; Ziv, E.; Blanco, A.; Kaplan, C.; Hiatt, R.; Fiscalini, A.S.; et al. The WISDOM study: A new approach to screening can and should be tested. Breast Cancer Res. Treat. 2021, 189, 593–598. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Hindmarch, S.; French, D.P.; Tischkowitz, M.; Moorthie, S.; Walter, F.M.; Dennison, R.A.; Stutzin Donoso, F.; Archer, S.; Taylor, L.; et al. Proactive breast cancer risk assessment in primary care: A review based on the principles of screening. Br. J. Cancer 2023, 128, 1636–1646. [Google Scholar] [CrossRef]
- Esserman, L.J.; Study, W.; Athena, I. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 2017, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.; Eklund, M.; Madlensky, L.; Sawyer, S.D.; Thompson, C.K.; Stover Fiscalini, A.; Ziv, E.; Van’t Veer, L.J.; Esserman, L.J.; Tice, J.A.; et al. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J. Natl. Cancer Inst. 2017, 109, djw290. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Nabi, H.H.; Andrulis, I.L.; Antoniou, A.C.; Chiquette, J.; Despres, P.; Devilee, P.; Dorval, M.; Droit, A.; Easton, D.F.; et al. Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). J. Pers. Med. 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Pace, L.E. Risk-Based Approaches to Breast Cancer Screening in China. JAMA Netw. Open 2022, 5, e2241448. [Google Scholar] [CrossRef]
- Bolze, A.; Cirulli, E.T.; Hajek, C.; Schnell Blitstein, J.M.; Grzymski, J.J. The Potential of Genetics in Identifying Women at Lower Risk of Breast Cancer. JAMA Oncol. 2024, 10, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; van den Hout, A.; Antoniou, A.C.; Shah, M.; Ficorella, L.; Steggall, E.; Easton, D.F.; Pharoah, P.D.P.; Pashayan, N. Estimation of age of onset and progression of breast cancer by absolute risk dependent on polygenic risk score and other risk factors. Cancer 2024, 130, 1590–1599. [Google Scholar] [CrossRef]
- Kelley-Jones, C.; Scott, S.; Waller, J. UK Women’s Views of the Concepts of Personalised Breast Cancer Risk Assessment and Risk-Stratified Breast Screening: A Qualitative Interview Study. Cancers 2021, 13, 5813. [Google Scholar] [CrossRef]
- Liow, J.J.K.; Lim, Z.L.; Sim, T.M.Y.; Ho, P.J.; Goh, S.A.; Choy, S.D.; Chew, Y.J.; Tan, B.K.; Tan, V.K.M.; Hartman, M.; et al. “It Will Lead You to Make Better Decisions about Your Health”-A Focus Group and Survey Study on Women’s Attitudes towards Risk-Based Breast Cancer Screening and Personalised Risk Assessments. Curr. Oncol. 2022, 29, 9181–9198. [Google Scholar] [CrossRef] [PubMed]
- Rainey, L.; van der Waal, D.; Jervaeus, A.; Wengstrom, Y.; Evans, D.G.; Donnelly, L.S.; Broeders, M.J.M. Are we ready for the challenge of implementing risk-based breast cancer screening and primary prevention? Breast 2018, 39, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wenger, N.; Stanton, A.L.; Sepucha, K.; Kaplan, C.; Madlensky, L.; Elashoff, D.; Trent, J.; Petruse, A.; Johansen, L.; et al. Risk estimation, anxiety, and breast cancer worry in women at risk for breast cancer: A single-arm trial of personalized risk communication. Psychooncology 2019, 28, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lowry, K.P.; Cott Chubiz, J.E.; Swan, J.S.; Motazedi, T.; Halpern, E.F.; Tosteson, A.N.A.; Gazelle, G.S.; Donelan, K. Breast cancer risk, worry, and anxiety: Effect on patient perceptions of false-positive screening results. Breast 2020, 50, 104–112. [Google Scholar] [CrossRef]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Bonanni, B.; Costantino, J.P.; Cummings, S.; DeCensi, A.; Dowsett, M.; Forbes, J.F.; Ford, L.; LaCroix, A.Z.; et al. Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet 2013, 381, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Fu, R.; Griffin, J.C.; Nygren, P.; Smith, M.E.; Humphrey, L. Systematic review: Comparative effectiveness of medications to reduce risk for primary breast cancer. Ann. Intern. Med. 2009, 151, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Laws, A.; Punglia, R.S. Endocrine Therapy for Primary and Secondary Prevention After Diagnosis of High-Risk Breast Lesions or Preinvasive Breast Cancer. J. Clin. Oncol. 2023, 41, 3092–3099. [Google Scholar] [CrossRef]
- Cuzick, J. Preventive therapy for cancer. Lancet Oncol. 2017, 18, e472–e482. [Google Scholar] [CrossRef]
- Hammarstrom, M.; Gabrielson, M.; Crippa, A.; Discacciati, A.; Eklund, M.; Lundholm, C.; Backlund, M.; Wengstrom, Y.; Borgquist, S.; Bergqvist, J.; et al. Side effects of low-dose tamoxifen: Results from a six-armed randomised controlled trial in healthy women. Br. J. Cancer 2023, 129, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Puntoni, M.; Provinciali, N.; Webber, T.B.; Briata, I.M.; D’Amico, M.; Giuliano, S.; Siri, G.; Cagnacci, S.; DeCensi, A. Estimating the magnitude of clinical benefit of systemic therapy in patients with DCIS or pre-invasive disease of the breast. Breast 2019, 48 (Suppl. S1), S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Saldanha, P. Risk Reduction Strategies in Breast Cancer Prevention. Eur. J. Breast Health 2017, 13, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Carbine, N.E.; Lostumbo, L.; Wallace, J.; Ko, H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 2018, 4, CD002748. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Norquist, B.M.; Swisher, E.M.; Yung, R.L. Preventing Breast Cancer in High-Risk Women: Is There Still a Role for Oophorectomy? JNCI Cancer Spectr. 2020, 4, pkz076. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer 2021, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Alsayer, R.M.; De Vol, E.B.; Almeharish, A.; Alfattani, A.; Alghamdi, A.J.; AlBehlal, L.B.; Alhaddab, S.; Altwaijri, Y. Ranking of Modifiable Lifestyle Risk Factors for Breast Cancer in Saudi Women: Population Attributable Risk and Nomogram. Breast Cancer 2024, 16, 545–554. [Google Scholar] [CrossRef]
- Neirich, L.; Yahiaoui-Doktor, M.; Lammert, J.; Basrai, M.; Seethaler, B.; Berling-Ernst, A.; Ramser, J.; Quante, A.S.; Schmidt, T.; Niederberger, U.; et al. Physical activity and Mediterranean diet as potential modulators of osteoprotegerin and soluble RANKL in gBRCA1/2 mutation carriers: Results of the lifestyle intervention pilot study LIBRE-1. Breast Cancer Res. Treat. 2021, 190, 463–475. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Stoll, C.R.; Anandarajah, A.; Doering, M.; Colditz, G.A. Modifiable risk factors in women at high risk of breast cancer: A systematic review. Breast Cancer Res. 2023, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chu, Y.; Gao, Y.; Cheng, W.; Gao, S. Efficacy and safety of endocrine therapy for breast-cancer prevention in high-risk premenopausal or postmenopausal women: A Bayesian network meta-analysis of nine randomized controlled trials. Menopause 2021, 28, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.B.; Hui, E.P.; Mok, T.S. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol. 2010, 11, 75–84. [Google Scholar] [CrossRef]
- Yap, Y.S.; Lu, Y.S.; Tamura, K.; Lee, J.E.; Ko, E.Y.; Park, Y.H.; Cao, A.Y.; Lin, C.H.; Toi, M.; Wu, J.; et al. Insights Into Breast Cancer in the East vs the West: A Review. JAMA Oncol. 2019, 5, 1489–1496. [Google Scholar] [CrossRef]
- Leong, S.P.; Shen, Z.Z.; Liu, T.J.; Agarwal, G.; Tajima, T.; Paik, N.S.; Sandelin, K.; Derossis, A.; Cody, H.; Foulkes, W.D. Is breast cancer the same disease in Asian and Western countries? World J. Surg. 2010, 34, 2308–2324. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.Y.; Tham, W.Y.; Nei, W.L.; Lim, C.; Miao, H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun. 2018, 38, 39. [Google Scholar] [CrossRef]
- August, D.A.; Rea, T.; Sondak, V.K. Age-related differences in breast cancer treatment. Ann. Surg. Oncol. 1994, 1, 45–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ritter, M.; Ling, B.M.; Oberhauser, I.; Montagna, G.; Zehnpfennig, L.; Levy, J.; Soysal, S.D.; Castrezana, L.L.; Muller, M.; Schwab, F.D.; et al. The impact of age on patient-reported outcomes after oncoplastic versus conventional breast cancer surgery. Breast Cancer Res. Treat. 2021, 187, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Vuong, B.; Jacinto, A.I.; Chang, S.B.; Kuehner, G.E.; Savitz, A.C. Contemporary Review of the Management and Treatment of Young Breast Cancer Patients. Clin. Breast Cancer 2024, 24, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Francks, L.; Hassanein, Z.M.; Lee, R.; Wilson, E. Breast cancer surgical decision-making. Experiences of Non-Caucasian women globally. A qualitative systematic review. Eur. J. Surg. Oncol. 2023, 49, 107109. [Google Scholar] [CrossRef] [PubMed]
- Pons-Vigues, M.; Puigpinos-Riera, R.; Rodriguez, D.; Fernandez de Sanmamed, M.J.; Pasarin, M.I.; Perez, G.; Borrell, C.; Casamitjana, M.; Benet, J. Country of origin and prevention of breast cancer: Beliefs, knowledge and barriers. Health Place 2012, 18, 1270–1281. [Google Scholar] [CrossRef]
- Abdullah, N.; Mohamed, N. Influence of cultural practices on breast cancer risks, stage at presentation and outcome in a multi-ethnic developing country. Oncol. Lett. 2021, 22, 806. [Google Scholar] [CrossRef] [PubMed]
- van Strien-Knippenberg, I.S.; Arjangi-Babetti, H.; Timmermans, D.R.M.; Schrauwen, L.; Fransen, M.P.; Melles, M.; Damman, O.C. Communicating the results of risk-based breast cancer screening through visualizations of risk: A participatory design approach. BMC Med. Inform. Decis. Mak. 2024, 24, 78. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Abe, A.; Fusegi, A.; Yoshimitsu, T.; Misaka, S.; Murakami, A.; Matsumoto, T.; Tsumura, S.; Kanno, M.; Aoki, Y.; et al. Impact of the coverage of risk-reducing salpingo-oophorectomy by the national insurance system for women with BRCA pathogenic variants in Japan. Sci. Rep. 2023, 13, 1018. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Ryu, J.M.; Park, H.S.; Park, J.S.; Kang, E.; Lee, S.; Lee, H.B.; Youn, H.J.; Yoo, T.K.; Kim, J.; et al. Trends in Risk-Reducing Mastectomy and Risk-Reducing Salpingo-Oophorectomy in Korean Carriers of the BRCA1/2 Mutation. J. Breast Cancer 2020, 23, 647–655. [Google Scholar] [CrossRef]
- Datta, N.R.; Samiei, M.; Bodis, S. Radiation therapy infrastructure and human resources in low- and middle-income countries: Present status and projections for 2020. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Sobue, T.; Inoue, M.; Tanaka, H. Cancer Registry and Epidemiological Study Working Group report. Jpn. J. Clin. Oncol. 2010, 40 (Suppl. S1), i76–i81. [Google Scholar] [CrossRef]
- Lim, J.F.Y.; Zafra, M.; Mocanu, J.D.; Umareddy, I.; de Lima Lopes, G.; Foo, R.; Jha, A.; Hickinbotham, L. Preparing health systems in Southeast and East Asia for new paradigms of care/personalized medicine in cancers: Are health systems ready for evolving cancer management? J. Asian Public Policy 2016, 10, 268–286. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Momani, S.M.; Malkawi, L.W.; AlRyalat, S.A.S. Comparing Bibliometric Analysis Using PubMed, Scopus, and Web of Science Databases. J. Vis. Exp. 2019, 152, e58494. [Google Scholar] [CrossRef]
- Helbach, J.; Pieper, D.; Mathes, T.; Rombey, T.; Zeeb, H.; Allers, K.; Hoffmann, F. Restrictions and their reporting in systematic reviews of effectiveness: An observational study. BMC Med. Res. Methodol. 2022, 22, 230. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Moassefi, M.; Nakhostin-Ansari, A.; Hosseini Asl, S.H.; Saghab Torbati, M.; Aghajani, R.; Maleki Ghorbani, Z.; Faghani, S. Abstract screening using the automated tool Rayyan: Results of effectiveness in three diagnostic test accuracy systematic reviews. BMC Med. Res. Methodol. 2022, 22, 160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chae, S.; Kim, E.-K.; Jang, Y.R.; Lee, A.S.; Kim, S.K.; Suh, D.H.; Kim, K.; No, J.H.; Kim, Y.-B.; Kim, S.-W.; et al. Effect of risk-reducing salpingo-oophorectomy on the quality of life in Korean BRCA mutation carriers. Asian J. Surg. 2021, 44, 1056–1062. [Google Scholar] [CrossRef]
- Chang, Y.J.; Cho, S.; Joo, J.; Ryu, K.H.; Lee, S.; Cho, J.; Lim, M.C.; Jung, S.-Y.; Han, J.H.; Lee, E.S.; et al. Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey. J. Pers. Med. 2022, 12, 818. [Google Scholar] [CrossRef]
- Courtney, E.; Chin, X.W.; Yuen, J.; Li, S.-T.; Chen, Y.; Allen, J.C.; Tan, V.; Lim, G.H.; Ngeow, J. Risk management adherence following genetic testing for hereditary cancer syndromes: A Singaporean experience. Fam. Cancer 2018, 17, 621–626. [Google Scholar] [CrossRef]
- Jeon, H.J.; Park, H.S.; Park, J.S.; Nam, E.J.; Lee, S.-T.; Han, J. Trends in contralateral prophylactic mastectomy rate according to clinicopathologic and socioeconomic status. Ann. Surg. Treat. Res. 2019, 97. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Ohtani, S.; Iwasaki, M.; Yamamoto, S.; Takamatsu, K.; Okamura, H.; Arai, M.; Nomura, T.; Ozaki, S.; Shibata, K.-i.; et al. The Japanese Breast Cancer Society clinical practice guidelines for epidemiology and prevention of breast cancer, 2022 edition. Breast Cancer 2023, 31, 166–178. [Google Scholar] [CrossRef]
- Kim, D.; Kang, E.; Hwang, E.; Sun, Y.; Hwang, Y.; Yom, C.K.; Kim, K.; No, J.H.; Kim, Y.-B.; Kim, S.-W. Factors affecting the decision to undergo risk-reducing salpingo-oophorectomy among women with BRCA gene mutation. Fam. Cancer 2013, 12, 621–628. [Google Scholar] [CrossRef]
- Kim, D.U.; Min, J.W.; Kim, Y.M.; Chang, M.C. Estimating the risks and benefits of tamoxifen for prophylactic breast cancer chemoprevention in Korea. J. Breast Cancer 2012, 15, 51–56. [Google Scholar] [CrossRef]
- Kwong, A.; Wong, C.H.N.; Shea, C.; Suen, D.T.K.; Choi, C.L.Y. Choice of Management of Southern Chinese BRCA Mutation Carriers. World J. Surg. 2010, 34, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-G.; Kang, H.J.; Lim, M.C.; Park, B.; Park, S.J.; Jung, S.-Y.; Lee, S.; Kang, H.-S.; Park, S.-Y.; Park, B.; et al. Different Patterns of Risk Reducing Decisions in Affected or Unaffected BRCA Pathogenic Variant Carriers. Cancer Res. Treat. 2019, 51, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Tsan, Y.-T.; Ho, W.-C.; Lin, M.-H.; Lee, C.-H.; Tseng, C.-D.; Guo, Y.-L.; Wang, J.-D.; Chen, P.-C. Angiotensin-converting enzyme inhibitors enhance the effect of cyclooxygenase inhibitors on breast cancer. J. Hypertens. 2012, 30, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, Y.; Oseto, K.; Katsuragi, R.; Yoshimoto, Y.; Takahara, S.; Yamauchi, A. Experience with Bilateral Risk-Reducing Mastectomy for an Unaffected BRCA Mutation Carrier. J. Breast Cancer 2016, 19. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Chen, C.; Lyu, S.-Y.; Lin, C.-E.; Tzeng, P.-C.; Wang, T.-F.; Chang, J.-C.; Shieh, Y.-H.; Chen, I.F.; Huang, S.K.; et al. Lovastatin lowers the risk of breast cancer: A population-based study using logistic regression with a random effects model. SpringerPlus 2016, 5, 1932. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Seo, H.J. Association between WHO First-Step Analgesic Use and Risk of Breast Cancer in Women of Working Age. Pharm. 2023, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, S.; Nakamura, S.; Miyata, H.; Watanabe, C.; Den, H.; Arai, M. Risk-reducing mastectomy for women with hereditary breast and ovarian cancer (HBOC): Analytical results of data from the Japanese Organization of HBOC. Jpn. J. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kim, D.; Kim, J.; Lee, B.Y.; Yoon, J.; Kim, S.-W. Attitudes toward Risk-Reducing Mastectomy and Risk-Reducing Salpingo-oophorectomy among Young, Unmarried, Healthy Women in Korea. Cancer Res. Treat. 2022, 54, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Res. Treat. 2014, 145, 785–790. [Google Scholar] [CrossRef]
- Tseng, C.-H. Sitagliptin May Reduce Breast Cancer Risk in Women With Type 2 Diabetes. Clin. Breast Cancer 2017, 17, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H. Rosiglitazone reduces breast cancer risk in Taiwanese female patients with type 2 diabetes mellitus. Oncotarget 2016, 8, 3042–3048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamauchi, H.; Nakagawa, C.; Kobayashi, M.; Kobayashi, Y.; Mano, T.; Nakamura, S.; Arai, M. Cost-effectiveness of surveillance and prevention strategies in BRCA1/2 mutation carriers. Breast Cancer 2017, 25, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Kornelius, E.; Chiou, J.-Y.; Lai, Y.-R.; Lo, S.-C.; Peng, C.-H.; Huang, C.-N. Low-Dose Aspirin Reduces Breast Cancer Risk in Women with Diabetes: A Nationwide Retrospective Cohort Study in Taiwan. J. Women’s Health 2017, 26, 1278–1284. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Kornelius, E.; Lo, S.-C.; Wang, Y.-H.; Huang, C.-N. Aspirin and Risk of Specific Breast Cancer Subtype in Women with Diabetes. J. Women’s Health 2023, 32, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.M.; Morris, M.; Gallagher, R.; Boyd, R.; Carson, H.; Harkin, D.P.; Wielogorska, E.; Elliott, C.; Savage, K.I.; McIntosh, S.A. Chemoprevention in BRCA1 mutation carriers (CIBRAC): Protocol for an open allocation crossover feasibility trial assessing mechanisms of chemoprevention with goserelin and anastrozole versus tamoxifen and acceptability of treatment. BMJ Open 2018, 8, e023115. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Bookwalter, D.B.; O’Brien, K.M.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann. Oncol. 2021, 32, 351–359. [Google Scholar] [CrossRef]
- Corleto, K.A.; Strandmo, J.L.; Giles, E.D. Metformin and Breast Cancer: Current Findings and Future Perspectives from Preclinical and Clinical Studies. Pharmaceuticals 2024, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Bakierzynska, M.; Cullinane, M.C.; Redmond, H.P.; Corrigan, M. Prophylactic aspirin intake and breast cancer risk; A systematic review and meta-analysis of observational cohort studies. Eur. J. Surg. Oncol. 2023, 49, 106940. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, T.; Gavras, I. Renin–Angiotensin Inhibition in Combating Malignancy: A Review. Anticancer Res. 2019, 39, 4597–4602. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Pierbon, M.; Lago, L.; Grotto, G.; Baldo, V. Breast Cancer Primary Prevention and Diet: An Umbrella Review. Int. J. Environ. Res. Public Health 2020, 17, 4731. [Google Scholar] [CrossRef] [PubMed]
- Mourouti, N.; Kontogianni, M.D.; Papavagelis, C.; Panagiotakos, D.B. Diet and breast cancer: A systematic review. Int. J. Food Sci. Nutr. 2015, 66, 1–42. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Basu, N.N.; Hodson, J.; Chatterjee, S.; Gandhi, A.; Wisely, J.; Harvey, J.; Highton, L.; Murphy, J.; Barnes, N.; Johnson, R.; et al. The Angelina Jolie effect: Contralateral risk-reducing mastectomy trends in patients at increased risk of breast cancer. Sci. Rep. 2021, 11, 2847. [Google Scholar] [CrossRef]
- Grandjean, B.; Scherz, A.; Rabaglio, M. Eleven Years of Oncogenetic Consultations in a Swiss Center: Patient and Testing Characteristics. Appl. Clin. Genet. 2023, 16, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Liede, A.; Cai, M.; Crouter, T.F.; Niepel, D.; Callaghan, F.; Evans, D.G. Risk-reducing mastectomy rates in the US: A closer examination of the Angelina Jolie effect. Breast Cancer Res. Treat. 2018, 171, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Ishikawa, T.; Asami, Y.; Hirose, Y.; Shimada, K.; Miyagami, S.; Mimura, T.; Miyamoto, S.; Onuki, M.; Morioka, M.; et al. Risk-reducing salpingo-oophorectomy for Japanese women with hereditary breast and ovarian cancer: A single-institution 10-year experience. Jpn. J. Clin. Oncol. 2023, 53, 472–479. [Google Scholar] [CrossRef]
- Arslan, B.; Tazeoglu, D.; Dag, A.; Berkesoglu, M.; Ozdemir, A.A. Is there an increasing trend of risk-reducing prophylactic mastectomy procedure in preventing breast cancer among women? Turk. J. Surg. 2021, 37, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Summerhayes, C.M.; Laws, S.; Rainsbury, R.M. Resource implications of risk-reducing mastectomy and reconstruction. Eur. J. Surg. Oncol. 2016, 42, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Oxley, S.; Sideris, M.; Kalra, A.; Sun, L.; Yang, L.; Legood, R.; Manchanda, R. Cost-Effectiveness of Risk-Reducing Surgery for Breast and Ovarian Cancer Prevention: A Systematic Review. Cancers 2022, 14, 6117. [Google Scholar] [CrossRef]
- Dowsett, M.; Bundred, N.J.; Decensi, A.; Sainsbury, R.C.; Lu, Y.; Hills, M.J.; Cohen, F.J.; Veronesi, P.; O’Brien, M.E.; Scott, T.; et al. Effect of raloxifene on breast cancer cell Ki67 and apoptosis: A double-blind, placebo-controlled, randomized clinical trial in postmenopausal patients. Cancer Epidemiol. Biomark. Prev. 2001, 10, 961–966. [Google Scholar]
- Lee, J.; Lee, J.S.; Lee, J.E.; Kim, Z.; Han, S.W.; Hur, S.M.; Choi, Y.J.; Park, S. A Nationwide Study on the Incidence of Breast Cancer in Korean Women with Osteoporosis Receiving Raloxifene Treatment. J. Breast Cancer 2021, 24, 280–288. [Google Scholar] [CrossRef]
- Cuzick, J.; Forbes, J.; Edwards, R.; Baum, M.; Cawthorn, S.; Coates, A.; Hamed, A.; Howell, A.; Powles, T.; IBIS investigators. First results from the International Breast Cancer Intervention Study (IBIS-I): A randomised prevention trial. Lancet 2002, 360, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Evans, D.G.; Howell, A.; et al. Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet 2020, 395, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.; Johnston, D.; Pater, J.; Goss, P. The National Cancer Institute of Canada Clinical Trials Group MAP.3 trial: An international breast cancer prevention trial. Curr. Oncol. 2007, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P.; Lasset, C.; Berthet, P.; Dugast, C.; Delaloge, S.; Fricker, J.P.; Tennevet, I.; Chabbert-Buffet, N.; This, P.; Baudry, K.; et al. Uptake of a randomized breast cancer prevention trial comparing letrozole to placebo in BRCA1/2 mutations carriers: The LIBER trial. Fam. Cancer 2012, 11, 77–84. [Google Scholar] [CrossRef]
- Iwuji, C.; Howells, L.; Thomasset, S.; Brown, K.; Steward, W.; Barwell, J.; Thomas, A. Cancer chemoprevention: Factors influencing attitudes towards chemopreventive agents in high-risk populations. Eur. J. Cancer Prev. 2014, 23, 594–601. [Google Scholar] [CrossRef]
- Garcia, C.; Wendt, J.; Lyon, L.; Jones, J.; Littell, R.D.; Armstrong, M.A.; Raine-Bennett, T.; Powell, C.B. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol. Oncol. 2014, 132, 428–433. [Google Scholar] [CrossRef]
- van der Kolk, D.M.; de Bock, G.H.; Leegte, B.K.; Schaapveld, M.; Mourits, M.J.E.; de Vries, J.; van der Hout, A.H.; Oosterwijk, J.C. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: High cancer incidence at older age. Breast Cancer Res. Treat. 2010, 124, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Kumar, A.; Lee, M.C.; Wang, X. Influential Factors on Risk-reduction Mastectomy in a High-risk Breast Cancer Population With Genetic Predispositions. Clin. Breast Cancer 2021, 21, e427–e433. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.A.; Lee, M.C.; Almanza, D.; Ahmed, K.A.; Sun, W.; Boulware, D.C.; Laronga, C. Trends in use of bilateral prophylactic mastectomy vs high-risk surveillance in unaffected carriers of inherited breast cancer syndromes in the Inherited Cancer Registry (ICARE). Breast Cancer Res. Treat. 2019, 174, 39–45. [Google Scholar] [CrossRef]
- Nazarali, S.A.; Narod, S.A. Tamoxifen for women at high risk of breast cancer. Breast Cancer 2014, 6, 29–36. [Google Scholar] [CrossRef]
- Brown, K. Breast cancer chemoprevention: Risk-benefit effects of the antioestrogen tamoxifen. Expert Opin. Drug Saf. 2002, 1, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Cecchini, R.S.; Cronin, W.M.; Robidoux, A.; Bevers, T.B.; Kavanah, M.T.; Atkins, J.N.; Margolese, R.G.; et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005, 97, 1652–1662. [Google Scholar] [CrossRef]
- Nayfield, S.G.; Karp, J.E.; Ford, L.G.; Dorr, F.A.; Kramer, B.S. Potential role of tamoxifen in prevention of breast cancer. J. Natl. Cancer Inst. 1991, 83, 1450–1459. [Google Scholar] [CrossRef]
- Powles, T.J.; Ashley, S.; Tidy, A.; Smith, I.E.; Dowsett, M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J. Natl. Cancer Inst. 2007, 99, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Forbes, J.F.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; International Breast Cancer Intervention Study, I.I. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J. Natl. Cancer Inst. 2007, 99, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Maisonneuve, P.; Rotmensz, N.; Bonanni, B.; Boyle, P.; Viale, G.; Costa, A.; Sacchini, V.; Travaglini, R.; D’Aiuto, G.; et al. Tamoxifen for the prevention of breast cancer: Late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J. Natl. Cancer Inst. 2007, 99, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Flippo-Morton, T.; Walsh, K.; Chambers, K.; Amacker-North, L.; White, B.; Sarantou, T.; Boselli, D.M.; White, R.L., Jr. Surgical Decision Making in the BRCA-Positive Population: Institutional Experience and Comparison with Recent Literature. Breast J. 2016, 22, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Padamsee, T.J.; Hils, M.; Muraveva, A. Understanding low chemoprevention uptake by women at high risk of breast cancer: Findings from a qualitative inductive study of women’s risk-reduction experiences. BMC Womens Health 2021, 21, 157. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Pan, K. Breast Cancer Prevention: Time for Change. JCO Oncol. Pract. 2021, 17, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Thorneloe, R.J.; Hall, L.H.; Walter, F.M.; Side, L.; Lloyd, K.E.; Smith, S.G.; Investigators, E. Knowledge of Potential Harms and Benefits of Tamoxifen among Women Considering Breast Cancer Preventive Therapy. Cancer Prev. Res. 2020, 13, 411–422. [Google Scholar] [CrossRef]
- Lee, S.I.; Curtis, H.; Qureshi, S.; Dutton, B.; Qureshi, N. Specialist recommendation for chemoprevention medications in patients at familial risk of breast cancer: A cross-sectional survey in England. J. Community Genet. 2021, 12, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; DeRoo, L.A.; Scharf, D.R.; Sandler, D.P. Risk-benefit profiles of women using tamoxifen for chemoprevention. J. Natl. Cancer Inst. 2015, 107, 354. [Google Scholar] [CrossRef]
- US Preventive Services Task Force Recommendation Statement; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 857–867. [Google Scholar] [CrossRef]
- Arevalo, M.V.P.N.; Maslog, E.A.S.; Manlongat, K.D.; Ornos, E.D.B.; Chitapanarux, I.; Eala, M.A.B.; Dee, E.C. Social determinants of sex disparities in cancer in Southeast Asia. iScience 2023, 26, 107110. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Thong, M.K.; Taib, N.A.; Yip, C.H.; Teo, S.H. Genetic counseling for patients and families with hereditary breast and ovarian cancer in a developing Asian country: An observational descriptive study. Fam. Cancer 2011, 10, 199–205. [Google Scholar] [CrossRef]
- Kwong, A. Genetic testing for hereditary breast cancer in Asia-moving forward. Chin. Clin. Oncol. 2016, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Mariano, L.; Pellegrino, G.; Ferrari, F.; Pesapane, F.; Bozzini, A.C.; Frassoni, S.; Bagnardi, V.; Pupo, D.; Mazzarol, G.; et al. Atypical Ductal Hyperplasia and Lobular In Situ Neoplasm: High-Risk Lesions Challenging Breast Cancer Prevention. Cancers 2024, 16, 837. [Google Scholar] [CrossRef]
- Sanders, M.E.; Podoll, M.B. Atypical Ductal Hyperplasia-Ductal Carcinoma In Situ Spectrum: Diagnostic Considerations and Treatment Impact in the Era of Deescalation. Surg. Pathol. Clin. 2022, 15, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Czene, K.; Strand, F.; Zackrisson, S.; Lindholm, P.; Lang, K.; Fornvik, D.; Sartor, H.; Mavaddat, N.; Easton, D.; et al. Identification of Women at High Risk of Breast Cancer Who Need Supplemental Screening. Radiology 2020, 297, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hietala, M.; Olsson, H.; Jernstrom, H. Prolactin levels, breast-feeding and milk production in a cohort of young healthy women from high-risk breast cancer families: Implications for breast cancer risk. Fam. Cancer 2008, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Eckert, S.; Krueger, K.A.; Grady, D.; Powles, T.J.; Cauley, J.A.; Norton, L.; Nickelsen, T.; Bjarnason, N.H.; Morrow, M.; et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999, 281, 2189–2197. [Google Scholar] [CrossRef]
- Cauley, J.A.; Norton, L.; Lippman, M.E.; Eckert, S.; Krueger, K.A.; Purdie, D.W.; Farrerons, J.; Karasik, A.; Mellstrom, D.; Ng, K.W.; et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res. Treat. 2001, 65, 125–134. [Google Scholar] [CrossRef]
- Mocellin, S.; Goodwin, A.; Pasquali, S. Risk-reducing medications for primary breast cancer: A network meta-analysis. Cochrane Database Syst. Rev. 2019, 4, CD012191. [Google Scholar] [CrossRef]
- Serrano, D.; Gandini, S.; Thomas, P.; Crew, K.D.; Kumar, N.B.; Vornik, L.A.; Lee, J.J.; Veronesi, P.; Viale, G.; Guerrieri-Gonzaga, A.; et al. Efficacy of Alternative Dose Regimens of Exemestane in Postmenopausal Women With Stage 0 to II Estrogen Receptor-Positive Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Afrin, S.; Jones, S.I.; Segars, J. Selective Progesterone Receptor Modulators-Mechanisms and Therapeutic Utility. Endocr. Rev. 2020, 41, bnaa012. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Kontos, M.; Spartalis, E.; Fentiman, I.S. The Role of NSAIDs in Breast Cancer Prevention and Relapse: Current Evidence and Future Perspectives. Breast Care 2016, 11, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Nangia, J.R.; Rimawi, M.F. Breast Cancer Prevention: Where Are We? JCO Oncol. Pract. 2021, 17, 720–722. [Google Scholar] [CrossRef] [PubMed]
| ID | Reference | Title | Year | Location | Aim | Type of Study | Participants | Risk Reduction Method | Key Findings |
|---|---|---|---|---|---|---|---|---|---|
| 4 | [70] | Effect of risk-reducing salpingo-oophorectomy on the quality of life in Korean BRCA mutation carriers | 2021 | Korea | This study aimed to compare the QOL, psychosocial status, sexual function, and menopausal symptoms between the risk-reducing salpingo-oophorectomy (RRSO) and non-RRSO groups comprising Korean women with BRCA mutation and to evaluate the effect of timing of RRSO (before and after menopause) on those aspects. | Cross-sectional | 52 women aged≥ 35 years, 47 of whom were affected and 5 of whom were unaffected carriers of BRCA mutation | Risk-reducing salpingo-oophorectomy | Opting for RRSO was associated with poorer physical quality of life only but did not negatively affect mental QOL, psychosocial status, sexual function, or menopausal symptoms. When comparing groups based on menopausal status, the only significant difference was that mental score component of QoL was higher in the premenopausal than in the postmenopausal group. |
| 5 | [71] | Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey | 2022 | Korea | This study aimed to compare the intent to undergo BRCA1/2 testing, risk-reducing salpingo-oophorectomy (RRSO), and risk-reducing mastectomy (RRM) among the general public, cancer patients, and healthcare professionals in Korea. | Cross-sectional | 3444 individuals; 1496 from the general public, 1500 cancer patients, 108 clinicians, and 340 researchers | Risk-reducing salpingo-oophorectomy and risk-reducing mastectomy | The main factor contributing to RRSO intent was “being at high risk of ovarian cancer” (72%). Among the barriers reported by those not intending to undergo RRSO, “the fact that I do not have breast cancer yet” (60%) was the most common. The most common reason behind the intent to undergo RRM was “test results indicated being at high risk of developing breast cancer” (67%). “The fact that I do not have breast cancer yet” (66%) was the most frequently reported reason among individuals not willing to undergo RRM. |
| 12 | [72] | Risk management adherence following genetic testing for hereditary cancer syndromes: a Singaporean experience | 2018 | Singapore | This study aims to examine adherence behavior to risk management guidelines among mutation carriers who have attended the Cancer Genetic Service (CGS) in Singapore. | Retrospective cohort study | 52 subjects with a confirmed presence of germline mutations in a gene associated with a hereditary cancer syndrome, 41 of whom were affected carriers | Risk-reducing mastectomy | The overall adherence rate was 96.2%, including 37 (74.0%) fully adherent and 13 (26.0%) partially adherent subjects. Adherence was similar across the age groups; however, it decreased with increasing age. Malay subjects had lower adherence compared with Chinese and other ethnic groups. Those who were the first in their family to undergo testing (proband testing) demonstrated lower adherence than those who underwent testing for a known familial mutation. |
| 24 | [73] | Trends in contralateral prophylactic mastectomy rate according to clinicopathologic and socioeconomic status | 2019 | Korea | This study aimed to examine trends in CPM (contralateral prophylactic mastectomy) rates according to clinicopathologic and socioeconomic status at a single institution in the Republic of Korea. | Retrospective cohort study | 128 participants diagnosed with BRCA mutations | Risk-reducing mastectomy | Women in the CPM group had significantly higher education levels. A total of 75% of participants who underwent CPM also underwent RRSO (compared to 12.5% in the non-CPM group). |
| 27 | [58] | Trends in Risk-Reducing Mastectomy and Risk-Reducing Salpingo-Oophorectomy in Korean Carriers of the BRCA1/2 Mutation | 2020 | Korea | The aim of this study was to investigate trends in risk-reducing surgery for the prevention of breast and ovarian cancer in Korean carriers of the BRCA1/2 mutation until August 2018. | Retrospective cohort study | 1238 affected and 514 unaffected BRCA mutation carriers | Risk-reducing salpingo-oophorectomy and risk-reducing mastectomy | Contralateral RRM among affected carriers increased approximately 5.8-fold, from 5 cases in 2013 to 29 cases in 2017. RRBSO among affected carriers also increased approximately 3.6-fold, from 22 cases in 2013 to 79 cases in 2017. Among unaffected carriers, bilateral RRM increased by 1 case in 2017, and RRBSO increased 8-fold from 2 cases in 2013 to 16 cases in 2017. |
| 28 | [74] | The Japanese Breast Cancer Society clinical practice guidelines for epidemiology and prevention of breast cancer, 2022 edition | 2024 | Japan | To provide a summary of the 2022 edition of the JBCS Clinical Practice Guidelines for Epidemiology and Prevention of Breast Cancer | Clinical practice guidelines | Not applicable | Chemoprevention, risk-reducing mastectomy, risk-reducing salpingo-oophorectomy | Prophylactic administration of selective estrogen receptor modulators (tamoxifen, raloxifen) or aromatase inhibitors (exemestane, anastrozole) to women at high risk of breast cancer has been found to prevent onset of breast cancer. However, a breast cancer risk model for Japanese women has not been established, so it is not possible to conclude whether administration of drugs to prevent development of breast cancer is or is not useful. Bilateral risk-reducing mastectomy (BRRM) is weakly recommended for breast cancer-naive women with BRCA pathologic variants. There is a weak recommendation for contralateral risk-reducing mastectomy (CRRM) in patients with a diagnosis of breast cancer with a BRCA pathologic variant. RRSO is strongly recommended for women with BRCA mutations. In Japan, the medical reimbursement revision in April 2020 allows for risk-reducing mastectomy (RRM) and risk-reducing surgery for oophorectomy (RRSO) covered by insurance. |
| 30 | [75] | Factors affecting the decision to undergo risk-reducing salpingo-oophorectomy among women with BRCA gene mutation | 2013 | Korea | This study evaluated the clinical and demographic factors that may affect the decision to undergo RRSO among South Korean women with BRCA gene mutations. | Retrospective cohort study | 71 BRCA mutation carriers older than 30, 51 of whom were affected carriers | Risk-reducing salpingo-oophorectomy | The RRSO rate of carriers in their fifth decade was significantly higher than that of the other age groups (p = 0.007). While 20 of 51 (39.2%) personally affected carriers had undergone RRSO, only 1 of the 20 (5.0%) personally unaffected carriers had undergone RRSO (p = 0.004). This revealed that whereas a personal history of breast cancer was a significant factor in the decision to undergo RRSO among the carriers, BMI and family history of ovarian cancer or breast cancer were not, and neither were alcohol intake, cigarette smoking, occupation, or economic status. |
| 31 | [76] | Estimating the Risks and Benefits of Tamoxifen for Prophylactic Breast Cancer Chemoprevention in Korea | 2012 | Korea | This study estimated the risks and benefits of tamoxifen using a Korean database in order to evaluate the feasibility of using tamoxifen for chemoprevention in Korean women. | Retrospective cohort study | Women from Korean National Insurance Database | Chemoprevention with tamoxifen | For the specific 5-year risk of breast cancer, the net benefit of tamoxifen was reduced based on increases in age because of the high risk of stroke in older women. Therefore, older Korean women had more risk than benefit from tamoxifen chemoprevention. Only women younger than age 40 had a positive risk–benefit index for the average 5-year risk of breast cancer. |
| 37 | [77] | Choice of Management of Southern Chinese BRCA Mutation Carriers | 2010 | Hong Kong | This study was designed to be the first to report the uptake of preventative and surveillance measures of Chinese women and men who were found to be carriers of the BRCA1 and BRCA2 mutations. | Cross-sectional | 31 affected carriers of the BRCA mutations and 83 of their family members | Risk-reducing surgeries and chemopreventative agents (tamoxifane, raloxifane, birth control) | Women who decided on prophylactic salpingo-oophorectomy were comparatively older than those who decided on prophylactic mastectomy. Interestingly, no female mutation carriers, excluding those who were already taking tamoxifen, agreed to have any chemoprevention. |
| 38 | [78] | Different Patterns of Risk Reducing Decisions in Affected or Unaffected BRCA Pathogenic Variant Carriers | 2019 | Korea | This study was designed to identify risk reduction management patterns in Korean carriers of BRCA1 and BRCA2 pathogenic variants. | Retrospective cohort study | 220 BRCA mutation carriers, 100 of whom were affected | Risk-reducing surgery and chemoprevention | Of the 179 female carriers, 98 (54.7%) underwent RR management, including 18 (10.1%) who received chemoprevention and 80 (44.7%) who underwent risk-reducing surgery (RRS), including RRSO in 76 patients, RRM in 1 patient, and both in 3 patients. Of the 79 unaffected female carriers, 39 (49.4%) received RR management, including 23 (29.1%) who underwent RRSO and 16 (20.3%) who received chemoprevention (p = 0.495). In affected female carriers with BC, older age was significantly associated with RR management (p = 0.011). However, in unaffected carriers, age, type of BRCA pathogenic variant, and family history were not significantly associated with RR management. |
| 39 | [79] | Angiotensin-converting enzyme inhibitors enhance the effect of cyclooxygenase inhibitors on breast cancer: a nationwide case-control study | 2012 | Taiwan | This study aimed to investigate the effects of NSAIDs, aspirin, and ACE inhibitors, alone or in combination, on the risk of developing breast cancer. | Case–control | A total of 16,847 breast cancer patients and 50,541 age-matched, cancer-free controls from the Taiwanese NHI database | Chemoprevention with NSAIDs, aspirin, and ACE inhibitors—alone or in combination | There were significantly lower rates of breast cancer among women who took aspirin and high doses of ACE inhibitors compared with women taking aspirin and lower doses of ACE inhibitors. Women taking NSAIDs and higher doses of ACE inhibitors also had significantly lower rates of breast cancer compared with women taking NSAIDs with lower doses of ACE inhibitors. Increasing doses of ACE inhibitors alone had no effect on breast cancer prevention. |
| 40 | [80] | Experience with Bilateral Risk-Reducing Mastectomy for an Unaffected BRCA Mutation Carrier | 2016 | Japan | This paper reports a case of a 38-year-old Japanese woman diagnosed with BRCA2 mutation that underwent prophylactic bilateral SSM. | Case report | A Japanese woman who was an unaffected carrier of BRCA2 mutation | Risk-reducing mastectomy | Not applicable; descriptive account of the case |
| 53 | [81] | Lovastatin lowers the risk of breast cancer: a population-based study using logistic regression with a random effects model | 2016 | Taiwan | This study aimed to statistically evaluate the risk of BC for statin users and non-users in Taiwan, as well as for each statin. | Case–control | A total of 4332 breast cancer patients and 21,660 cancer-free controls from the Taiwanese NHI database | Chemoprevention with statins (atorvastatin, fluvastatin, lovastatin, pravastatin, simvastatin) | The adjusted odds ratio for BC among lovastatin users was 0.596 (95% CI 0.497–0.714), lower than that for subjects who did not use lovastatin. Simvastatin, pravastatin, fluvastatin, and atorvastatin did not exhibit a statistically significant protective effect against BC, although atorvastatin showed a protective tendency against BC that did not reach statistical significance (adj OR 0.887; 95% CI 0.776–1.013; p < 0.077). |
| 59 | [57] | Impact of the coverage of risk-reducing salpingo-oophorectomy by the national insurance system for women with BRCA pathogenic variants in Japan | 2023 | Japan | To determine the impact of the coverage of risk-reducing salpingo-oophorectomy (RRSO) by the national insurance system by comparing cases before and after the coverage date | Retrospective cohort study | 134 women underwent with confirmed BRCA mutations | Risk-reducing salpingo-oophorectomy | Before insurance coverage, 45 women underwent RRSO in 36 months, whereas 89 women underwent RRSO in 24 months after coverage. The median age, BRCA status, personal BC history, and family history of OC to within the third degree between the groups were not statistically different. There were significantly more parous women after coverage. |
| 61 | [82] | Association between WHO First-Step Analgesic Use and Risk of Breast Cancer in Women of Working Age | 2023 | Korea | To analyze the association between economic activity, regular analgesic use, and breast cancer development in women of specific age groups to provide a basis for improving cancer prevention practices | Retrospective cohort study | Women of working age from The Korean National Health Insurance Service—National Sample Cohort database, 1347 of whom were cancer patients and 5388 of whom were not | Chemoprevention with analgesics | The economically active group had a higher risk of breast cancer than the inactive group (p < 0.001), possibly due to higher levels of stress and lower engagement in health protective behaviours. The risk of breast cancer was higher in the high-income group than in the low-income group (p < 0.001). The risk of breast cancer was lower in participants with regular analgesic use compared to those with no analgesic use (HR = 0.748, 95% CI: 0.614–0.912, p < 0.05). Regular analgesic use may be beneficial for inhibiting cancer development in the young age group (24~39 years). |
| 62 | [83] | Risk-reducing mastectomy for women with hereditary breast and ovarian cancer (HBOC): analytical results of data from the Japanese Organization of HBOC | 2022 | Japan | To confirm the rate of risk-reducing mastectomy in women with BRCA pathogenic variants before the approval of National Medical Insurance coverage | Retrospective cohort study | 687 women with confirmed BRCA mutations | Risk-reducing mastectomy | The rates of RRM were statistically significantly higher in women with a personal (positive 15.4% vs. negative 5.2%, p = 0.001) or family history (positive 16.6% vs. negative 10.5%, p = 0.042) of breast cancer than those without them, in mothers compared with those without children (mothers 15.1% vs. women without children 7.9%, p = 0.023), in women who were receiving surveillance with MRI than women who did not (surveillance +23.9% vs. surveillance −9.7%, p < 0.001), and in women who received RRSO compared with women who did not (RRSO +36.7% vs. RRSO −7.3%, p < 0.001). |
| 63 | [84] | Attitudes toward Risk-Reducing Mastectomy and Risk-Reducing Salpingo-oophorectomy among Young, Unmarried, Healthy Women in Korea | 2022 | Korea | To investigate the attitudes toward risk-reducing mastectomy (RRM) and risk-reducing salpingo-oophorectomy (RRSO) as cancer prevention options for BRCA1/2 carriers in healthy, young, unmarried Korean women | Cross-sectional | 600 cancer-free unmarried women aged 20–39 from different parts of South Korea | Risk-reducing mastectomy and risk-reducing salpingo-oophorectomy | Among 600 women aged 20–39 years, 54.7% and 57.7% had the intention to undergo RRM and RRSO, respectively, on the assumption that they were BRCA1/2 carriers. As age increased, the proportion of women with the intention to undergo RRM or RRSO decreased from >65% in the 20–24-year-old age group to around 40% in the 35–39-year-old age group. Those who wanted to undergo screening for BRCA1/2 mutations had a significantly high intention to undergo risk-reducing surgeries (p < 0.05). |
| 95 | [85] | Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes | 2014 | Taiwan | To evaluate whether metformin use in the Taiwanese women with T2DM would affect the risk of breast cancer | Retrospective cohort study | 476,282 patients with a diagnosis of newly onset type II diabetes mellitus | Chemoprevention with metformin | For the overall hazard ratios comparing ever users versus never users, there was a significantly lower risk of breast cancer associated with metformin use. In the dose–response analyses adjusted for age, all categories of exposure to metformin were associated with a significantly reduced risk. In the fully adjusted models, although a significantly reduced risk was observed with increasing cumulative duration and cumulative dose, the first tertiles of the dose–response parameters showed a significantly higher risk associated with metformin use. |
| 96 | [86] | Sitagliptin May Reduce Breast Cancer Risk in Women With Type 2 Diabetes | 2017 | Taiwan | To evaluate whether sitagliptin use in Taiwanese female patients with T2DM would affect the risk of breast cancer | Retrospective cohort study | 428,478 newly diagnosed female patients with type II diabetes mellitus with onset at the age of 25 or later | Chemoprevention with sitagliptin | For cumulative duration and cumulative dose of sitagliptin use, the hazard ratios for the first and second tertiles were not significant, but a significantly reduced risk was observed in the third tertile of cumulative duration and cumulative dose. In the matched cohort, although the overall HR was not significant, a lower risk of breast cancer associated with sitagliptin use could also be observed. In the tertile analyses in the matched cohort, significantly reduced risk could also be observed for the third tertile of cumulative duration and cumulative dose. |
| 97 | [87] | Rosiglitazone reduces breast cancer risk in Taiwanese female patients with type 2 diabetes mellitus | 2017 | Taiwan | To study the association between rosiglitazone and breast cancer in Taiwanese female patients with type 2 diabetes mellitus | Retrospective cohort study | 431,447 female patients with type II diabetes mellitus | Chemoprevention with oral antidiabetic agents or insulin | The overall hazard ratios for ever users versus never users showed a significantly lower risk in all models. While evaluating the dose–response relationship, a lower risk was observed in the third tertiles of both cumulative duration and cumulative dose in all models, and all p values for the trend were significant. There was a significant interaction between rosiglitazone and metformin. The lowest risk was observed in users of both drugs (hazard ratio 0.812, 95% confidence interval: 0.705–0.934). |
| 110 | [88] | Cost-effectiveness of surveillance and prevention strategies in BRCA1/2 mutation carriers | 2018 | Japan | This study aimed to conduct a cost-effectiveness analysis of surveillance and prevention strategies in BRCA1/2 mutation carriers with quality adjustment. | Economic evaluation | A simulated cohort of female BRCA1/2 mutation carriers aged 35–70 years, who had no cancer diagnosis at baseline | Risk-reducing mastectomy and risk-reducing salpingo-oophorectomy | Compared with surveillance, RRSO and RRM&RRSO were dominant (cost-saving and more effective) and RRM was also more cost-effective in BRCA1 mutation carriers. RRM and RRM&RRSO were dominant and RRSO was also more cost-effective in BRCA2 mutation carriers, with quality adjustment based on preference ratings. |
| 111 | [89] | Low-Dose Aspirin Reduces Breast Cancer Risk in Women with Diabetes: A Nationwide Retrospective Cohort Study in Taiwan | 2017 | Taiwan | This study aimed to investigate the relationship between low-dose aspirin use and breast cancer incidence in women with diabetes. | Retrospective cohort study | 48,202 female patients with type II diabetes mellitus | Chemoprevention with aspirin | The risk of breast cancer was first found to be lower in aspirin users (HR, 0.77; 95% CI, 0.68–0.89; p < 0.01) in the unadjusted model. After adjustment for age, comorbidities, hypertension, and hyperlipidemia (Model 1), and modification of Model 1 with the addition of antidiabetic agents (Model 2), the risk of breast cancer was still found to be lower in aspirin users (HR, 0.78 and 0.81; p < 0.01 for Model 1 and Model 2, respectively). Only a high cumulative dose of aspirin (>88,900 mg) for a mean period of 8.5 years was found to reduce the risk of breast cancer (HR, 0.53; 95% CI, 0.43–0.67; p < 0.01). The incidence of breast cancer seemed to decrease after 1 year of aspirin use, with a continued divergence in the cumulative curves throughout the follow-up period. |
| 112 | [90] | Aspirin and Risk of Specific Breast Cancer Subtype in Women with Diabetes | 2023 | Taiwan | This study aimed to explore the association between aspirin use and the risk of specific breast cancer subtypes in women with diabetes. | Retrospective cohort study | 48,202 female patients with type II diabetes mellitus | Chemoprevention with aspirin | The effect of ever use of aspirin appeared limited to hormone receptor-positive breast cancer. For ever use of aspirin, hormone receptor-positive breast cancer risk was significant (HR: 0.73; 95% CI: 0.59–0.91); however, it was not significant for hormone receptor-negative breast cancer risk (HR: 0.88; 95% CI: 0.74–1.05). A cumulative dose of aspirin use of more than 8600 mg for a mean period of 8.5 years was found to reduce the risk of hormone receptor-positive breast cancer by 31% (HR: 0.69; 95% CI: 0.50–0.97). A cumulative dose of aspirin use of more than 88,900 mg was found to reduce the risk of both hormone receptor-positive and hormone receptor-negative breast cancer. Among women aged ≤ 50 years old, aspirin use was associated with an increased risk of hormone receptor-positive breast cancer (HR: 1.44; 95% CI: 1.04–1.99). For women aged >50 years old, aspirin use was associated with decreased risk for both hormone receptor-positive and -negative breast cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpeza, F.; Loo, C.K.Y.; Zhuang, Q.; Hartman, M.; Goh, S.S.N.; Li, J. A Scoping Review of Primary Breast Cancer Risk Reduction Strategies in East and Southeast Asia. Cancers 2025, 17, 168. https://doi.org/10.3390/cancers17020168
Alpeza F, Loo CKY, Zhuang Q, Hartman M, Goh SSN, Li J. A Scoping Review of Primary Breast Cancer Risk Reduction Strategies in East and Southeast Asia. Cancers. 2025; 17(2):168. https://doi.org/10.3390/cancers17020168
Chicago/Turabian StyleAlpeza, Filipa, Christine Kim Yan Loo, Qingyuan Zhuang, Mikael Hartman, Serene Si Ning Goh, and Jingmei Li. 2025. "A Scoping Review of Primary Breast Cancer Risk Reduction Strategies in East and Southeast Asia" Cancers 17, no. 2: 168. https://doi.org/10.3390/cancers17020168
APA StyleAlpeza, F., Loo, C. K. Y., Zhuang, Q., Hartman, M., Goh, S. S. N., & Li, J. (2025). A Scoping Review of Primary Breast Cancer Risk Reduction Strategies in East and Southeast Asia. Cancers, 17(2), 168. https://doi.org/10.3390/cancers17020168







