Diagnostic Performance of PIVKA-II in Italian Patients with Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Sample Collection and Marker Quantification
2.4. Statistical Analysis
3. Results
3.1. Distribution of PIVKA-II Serum Levels Across Different Comorbidities
3.2. PIVKA-II Serum Levels in Relation to Nodules Number and Size
3.3. PIVKA-II Serum Levels According to HCC Type and Grading
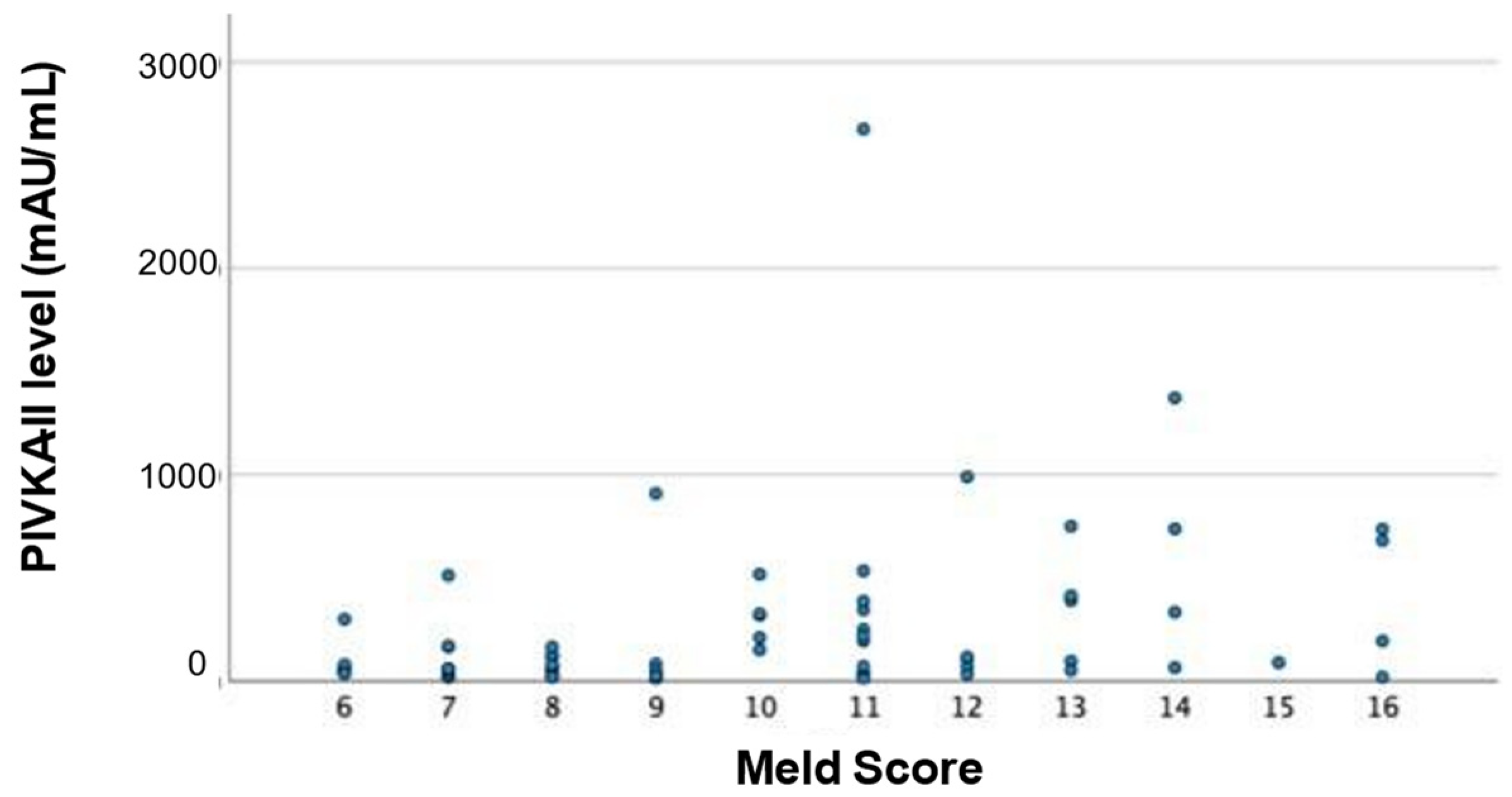
3.4. PIVKA-II Levels in Relation to HCC Stage
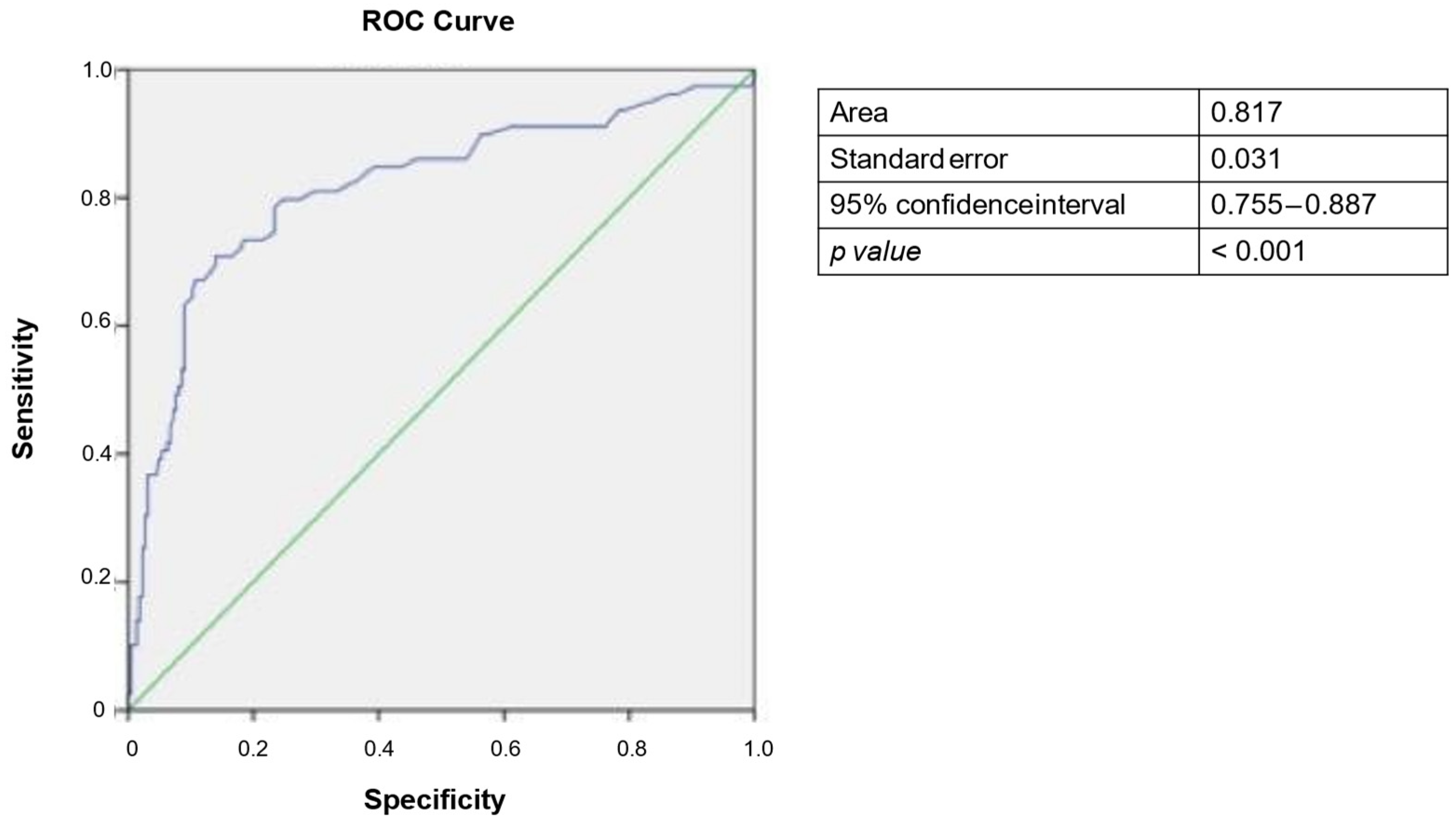
3.5. Analysis of Diagnostic Threshold for PIVKA-II
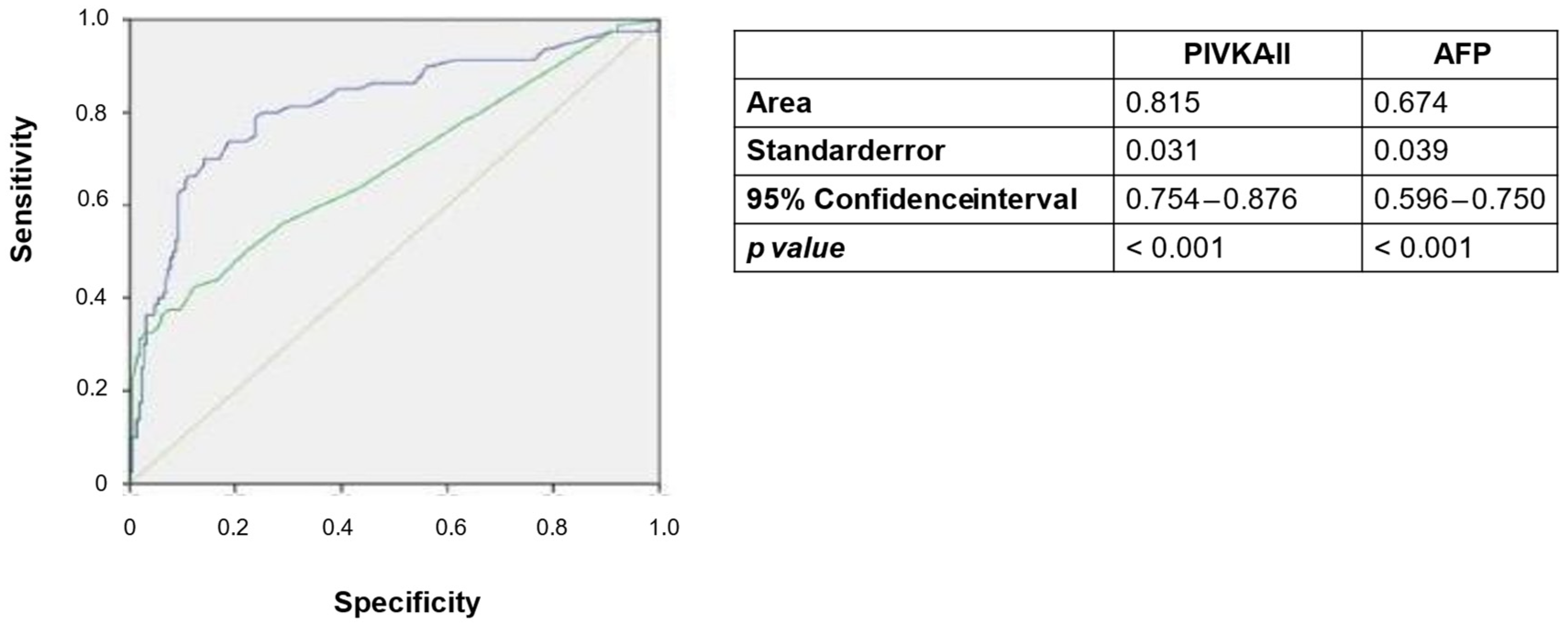
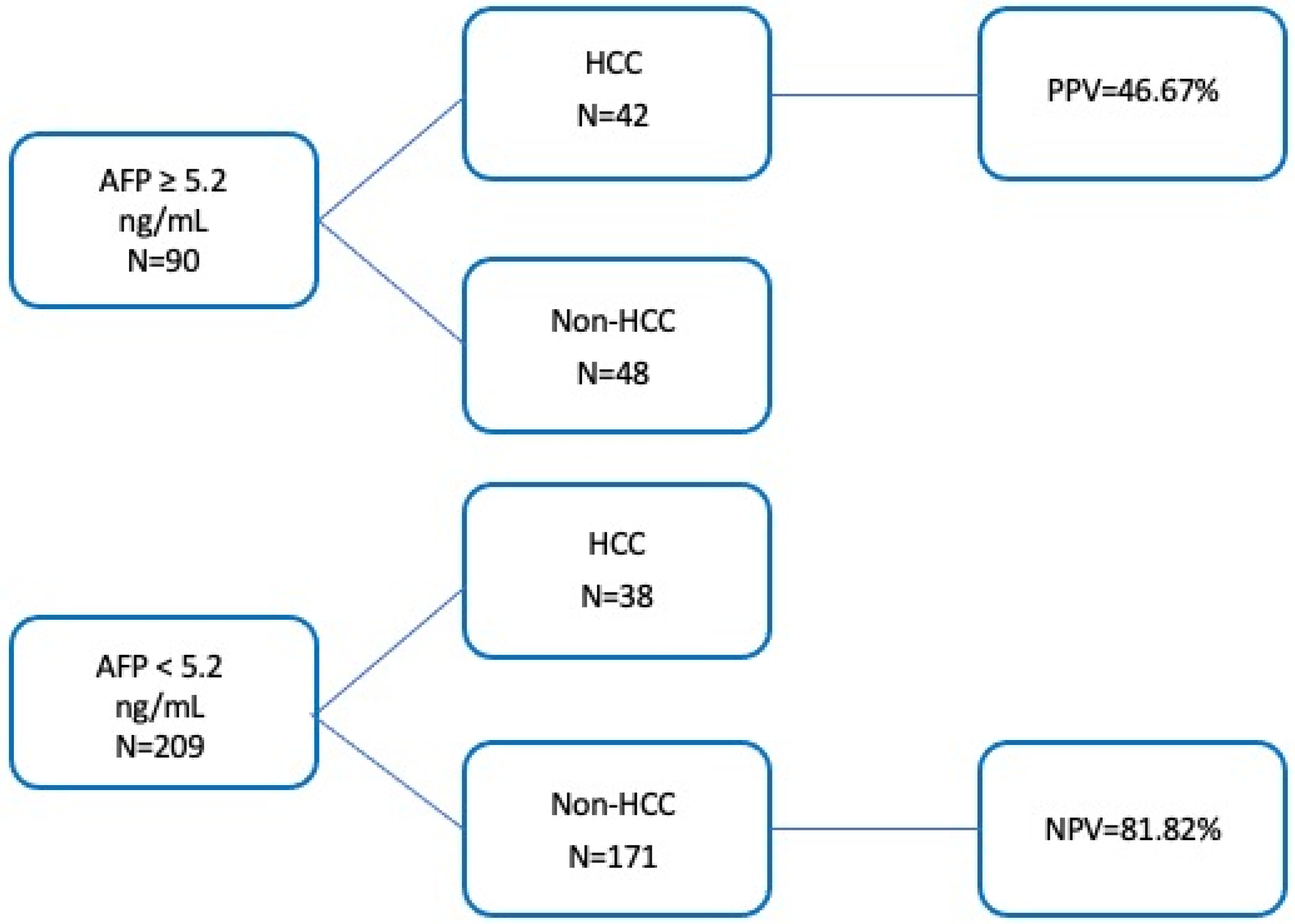
3.6. Comparison of Diagnostic Thresholds for PIVKA-II and AFP
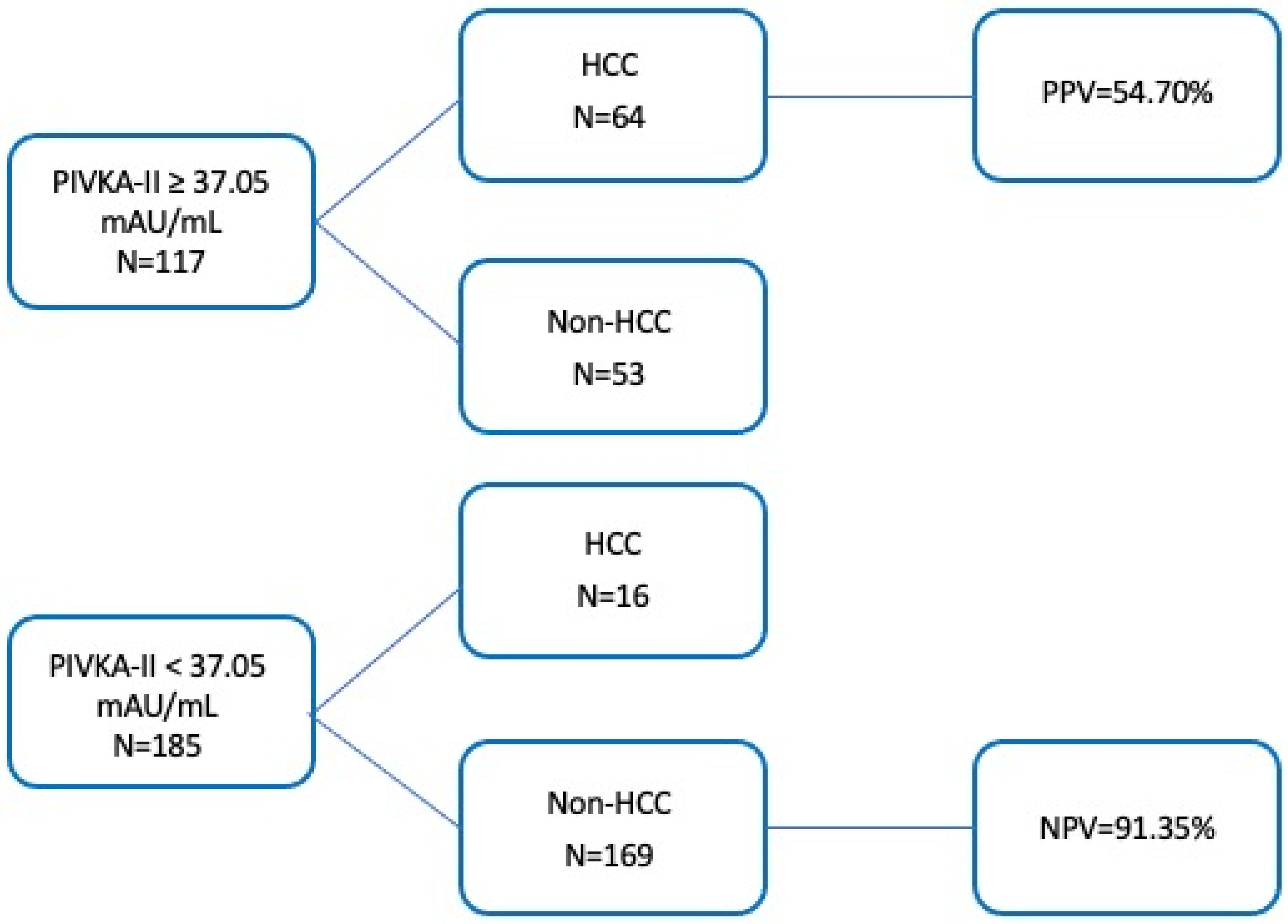
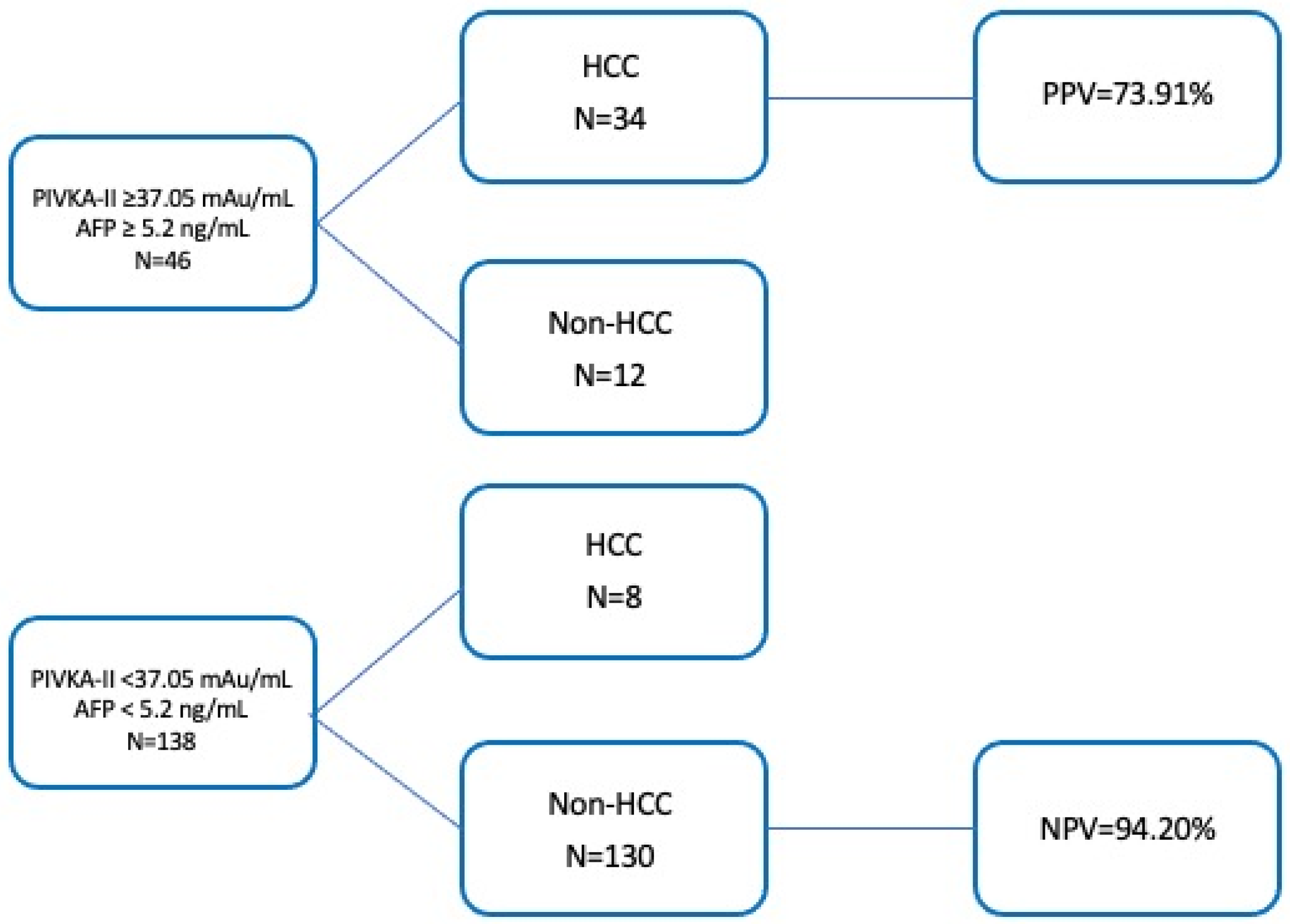
3.7. Combination of PIVKA-II and AFP
3.8. Other Considerations
4. Discussion
4.1. What Does the Study Add to Current Knowledge?
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alqahtani, A.; Khan, Z.; Alloghbi, A.; Said Ahmed, T.S.; Ashraf, M.; Hammouda, D.M. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina 2019, 55, 526. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, E.; Sarkar, D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 2798. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Zhang, X.; Addissie, B.D.; Mohamed, E.A.; Harmsen, W.S.; Theobald, P.J.; Peters, B.E.; Balsanek, J.G.; Ward, M.M.; Giama, N.H.; et al. Combinations of Biomarkers and Milan Criteria for Predicting Hepatocellular Carcinoma Recurrence after Liver Transplantation. Liver Transpl. 2015, 21, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-H.; Yang, B.-H.; Tang, Z.-Y. Randomized Controlled Trial of Screening for Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.a.M.; Marrero, J.A. Meta-Analysis: Surveillance with Ultrasound for Early-Stage Hepatocellular Carcinoma in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Mizejewski, G.J. Alpha-Fetoprotein Structure and Function: Relevance to Isoforms, Epitopes, and Conformational Variants. Exp. Biol. Med. 2001, 226, 377–408. [Google Scholar] [CrossRef]
- Suttie, J.W. Vitamin K-Dependent Carboxylase. Annu. Rev. Biochem. 1985, 54, 459–477. [Google Scholar] [CrossRef]
- Liebman, H.A. Isolation and Characterization of a Hepatoma-Associated Abnormal (Des-Gamma-Carboxy)Prothrombin. Cancer Res. 1989, 49, 6493–6497. [Google Scholar] [PubMed]
- Pozzi, N.; Chen, Z.; Gohara, D.W.; Niu, W.; Heyduk, T.; Di Cera, E. Crystal Structure of Prothrombin Reveals Conformational Flexibility and Mechanism of Activation. J. Biol. Chem. 2013, 288, 22734–22744. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.Y.; Vogel, A.; Berg, T.; De Toni, E.N.; Kudo, M.; Trojan, J.; Eiblmaier, A.; Klein, H.-G.; Hegel, J.K.; Sharma, A.; et al. Performance Evaluation of the Elecsys PIVKA-II and Elecsys AFP Assays for Hepatocellular Carcinoma Diagnosis. JGH Open 2022, 6, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Widdershoven, J.; van Munster, P.; De Abreu, R.; Bosman, H.; van Lith, T.; van der Putten-van Meyel, M.; Motohara, K.; Matsuda, I. Four Methods Compared for Measuring Des-Carboxy-Prothrombin (PIVKA-II). Clin. Chem. 1987, 33, 2074–2078. [Google Scholar] [CrossRef]
- Suzuki, H.; Murata, K.; Gotoh, T.; Kusano, M.; Okano, H.; Oyamada, T.; Yasuda, Y.; Imamura, M.; Kudo, M.; Mizokami, M.; et al. Phenotype-Dependent Production of Des-γ-Carboxy Prothrombin in Hepatocellular Carcinoma. J. Gastroenterol. 2011, 46, 1219–1229. [Google Scholar] [CrossRef]
- Ono, M.; Ohta, H.; Ohhira, M.; Sekiya, C.; Namiki, M. Measurement of Immunoreactive Prothrombin, Des-Gamma-Carboxy Prothrombin, and Vitamin K in Human Liver Tissues: Overproduction of Immunoreactive Prothrombin in Hepatocellular Carcinoma. Am. J. Gastroenterol. 1990, 85, 1149–1154. [Google Scholar]
- Zhang, S.-G.; Huang, Y. Usefulness of AFP, PIVKA-II, and Their Combination in Diagnosing Hepatocellular Carcinoma Based on Upconversion Luminescence Immunochromatography. Lab. Med. 2022, 53, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, G.; Lu, Z.; Liu, Y.; Kong, J.; Liu, J. Progression of Prothrombin Induced by Vitamin K Absence-II in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 726213. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Troshina, G.; Carucci, P.; Rolle, E.; Risso, A.; Burlone, M.E.; Albè, A.; Crevola, M.; Musso, E.C.; et al. Identification of the Best Cut-Off Value of PIVKA-II for the Surveillance of Patients at Risk of Hepatocellular Carcinoma Development. Biology 2023, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Loglio, A.; Iavarone, M.; Facchetti, F.; Di Paolo, D.; Perbellini, R.; Lunghi, G.; Ceriotti, F.; Galli, C.; Sandri, M.T.; Viganò, M.; et al. The Combination of PIVKA-II and AFP Improves the Detection Accuracy for HCC in HBV Caucasian Cirrhotics on Long-Term Oral Therapy. Liver Int. 2020, 40, 1987–1996. [Google Scholar] [CrossRef]
- Degasperi, E.; Perbellini, R.; D’Ambrosio, R.; Uceda Renteria, S.C.; Ceriotti, F.; Perego, A.; Orsini, C.; Borghi, M.; Iavarone, M.; Bruccoleri, M.; et al. Prothrombin Induced by Vitamin K Absence or Antagonist-II and Alpha Foetoprotein to Predict Development of Hepatocellular Carcinoma in Caucasian Patients with Hepatitis C-Related Cirrhosis Treated with Direct-Acting Antiviral Agents. Aliment. Pharmacol. Ther. 2022, 55, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Poté, N.; Cauchy, F.; Albuquerque, M.; Voitot, H.; Belghiti, J.; Castera, L.; Puy, H.; Bedossa, P.; Paradis, V. Performance of PIVKA-II for Early Hepatocellular Carcinoma Diagnosis and Prediction of Microvascular Invasion. J. Hepatol. 2015, 62, 848–854. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.; Kanwal, F.; Ning, J.; Powell, H.; Khaderi, S.; Singal, A.G.; Asrani, S.; Marrero, J.A.; Amos, C.I.; Thrift, A.P.; et al. Serum Biomarker Signature Is Predictive of the Risk of Hepatocellular Cancer in Patients with Cirrhosis. Gut 2024, 73, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, G.-A.; Han, S.; Lee, W.; Chun, S.; Lim, Y.-S. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology 2019, 69, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Cai, L.; Ouyang, J.; Wang, F.; Li, Z.; Liu, M.; Wang, Y.; Zhou, Y.; Hu, E.; Huang, C.; et al. Proteomics-Driven Noninvasive Screening of Circulating Serum Protein Panels for the Early Diagnosis of Hepatocellular Carcinoma. Nat. Commun. 2023, 14, 8392. [Google Scholar] [CrossRef]
- Marrero, J.A.; Su, G.L.; Wei, W.; Emick, D.; Conjeevaram, H.S.; Fontana, R.J.; Lok, A.S. Des-Gamma Carboxyprothrombin Can Differentiate Hepatocellular Carcinoma from Nonmalignant Chronic Liver Disease in American Patients. Hepatology 2003, 37, 1114–1121. [Google Scholar] [CrossRef]
- Saitta, C.; Raffa, G.; Alibrandi, A.; Brancatelli, S.; Lombardo, D.; Tripodi, G.; Raimondo, G.; Pollicino, T. PIVKA-II Is a Useful Tool for Diagnostic Characterization of Ultrasound-Detected Liver Nodules in Cirrhotic Patients. Medicine 2017, 96, e7266. [Google Scholar] [CrossRef] [PubMed]
- Syriha, A.; Pantzios, S.; Mandilara, D.; Galanis, P.; Stathopoulou, I.; Barla, G.; Elefsiniotis, I. Diagnostic Accuracy of Serum Protein Induced by Vitamin K Absence (PIVKA-II), Serum a-Fetoprotein and Their Combination for Hepatocellular Carcinoma among Caucasian Cirrhotic Patients with Diagnostic or Non-Diagnostic Serum a-Fetoprotein Levels. Cancer Med. 2024, 13, e6825. [Google Scholar] [CrossRef]
- Nakao, A.; Suzuki, Y.; Isshiki, K.; Kimura, Y.; Takeda, S.; Kishimoto, W.; Nonami, T.; Harada, A.; Takagi, H. Clinical Evaluation of Plasma Abnormal Prothrombin (Des-Gamma-Carboxy Prothrombin) in Hepatobiliary Malignancies and Other Diseases. Am. J. Gastroenterol. 1991, 86, 62–66. [Google Scholar] [PubMed]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma: A 2017 Update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Gentile, I.; Buonomo, A.R.; Scotto, R.; Zappulo, E.; Carriero, C.; Piccirillo, M.; Izzo, F.; Rizzo, M.; Cerasuolo, D.; Borgia, G.; et al. Diagnostic Accuracy of PIVKA-II, Alpha-Fetoprotein and a Combination of Both in Diagnosis of Hepatocellular Carcinoma in Patients Affected by Chronic HCV Infection. In Vivo 2017, 31, 695–700. [Google Scholar] [CrossRef]
- Gao, H.; Xie, C.; Wang, J.; Ma, J.; Liu, S.; Xie, L.; Zheng, Y.; Dong, R.; Wang, S.; Fang, Y.; et al. PIVKA-II Combined with Alpha-Fetoprotein for the Diagnostic Value of Hepatic Tumors in Children: A Multicenter, Prospective Observational Study. Hepatol. Int. 2024, 18, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Ribaldone, D.G.; Abate, M.L.; Ciancio, A.; Pellicano, R.; Smedile, A.; Saracco, G.M. Performance of Protein Induced by Vitamin K Absence or Antagonist-II Assessed by Chemiluminescence Enzyme Immunoassay for Hepatocellular Carcinoma Detection: A Meta-Analysis. Scand. J. Gastroenterol. 2018, 53, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, R.; Wei, Q.; Xu, X. The Landscape Of Alpha Fetoprotein In Hepatocellular Carcinoma: Where Are We? Int. J. Biol. Sci. 2022, 18, 536–551. [Google Scholar] [CrossRef] [PubMed]
| Variable | HCC N = 80 | LC N = 111 | CHC N = 111 |
|---|---|---|---|
| Median Age (Years) | 66 [48–85] | 63 [20–83] | 59 [28–84] |
| Male/female: N [%] | 65/15 (81%/19%) | 67/44 (60%/40%) | 41/70 (37%/63%) |
| Liver Disease Etiology | |||
| 19 (23.75%) | 46 (42%) | 104 (94%) |
| 7 (8.75%) | 11 (10%) | 0 (0%) |
| 10 (12.5%) | 5 (4%) | 0 (0%) |
| 4 (5%) | 9 (8%) | 0 (0%) |
| 2 (2.5%) | 2 (2%) | 0 (0%) |
| Multifactorial disease: | |||
| 2 (2.5%) | 14 (13%) | 1 (1%) |
| 10 (12.5%) | 5 (4%) | 0 (0%) |
| 3 (3.75%) | 13 (12%) | 6 (5%) |
| 1 (1.25%) | 1 (1%) | 0 (0%) |
| 23 (28.75%) | 5 (4%) | 0 (%) |
| CTP Score | |||
| 63 (78.75%) | 102 (92%) | 110 (99%) |
| 17 (21.25%) | 9 (8%) | 1 (1%) |
| Meld Score | 9 [3–16] | 8 [1–22] | 5 [0–9] |
| BCLC Score | |||
| 25 (31.25%) | ||
| 33 (41.25%) | ||
| 22 (27.5%) |
| Variable | HCC N = 80 | LC N = 111 | CHC N = 111 |
|---|---|---|---|
| Type 2 Diabetes Mellitus | 30 (37.5%) | 29 (26%) | 8 (7%) |
| AFP (ng/mL) | 5.5 [1.3–43,857] | 3 [1–109] | 3 [1–33] |
| ALT (U/L) | 24 [9–173] | 23 [4–225] | 42 [10–277] |
| AST (U/L) | 30 [11–175] | 28 [12–174] | 34 [13–232] |
| Total bilirubin (mg/dL) | 1.03 [0.33–3.2] | 0.96 [0.2–3.93] | 0.7 [0.2–1.5] |
| Creatinine (mg/dL) | 0.82 [0.44–2.57] | 0.79 [0.47–2.6] | 0.7 [0.4–1.2] |
| GRF (mL/min) | 90.09 [26–117.24] | 92.05 [22.45–134.29] | 95.21 [51.07–126.41] |
| INR | 1.135 [0.96–1.5] | 1.17 [0.9–1.73] | 1.01 [0.8–1.16] |
| Albumine (g/dL) | 3.81 [2.7–4.7] | 4 [2.74–4.8] | 4.1 [3.4–4.8] |
| Neutrophils (n./mm3) | 3.19 [1.01–8.58] | 2.8 [0.96–7.99] | 3.34 [1.3–10.6] |
| Lymphocytes (n./mm3) | 1.345 [0.4–8.1] | 1.46 [0.38–5.44] | 1.92 [0.69–4.7] |
| Platelets (n.×109/L) | 113 [35–290] | 108 [21–303] | 229 [47–430] |
| Analysis | First Variable | Second Variable | z Score | p Value |
|---|---|---|---|---|
| Mann-Whitney | HCC | LC | −6.810 | <0.001 |
| HCC | CHC | −40.235 | <0.001 | |
| CHC | LC | −40.235 | <0.001 | |
| Multiple nodules | Single nodule | −0.361 | 0.719 | |
| 0 | A | −0.934 | 0.352 | |
| 0 | B | −2.042 | 0.041 | |
| A | B | −2.035 | 0.042 | |
| Kruskal–Wallis | H score | p value | ||
| HCC-LC-CHC | 474.269 | <0.001 | ||
| 0-A-B | 5.738 | 0.057 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarneri, V.; Loggi, E.; Ramacieri, G.; Serra, C.; Vukotic, R.; Vitale, G.; Scuteri, A.; Cursaro, C.; Margotti, M.; Galli, S.; et al. Diagnostic Performance of PIVKA-II in Italian Patients with Hepatocellular Carcinoma. Cancers 2025, 17, 167. https://doi.org/10.3390/cancers17020167
Guarneri V, Loggi E, Ramacieri G, Serra C, Vukotic R, Vitale G, Scuteri A, Cursaro C, Margotti M, Galli S, et al. Diagnostic Performance of PIVKA-II in Italian Patients with Hepatocellular Carcinoma. Cancers. 2025; 17(2):167. https://doi.org/10.3390/cancers17020167
Chicago/Turabian StyleGuarneri, Valeria, Elisabetta Loggi, Giuseppe Ramacieri, Carla Serra, Ranka Vukotic, Giovanni Vitale, Alessandra Scuteri, Carmela Cursaro, Marzia Margotti, Silvia Galli, and et al. 2025. "Diagnostic Performance of PIVKA-II in Italian Patients with Hepatocellular Carcinoma" Cancers 17, no. 2: 167. https://doi.org/10.3390/cancers17020167
APA StyleGuarneri, V., Loggi, E., Ramacieri, G., Serra, C., Vukotic, R., Vitale, G., Scuteri, A., Cursaro, C., Margotti, M., Galli, S., Caracausi, M., Brodosi, L., Gabrielli, F., & Andreone, P. (2025). Diagnostic Performance of PIVKA-II in Italian Patients with Hepatocellular Carcinoma. Cancers, 17(2), 167. https://doi.org/10.3390/cancers17020167






